Synthesis method of chesulfamide
The technology of a cyclohexasulfame and its synthesis method is applied in the direction of sulfonamide preparation, sulfonate preparation, organic chemistry, etc. It can solve the problems of difficult operation, many wastes in the synthesis technology, harsh conditions, etc., and achieves mild process conditions, The effect of reducing the discharge of three wastes and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
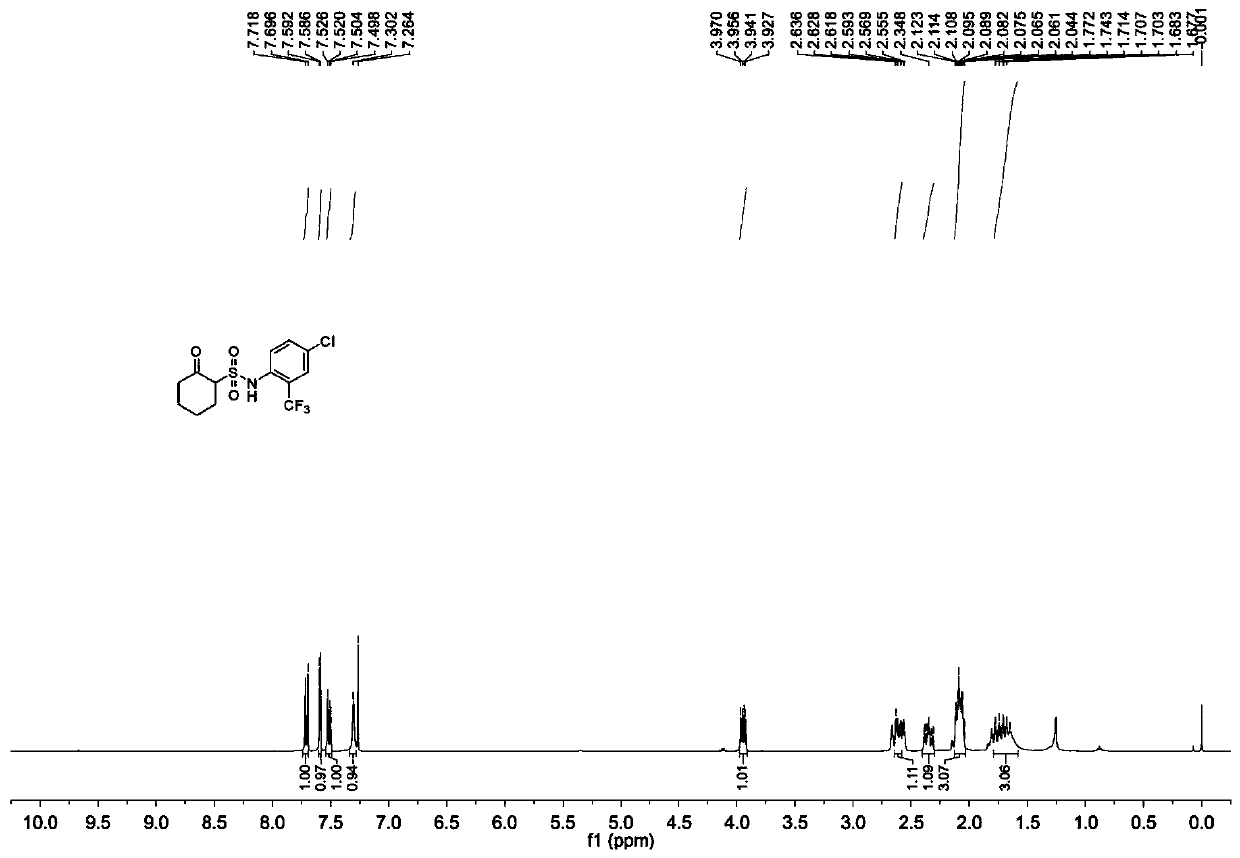
[0032] In the present invention, the specific synthetic method of cyclohexasulfame can be decomposed into following 3 technological engineerings:
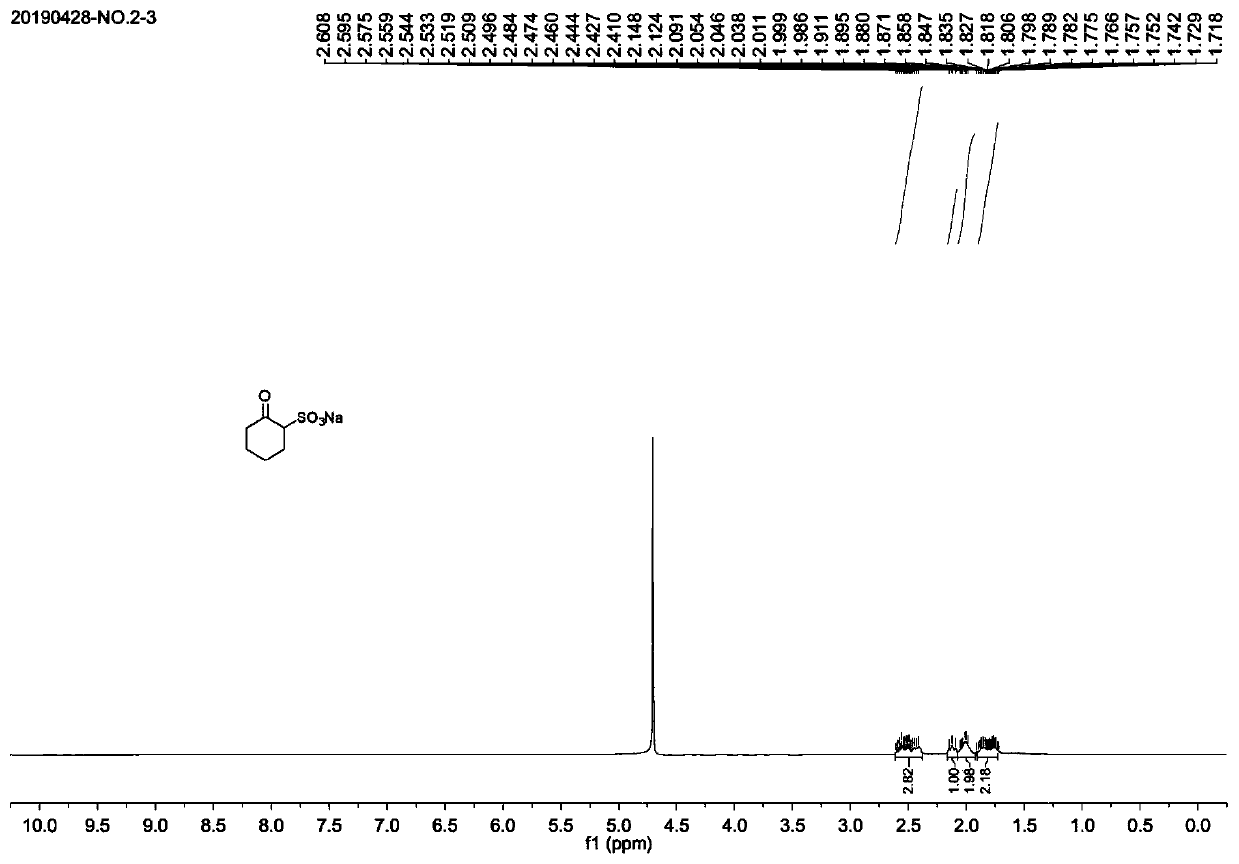
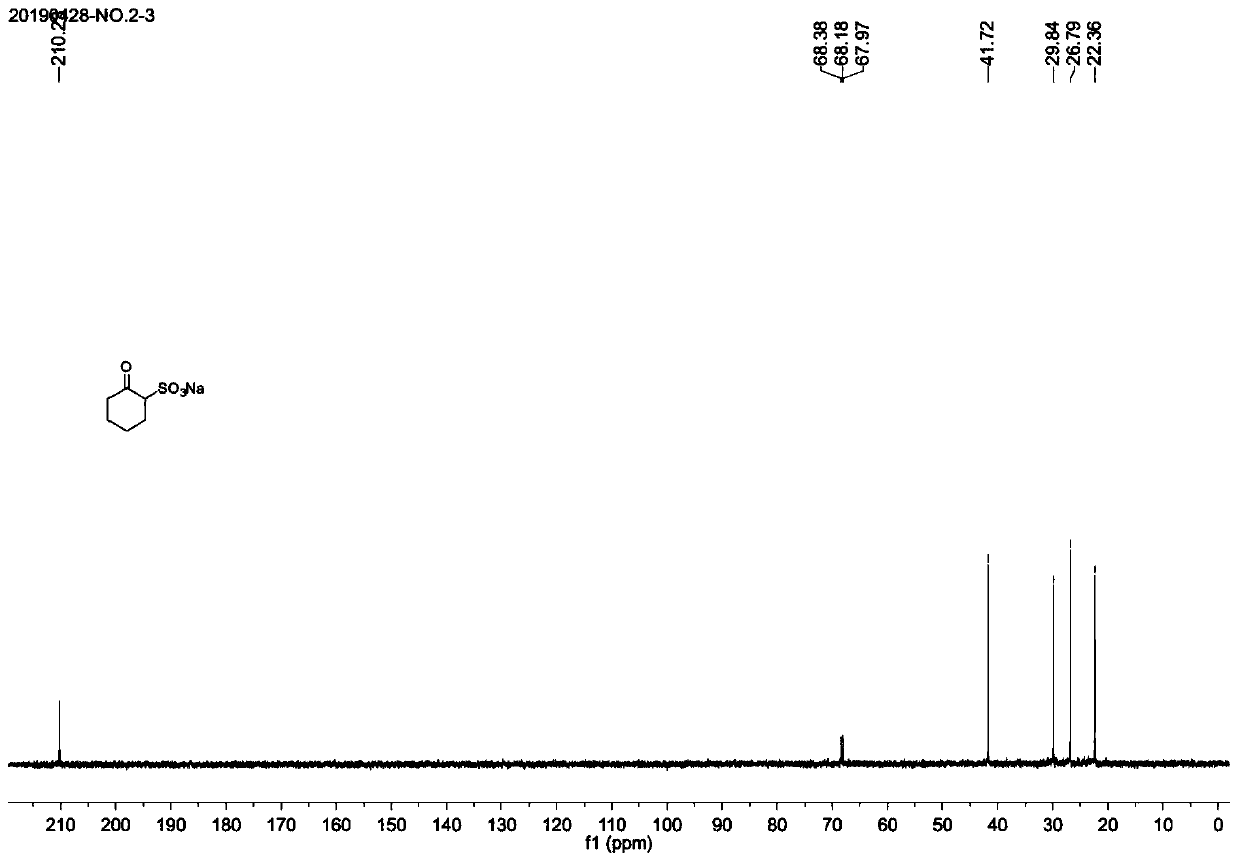
[0033] (1) Preparation of sodium 2-oxocyclohexylsulfonate
[0034] 13.26 g (100 mmol) of 2-chlorocyclohexanone, 18.91 g (150 mmol) of anhydrous sodium sulfite and 300 ml of water were mixed and charged into a 500 ml round-bottomed flask, and stirred at reflux at 100°C for 20 hours. After the reaction is complete, when the reaction liquid is cooled to about 40°C, the solvent is rotovapped to dryness. Add 500ml of absolute ethanol to the raffinate, evaporate the remaining water to dryness, add 200ml of 1:1 anhydrous ether-n-hexane, stir at room temperature for a period of time, filter with suction, and dry under reduced pressure to obtain 2-oxocyclohexyl The crude sodium sulfonate was directly used in the next reaction.
[0035] (2) Preparation of 2-oxocyclohexylsulfonyl chloride
[0036] Add sodium 2-oxocyclohexylsulfonate obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com