Large steric hindrance ligand Pd complex catalyst as well as preparation method and application thereof
A technology of catalysts and complexes, applied in the chemical field, can solve the problems of difficult separation of palladium catalysts and difficult recovery and reuse of palladium black, and achieve good stability, catalysis, and high catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
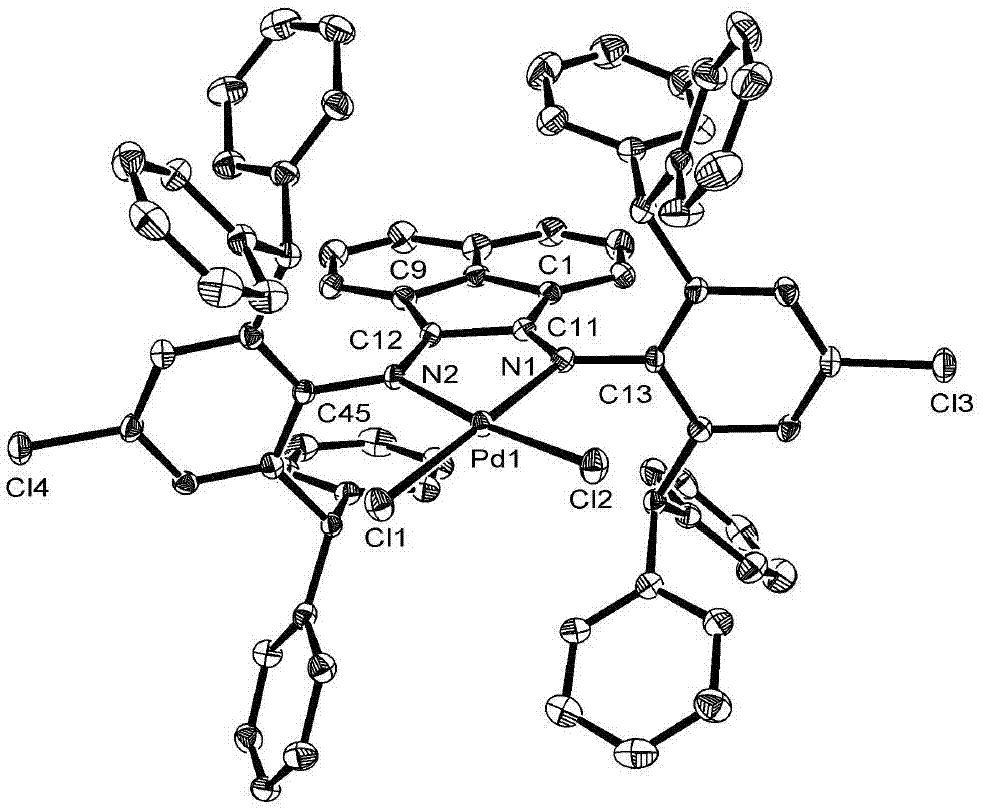
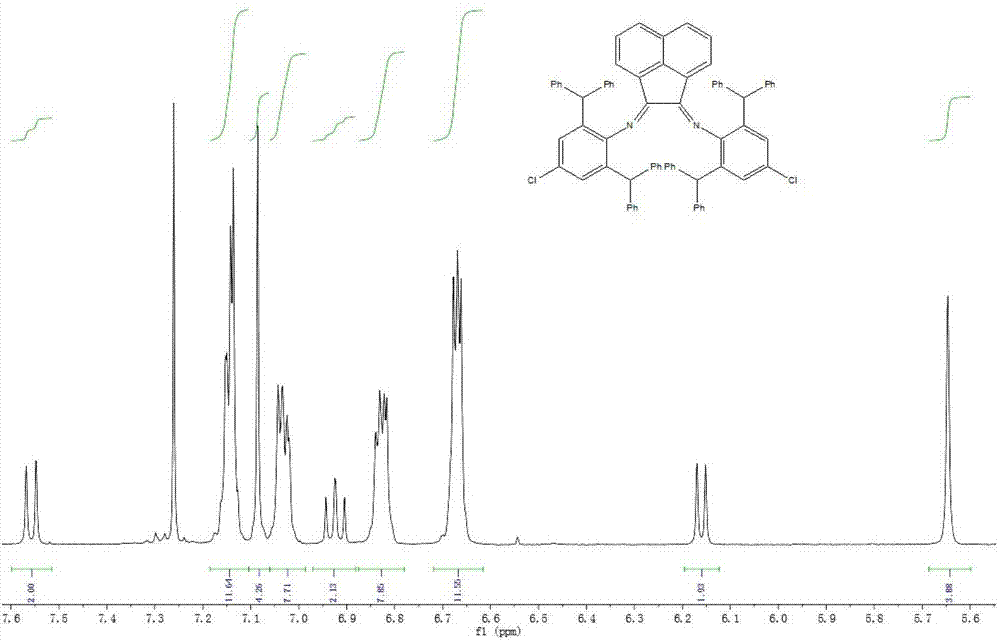
[0050] Ligand 3 (R 1 Benzhydryl; R 2 Chlorine): Preparation of 2,6-bis(benzhydryl)-4-chloroaniline (1.06 g, 2.3 mmol) and 2-(2,6-bis(benzhydryl)-4-chlorophenylimine ) acenaphthylen-1-one (1.44g, 2.3mmol) was added with 150mg p-toluenesulfonic acid as a catalyst, stirred at reflux in 100mL toluene for 6h, filtered and removed the solvent, and the residue was dissolved in dichloromethane, passed through a silica gel column, and used petroleum After rinsing with ether / ethyl acetate (50:1), the second fraction was separated into the product, and the solvent was removed to obtain 0.25 g of a yellow solid with a yield of 10.2%. Melting point: 257–258°C. IR(KBr):3061,3029,2894,1655,1592,1493,1423,1180,1034,890,765,728,696cm -1 . 1 H NMR (400MHz, CDCl 3 ,TMS):δ=7.56(d,J=8.25Hz,2H),7.18-7.13(m,12H),7.09(s,4H),7.03(m,8H),6.92(t,J=7.55Hz, 2H),6.83(m,8H),6.67(t,J=8.64Hz,12H),6.16(d,J=7.15Hz,2H),5.65(s,4H)ppm. 13 C NMR (100MHz, CDCl 3 ,TMS): δ=163.8,147.5,142.9,141.8,134.0,129.8,12...
Embodiment 2
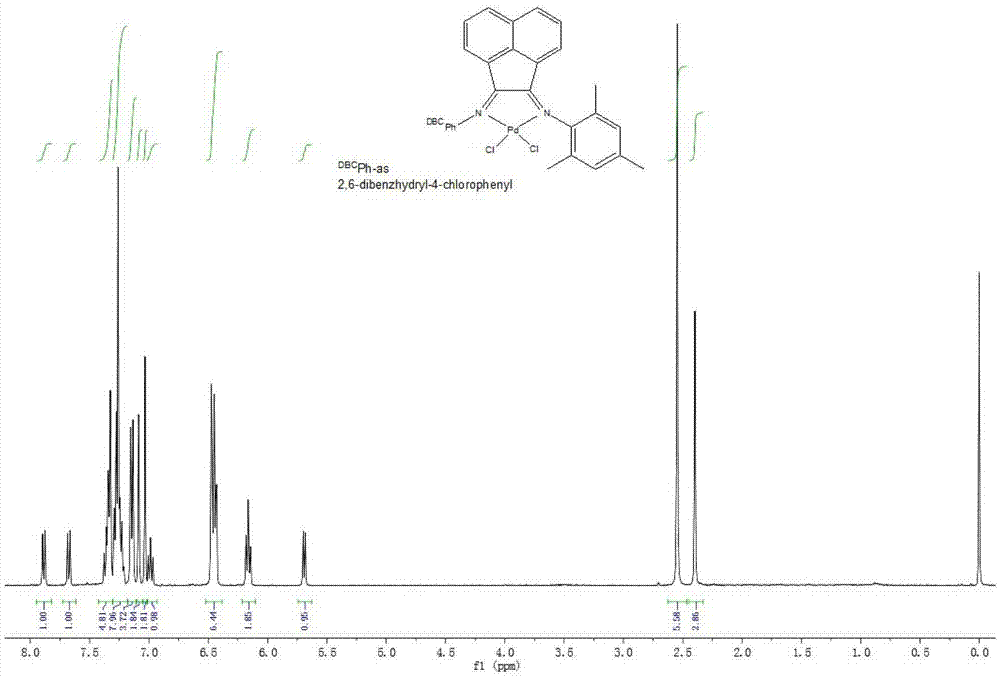
[0053] Pd complex catalyst 1 (R 1 is methyl; R 2 for the preparation of methyl): the N 2 Under protection, the PdCl 2 (CH 3 EN) 2 (0.04g, 0.14mmol) and ligand 4 (compound of formula II, R 1 is methyl; R 2 Methyl) (0.14mmol) was added to 10mL of dichloromethane, stirred at room temperature for 12h, passed through a silica gel column, and washed with petroleum ether / ethyl acetate (5:1). Rinse with methane, collect the second fraction, and the obtained is a Pd complex catalyst. After removing the solvent, 68 mg of a red solid is obtained, with a yield of 52.7%. IR(KBr):3056,2961,2871,1623,1600,1576,1490,1442,1299,1182,1076,768,742,698cm -1 . 1 H NMR (400MHz, CDCl 3 ,TMS):δ=7.89(d,J=8.31Hz,1H),7.68(d,J=8.30Hz,1H),7.35(m,5H),7.29–7.21(m,6H),7.14(d, J=7.48Hz,4H),7.03(s,2H),7.01(s,2H),6.99(d,J=7.73Hz,1H),6.45(t,J=9.88Hz,7H),6.16(t, J=7.40Hz,2H),5.69(d,J=7.22Hz,1H),2.55(s,6H),2.40(s,3H)ppm. 13 C NMR (100MHz, CDCl 3 ,TMS):δ=178.2,176.3,146.3,141.5,141.0,139.7,138.7,143.2,1...
Embodiment 3
[0055] Pd complex catalyst 2 (R 1 is ethyl; R 2 is methyl) is prepared with embodiment 2, and the difference is that ligand is ligand 2 (compound of formula II, R 1 is ethyl; R 2 For methyl), 82 mg of red solid was obtained, with a yield of 62.1%. IR (KBr):3060,2965,2933,2871,1625,1603,1581,1489,1442,1301,1182,1069,767,745,699cm -1 . 1 HNMR (400MHz, CDCl 3 ,TMS):δ=7.88(d,J=8.30Hz,1H),7.66(d,J=8.32Hz,1H),7.35(d,J=7.27Hz,4H),7.30-7.18(m,8H) ,7.13(d,J=5.57Hz,6H),7.03(s,2H),6.97(t,J=7.90Hz,1H),6.46(t,J=6.80Hz,6H),6.18(t,J= 7.34Hz, 2H), 5.69(d, J=7.10Hz, 1H), 3.15(m, 2H), 2.80(m, 2H), 2.44(s, 3H), 1.38(t, J=7.50Hz, 6H) ppm. 13 C NMR (100MHz, CDCl 3,TMS):δ=177.8,176.3,145.8,141.1,140.2,139.1,138.7,138.4,134.1,133.5,132.1,129.1,129.0,128.9,128.2,128.0,127.9,127.2,126.8,125.2 122.8, 122.1, 53.0, 24.3, 21.1, 13.7ppm. Elemental analysis C 55 h 45 Cl 3 N 2 Pd Theoretical value (%): C, 69.78, H, 4.79, N, 2.96; Experimental value (%): C, 69.39, H, 4.97, N, 2.75. Its NMR spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com