Synthetic method of drug intermediate of hemosanone, parent nucleus of hemosanone
A synthetic method, the technology of hemosanone, applied in the field of organic synthesis, can solve problems such as low efficiency and by-product pollution, and achieve the effects of low cost, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
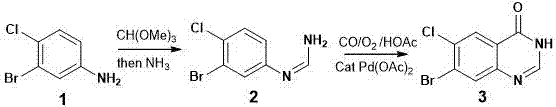
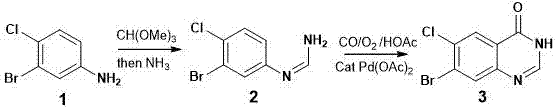
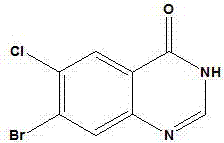
[0030] The synthetic method of fushanone parent nucleus, the step is:
[0031] 1) In the presence of a catalyst, heat 3-bromo-4-chloroaniline and trimethyl orthoformate to fully react, cool, and then react with NH 3 Fully reacted, separated and preliminarily purified to obtain a solid crude product;
[0032] 2) In the presence of a catalyst, the solid crude product obtained in the previous step was mixed with CO, O 2 Heating for sufficient reaction, separation and purification to obtain the final product.
[0033] Preferably, in step 1), the catalyst is a protonic acid catalyst; more preferably, it is one of p-toluenesulfonic acid, solid superacid, and dodecylbenzenesulfonic acid; even more preferably, it is p-toluenesulfonic acid toluenesulfonic acid;
[0034] Preferred, NH 3 The use form is saturated methanol solution of ammonia;
[0035] Preferably, the dosage ratio of 3-bromo-4-chloroaniline, trimethyl orthoformate and saturated methanol solution of ammonia is (1-5) m...
Embodiment
[0047] The synthetic method of fushanone parent nucleus, the step is:
[0048] 1. Preparation of Compound 2
[0049] 3-Bromo-4-chloroaniline (compound 1, 0.2 g) was heated and mixed with trimethyl orthoformate (5 mL), a catalytic amount of p-toluenesulfonic acid (2 mg) was added, heated to reflux under stirring, and the Analysis (TLC) followed the reaction and detected by ultraviolet light until compound 1 disappeared completely. After cooling to room temperature, a saturated methanol solution of ammonia (2 mL) was added and stirred at room temperature for 10 hours. Methanol and remaining trimethyl orthoformate were distilled off to obtain a crude compound 2 (0.22 g) as a solid. Wash once with water (10 mL) and air dry. The product was directly used in the next reaction without further purification.
[0050] 2. Synthesis of hemosanone mother nucleus
[0051] The above reaction solid crude product (0.22 g) was mixed with glacial acetic acid (10 mL) and palladium acetate (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com