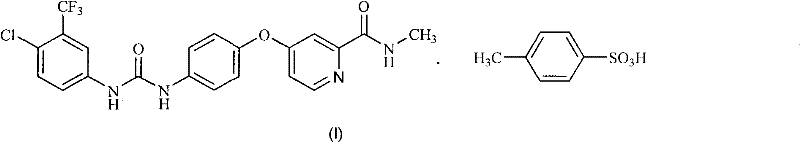
Method for preparing sorafenib
An inert solvent, stage technology, applied in the field of medicinal chemistry, can solve the problems of difficult storage, long reaction time, poor stability, etc., and achieve the effects of easy transportation and storage, short reaction time, and easy metering.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
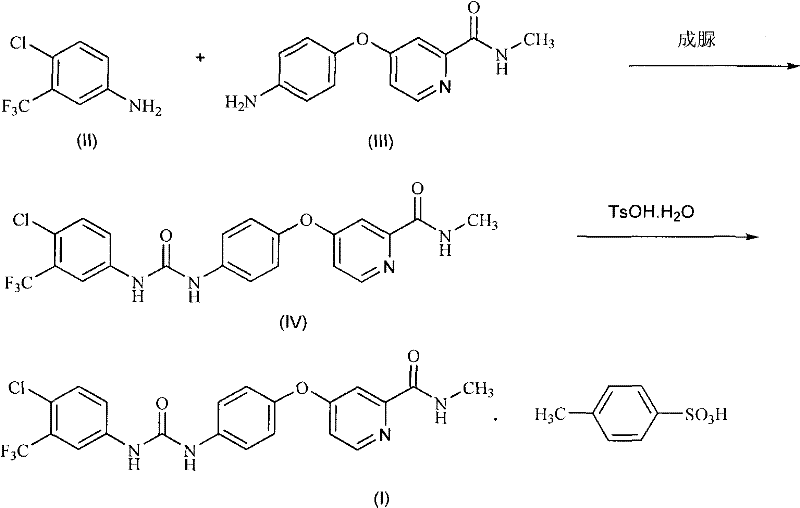
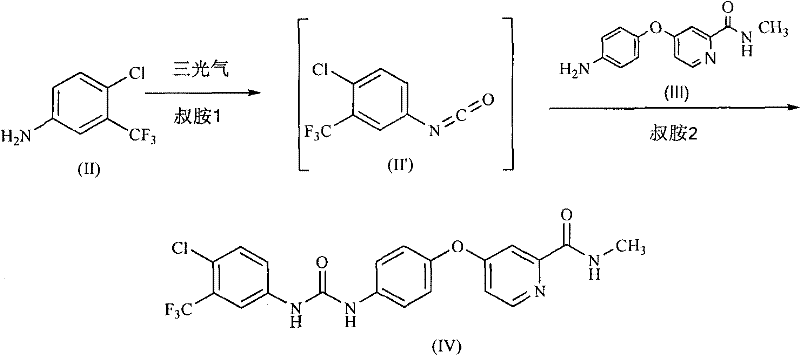
[0054] Example 1: 4-(4-{3-[3-(trifluoromethyl)-4-chlorophenyl]ureido}phenoxy)-N-methylpyridine-2-carboxamide (sorafil Ni, the preparation of IV)
[0055] Under nitrogen, dissolve triphosgene (0.55g, 1.85mmol) in anhydrous dichloromethane (10ml), and add 3-trifluoromethyl-4-chloroaniline (II) (0.98g , 5 mmol) and diisopropylethylamine (0.78 g, 6 mmol) in anhydrous dichloromethane (10 ml). After dropping, the mixture was stirred at the same temperature for another 30 minutes. Add 4-(4-aminophenoxy)-N-methyl-2- A solution of picolinamide (III) (1.1 g, 4.5 mmol) and diisopropylethylamine (0.7 g, 5.4 mmol) in anhydrous dichloromethane (10 ml). After dropping, stir at room temperature for 1 hour. After washing the obtained reaction solution with water, water (15 ml) was added, and sorafenib (IV) seed crystals were inoculated into the obtained two-phase system. Stir and crystallize at room temperature for 4 hours, filter and wash, and dry in vacuo to obtain Sorafenib (IV) (1.76 ...
Embodiment 2
[0058] Embodiment 2: the preparation of sorafenib p-toluenesulfonate (I)
[0059] Get the obtained Sorafenib of Example 1 (1.16g, 2.5mmol) and ethyl acetate (10ml) and water (0.25ml) mix, after heating to 70 ℃, dropwise add p-toluenesulfonic acid monohydrate (0.57g, 3mmol ) in ethyl acetate (0.5ml) and water (0.15ml), then concentrated under reduced pressure. Add absolute ethanol (22ml) to the resulting residue, reflux for 30 minutes, then cool to room temperature within 2 hours, filter with suction, wash the filter cake with absolute ethanol, and vacuum-dry at 50°C to obtain Sorafenib p-toluenesulfonate (I), as a slightly brown crystalline powder (1.45 g, 91%):
[0060] 1 H-NMR (DMSO-d 6 )δ: 9.33(s, 1H, exchangeable), 9.13(s, 1H, exchangeable), 8.86(d, J=4.4Hz, 1H, exchangeable), 8.55(d, J=5.6Hz, 1H), 8.11(d , J=2.4Hz, 1H), 7.68(dd, J=2.4, 8.8Hz, 1H), 7.57-7.63(m, 4H), 7.54(d, J=8.0Hz, 2H), 7.22(dd, J= 2.4, 5.6Hz, 1H), 7.18(d, J=9.2Hz, 2H), 7.14(d, J=8.0Hz, 2H), 2.82(d, ...
Embodiment 3
[0061] Example 3: 4-(4-{3-[3-(trifluoromethyl)-4-chlorophenyl]ureido}phenoxy)-N-methylpyridine-2-carboxamide (sorafil Ni, the preparation of IV)
[0062] Under nitrogen, triphosgene (0.55g, 1.85mmol) was dissolved in anhydrous 2-methyltetrahydrofuran (9ml), and 3-trifluoromethyl-4-chloroaniline (II) was added dropwise at 24~30°C ( 0.98g, 5mmol) and diisopropylethylamine (0.78g, 6mmol) in dry 2-methyltetrahydrofuran (10ml). After dropping, the mixture was stirred at the same temperature for another 30 minutes. Add 4-(4-aminophenoxy)-N-methyl-2- A solution of picolinamide (III) (1.1 g, 4.5 mmol) and diisopropylethylamine (0.7 g, 5.4 mmol) in anhydrous 2-methyltetrahydrofuran (10 ml). After dropping, the mixture was stirred at about 40°C for 1 hour. The resulting reaction solution was washed successively with a mixture of saturated aqueous sodium bicarbonate (2ml) and water (4ml), and water (6ml), then concentrated under reduced pressure to about half the volume, and inoculat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com