Preparation method of 4-Chloro-2,5-dimethoxyacetoace tanilide
A technology of chloroacetoacetanilide and dimethoxy, which is applied in 2 fields and can solve the problems of large energy consumption and large material loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
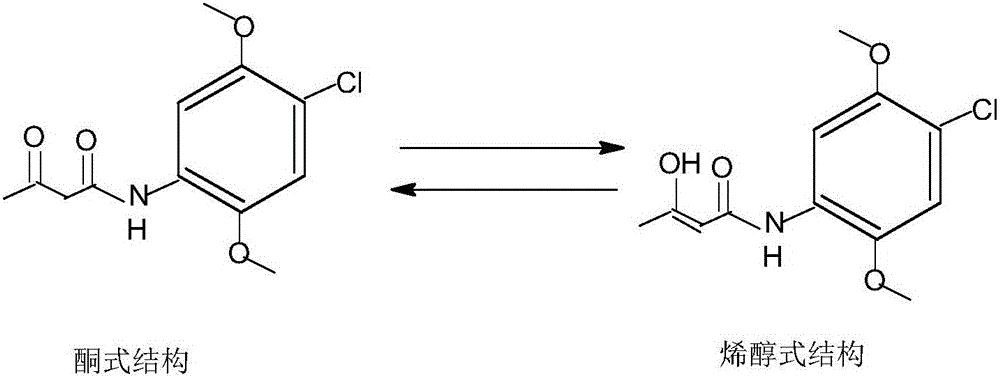
[0029] Add ethanol and 100 grams of 2,5-dimethoxy-4-chloroaniline to a 500ml three-necked flask equipped with a stirrer and a thermometer, control the temperature at 50°C, add 44.8 grams of diketene dropwise, and continue After 4 hours of reaction, the reaction was completed. The pH of the reaction solution was adjusted to 3.0 with hydrochloric acid, and the reaction solution was rapidly cooled at a rate of 35°C / min. Filter, wash and dry at 5°C to obtain the product. According to HPLC analysis, the content of enol isomer in 2,5-dimethoxy-4-chloroacetoacetanilide is 28 ppm.
Embodiment 2
[0031] Add methanol and 100 grams of 2,5-dimethoxy-4-chloroaniline to a 500ml three-necked flask equipped with a stirrer and a thermometer, control the temperature at 25°C, add 53.7 grams of diketene dropwise, and continue After reacting for 2 hours, the reaction was completed. The pH of the reaction solution was adjusted to 3.0 with hydrochloric acid, and the reaction solution was rapidly cooled at a speed of 30°C / min. Filter, wash and dry at 10°C to obtain the product. According to HPLC analysis, the content of enol isomer in 2,5-dimethoxy-4-chloroacetoacetanilide is 20 ppm.
Embodiment 3
[0033] Add 100 grams of isopropanol and 2,5-dimethoxy-4-chloroaniline to a 500ml three-necked flask equipped with a stirrer and a thermometer, control the temperature at 60°C, add 51.2 grams of diketene dropwise, and continue After reacting for 2.5 hours, the reaction was completed. The pH of the reaction solution was adjusted to 2.5 with hydrochloric acid, and it was rapidly cooled at a rate of 40°C / min. °C, filtered, washed, and dried to obtain the product. According to HPLC analysis, the content of enol isomer in 2,5-dimethoxy-4-chloroacetoacetanilide was 34 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com