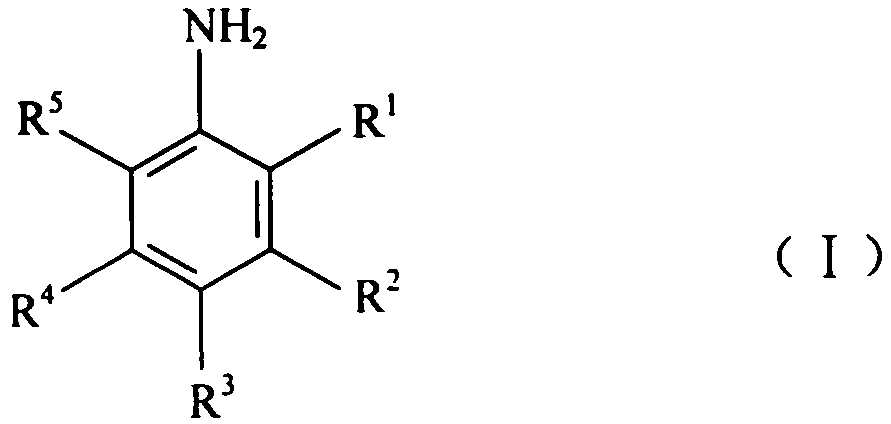
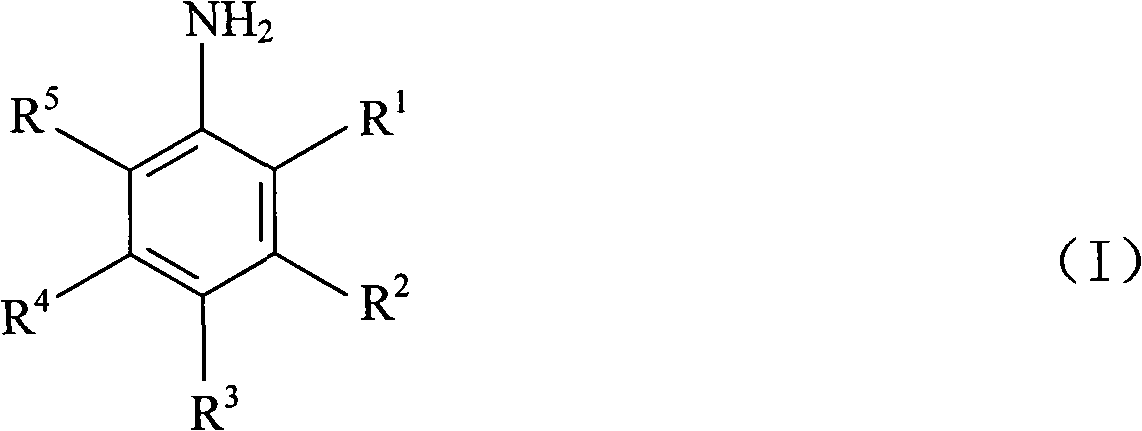Method for converting arylamine polyhalide
A polychlorinated aromatic amine and compound technology, which is applied in the preparation of organic compounds, the preparation of amino compounds, chemical instruments and methods, etc., to achieve the effect of reducing environmental pollution and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This example is used to illustrate the preparation of the catalyst.
[0024] According to the final composition of the catalyst, an appropriate amount of Group VIII metal chloride (such as palladium chloride) was weighed and dissolved in 20 ml of concentrated hydrochloric acid, and diluted to 100 ml with water. Take 50ml of the prepared mixed solution, add it to 100g of activated carbon carrier treated with nitric acid, soak it at 25°C for 8 hours, filter it, evaporate it to dryness on a water bath in an evaporating dish, and finally place it in an oven for drying at 120°C. That is, an activated carbon-supported catalyst with Group VIII metal as an active component is obtained. The catalyst needs to pass through H before use 2 reduction.
Embodiment 2
[0026] This example illustrates the effect of the method of the invention.
[0027] Add 100 grams of 2,4,6-trichloroaniline, 0.38 grams of activated carbon-supported palladium catalyst (containing 0.1% by weight of palladium), 300 ml of ethanol solvent, and 10 grams of sodium hydroxide into a 1 L autoclave. Under the hydrogen pressure of 3.5MPa, the temperature was controlled at 120° C., stirred, and a partial hydrodechlorination reaction was carried out. After the reaction was carried out for 4 hours, 85 grams of the final reaction product were obtained, including 12% of dichloroaniline and 88% of monochloroaniline.
Embodiment 3
[0029] Add 100 grams of 2,6-dichloroaniline, 0.2 grams of activated carbon-supported palladium catalyst (containing 0.1% by weight of palladium), 300 ml of ethanol solvent, and 10 grams of sodium hydroxide into a 1 L autoclave. Under the hydrogen pressure of 2.5 MPa, the temperature was controlled at 100° C., stirred, and a partial hydrodechlorination reaction was carried out. After the reaction was carried out for 5 hours, 80 g of the final reaction product was obtained, of which 100% monochloroaniline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com