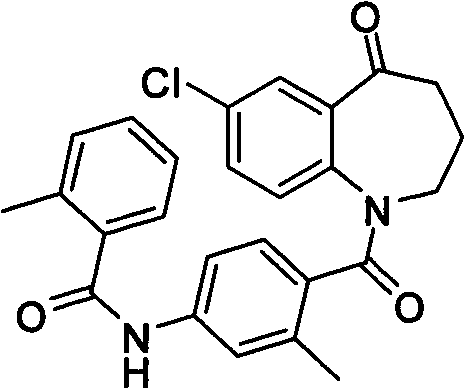
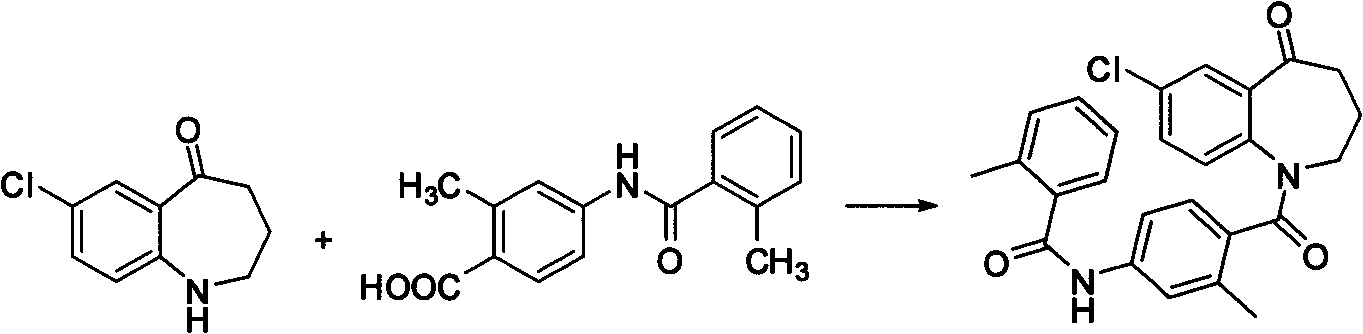
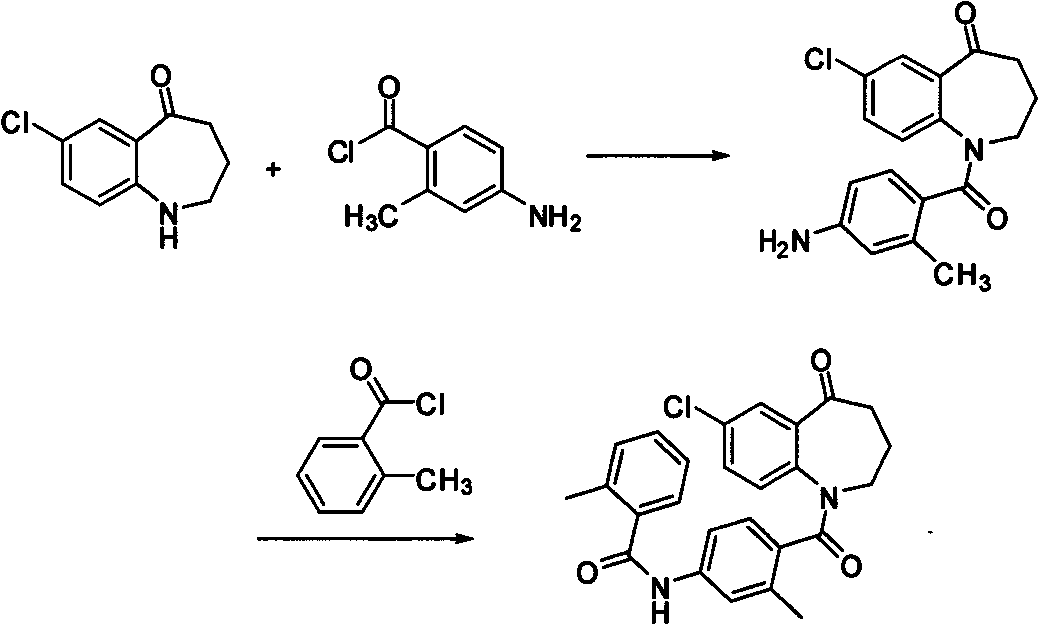
Method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzoazepine-2,5-diketone
A kind of technology of benzoazepine and benzodiazepines, which is applied in the field of preparation of benzoazepine compounds, can solve the problems of unsuitable application of industrialized production, low yield, high cost, etc. Easy to obtain, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of Compound 4:
[0049] Put 4-chloroaniline (12.7g, 0.1mol) in a 250mL reaction flask, add toluene (100mL), stir to dissolve 4-chloroaniline, add succinic anhydride (10.0g, 0.1mol) at room temperature, stir for 10min, and the reaction solution Gradually was a paste. The reaction solution was heated to reflux for 1 hour. The reaction solution was allowed to cool to room temperature, filtered, and a little toluene washed the filter cake. The obtained solid was air-dried at 50° C. to obtain a white flaky solid, compound 4 (22.5 g, 0.0988 mol), with a yield of 98.8%. Melting point: 159.1-161.4°C.
Embodiment 2
[0051] Preparation of compound 4:
[0052] Put 4-chloroaniline (12.7g, 0.1mol) in a 250mL reaction flask, add dichloromethane (100mL), stir to dissolve 4-chloroaniline, add succinic anhydride (10.0g, 0.1mol) at room temperature, stir for 10min, The reaction solution gradually became a paste. The reaction solution was heated to reflux for 1.5 hours. Dichloromethane was distilled off under reduced pressure, anhydrous methanol (90 mL) was added, the temperature was raised to reflux, slowly cooled to room temperature, cooled to 0-5°C in an ice-water bath, stirred for 30 min, filtered, and a little methanol washed the filter cake. The obtained solid was air-dried at 50° C. to obtain a white flaky solid, compound 4 (21.1 g, 0.0927 mol), with a yield of 92.7%. Melting point: 159.7-161.5°C.
Embodiment 3
[0054] Preparation of Compound 4:
[0055] Put 4-chloroaniline (12.7g, 0.1mol) in a 250mL reaction flask, add dichloromethane (40mL), stir to dissolve 4-chloroaniline, add succinic anhydride (11.0g, 0.11mol) at room temperature, stir for 10min, The reaction solution gradually became a paste. The reaction was carried out at room temperature for 2 hours. Dichloromethane was distilled off under reduced pressure, anhydrous methanol (90 mL) was added, the temperature was raised to reflux, slowly cooled to room temperature, cooled to 0-5°C in an ice-water bath, stirred for 30 min, filtered, and a little methanol washed the filter cake. The obtained solid was air-dried at 50° C. to obtain a white flaky solid, compound 4 (20.8 g, 0.0915 mol), with a yield of 91.5%. Melting point: 159.0-161.5°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com