Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Tetramethylbenzenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
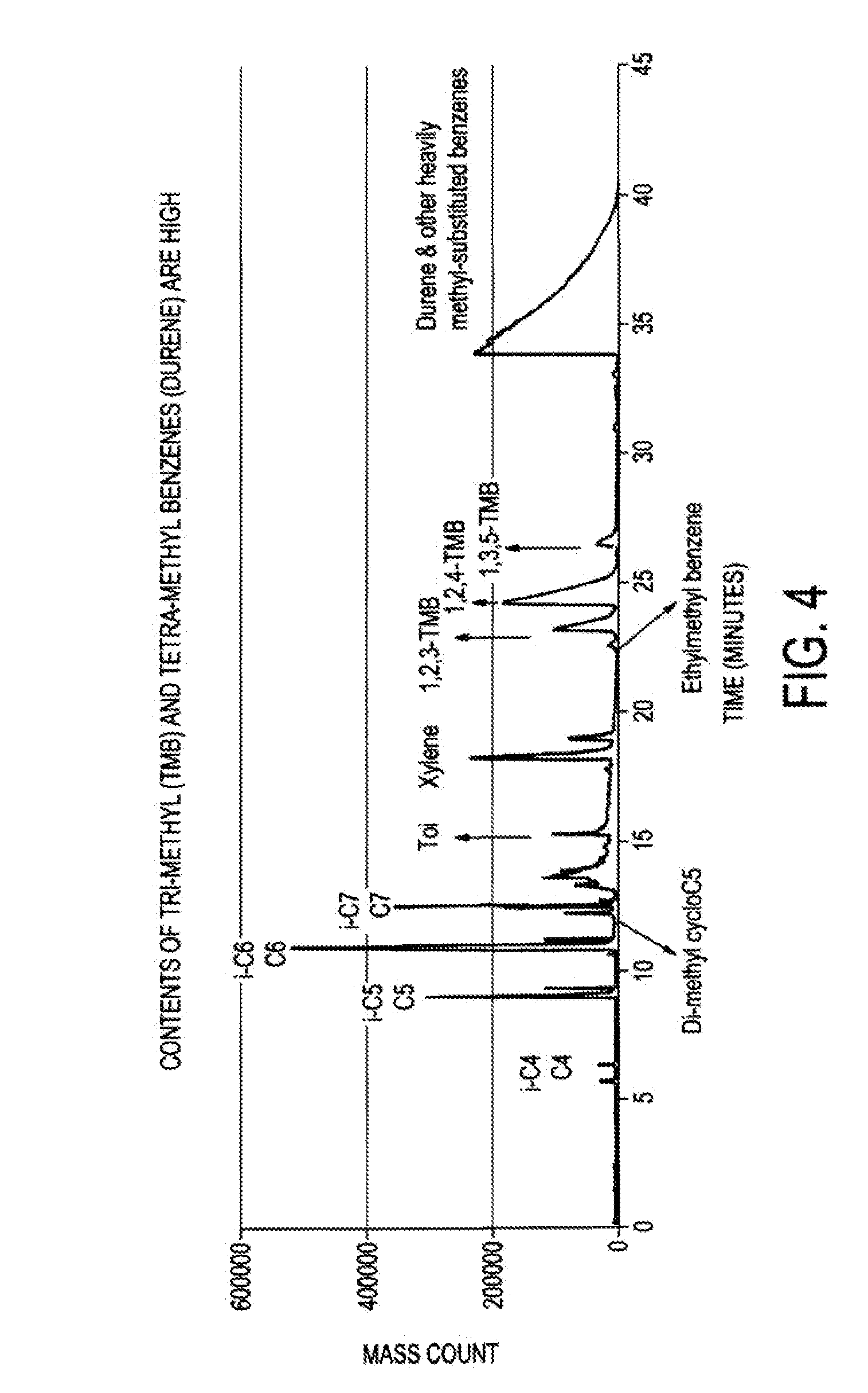
The tetramethylbenzenes constitute a group of substances of aromatic hydrocarbons, which structure consists of a benzene ring with four methyl groups (–CH₃) as a substituent. Through their different arrangement, they form three structural isomers with the molecular formula C₁₀H₁₄. They also belong to the group of C₄-benzenes. The best-known isomer is durene.
Room-temperature self-crosslinking water-soluble polyurethane acrylic resin and preparation method and application thereof
InactiveCN101544738ALow viscosityImprove conversion ratePolyureas/polyurethane adhesivesInksPolymer scienceAcrylic resin
The invention discloses a room-temperature self-crosslinking water-soluble polyurethane acrylic resin and a preparation method and an application thereof. The preparation method adopts an in-situ emulsion polymerization method and improves the prior preparation method, introduces tetramethyl benzene dimethylene diisocyanate or 2, 4 methyl cyclohexyl diisocyanate, and dipropylene glycol or neopentyl glycol functioning as chain extendor in raw materials, thereby lowering the viscosity of polyurethane prepolymer, and achieving the purpose of performing the synthetic reaction without organic solvent; oil-soluble evocating agent and water-soluble evocating agent are adopted to together evocate radical copolymerization so that oil-soluble monomer and water-soluble monomer can more effectively perform the radical copolymerization, thereby improving the reaction conversion rate. In addition, the combination of the two evocating agents also leads emulsion in the reaction to keep stable without easily jellifying and layering. The resin can be used for preparing plastics, aluminum foil package compound gel, paper-plastics compound gel, water color ink, water oil polish, water wooden ware paint or water plastic paint.
Owner:SUN YAT SEN UNIV
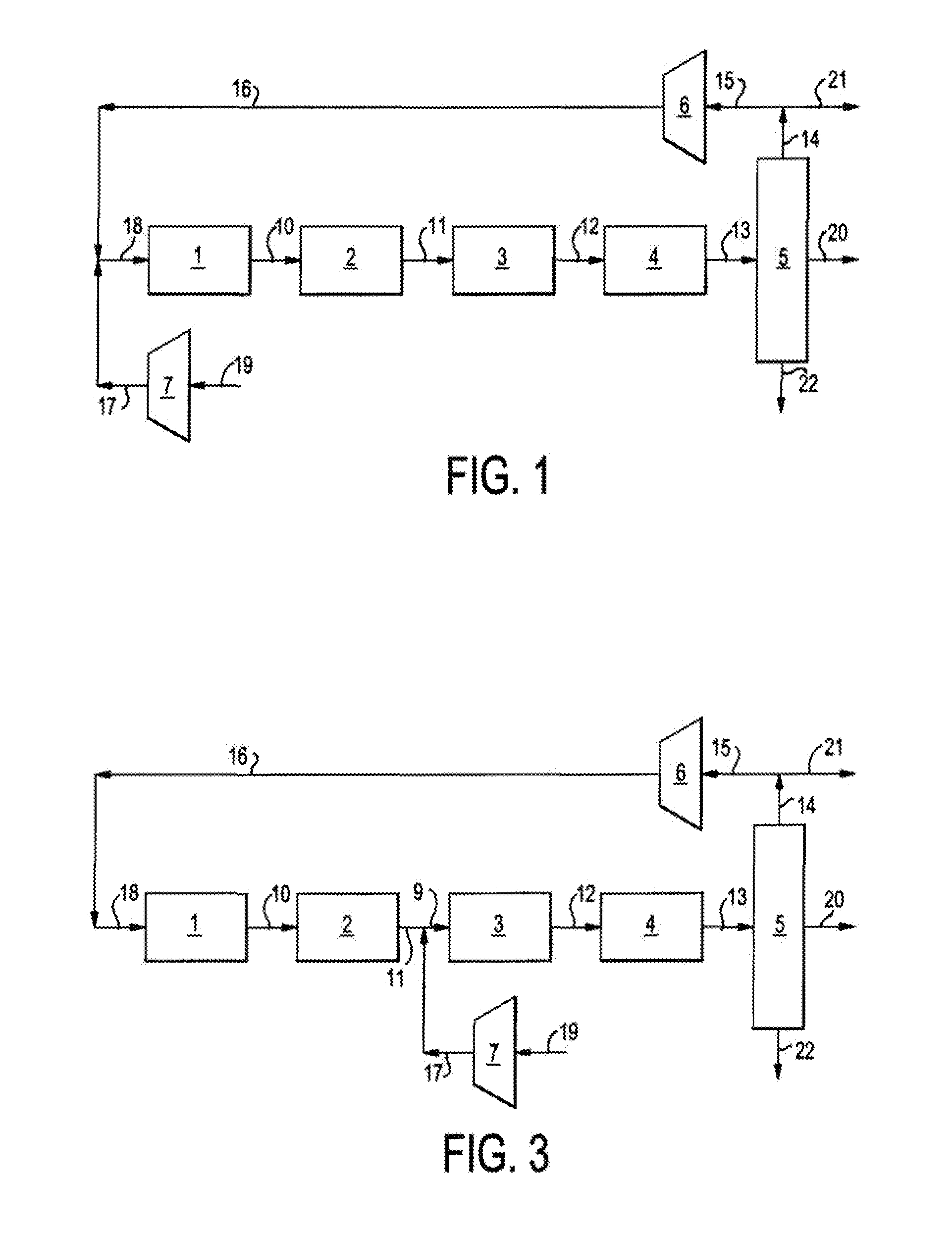
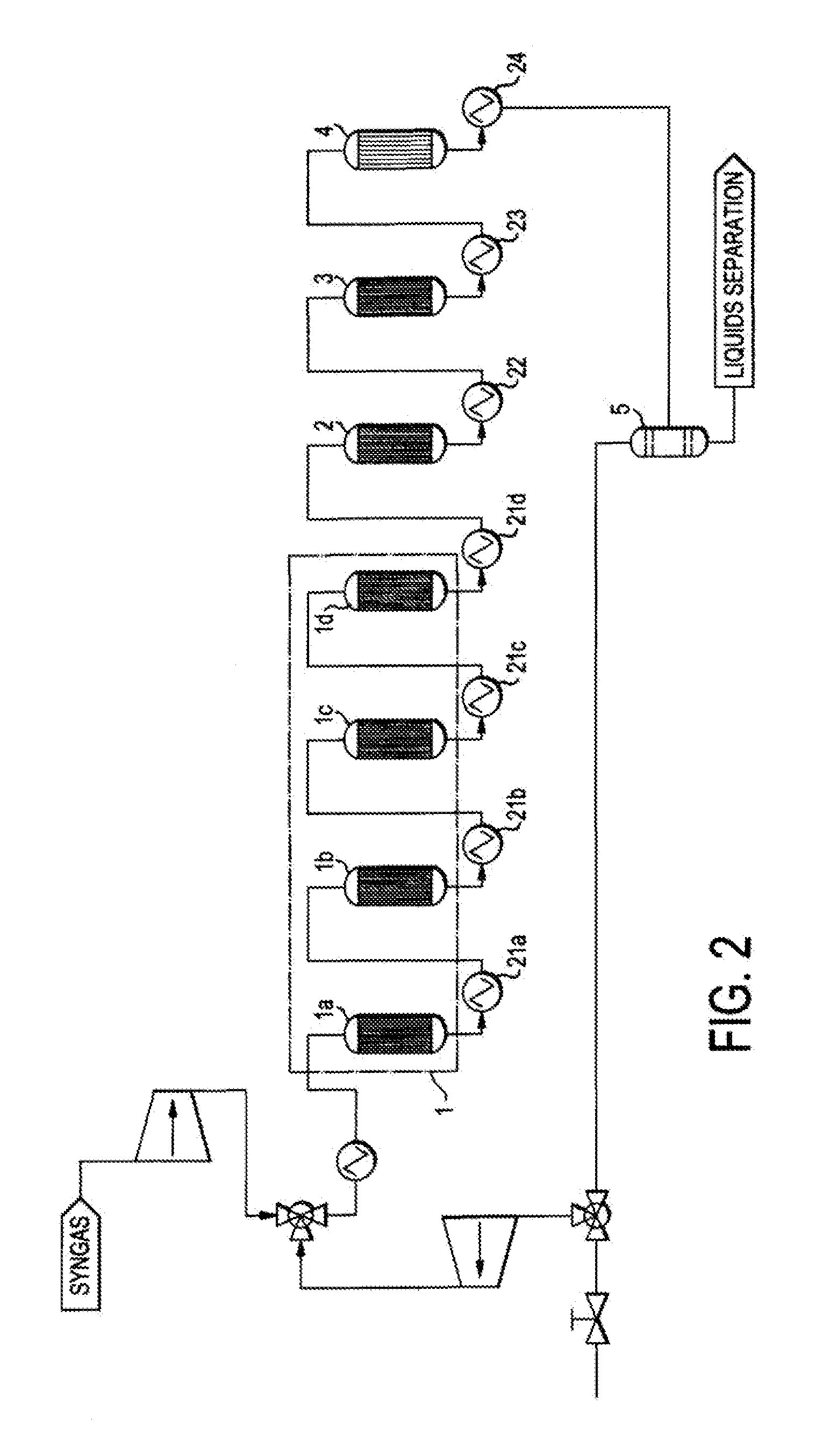
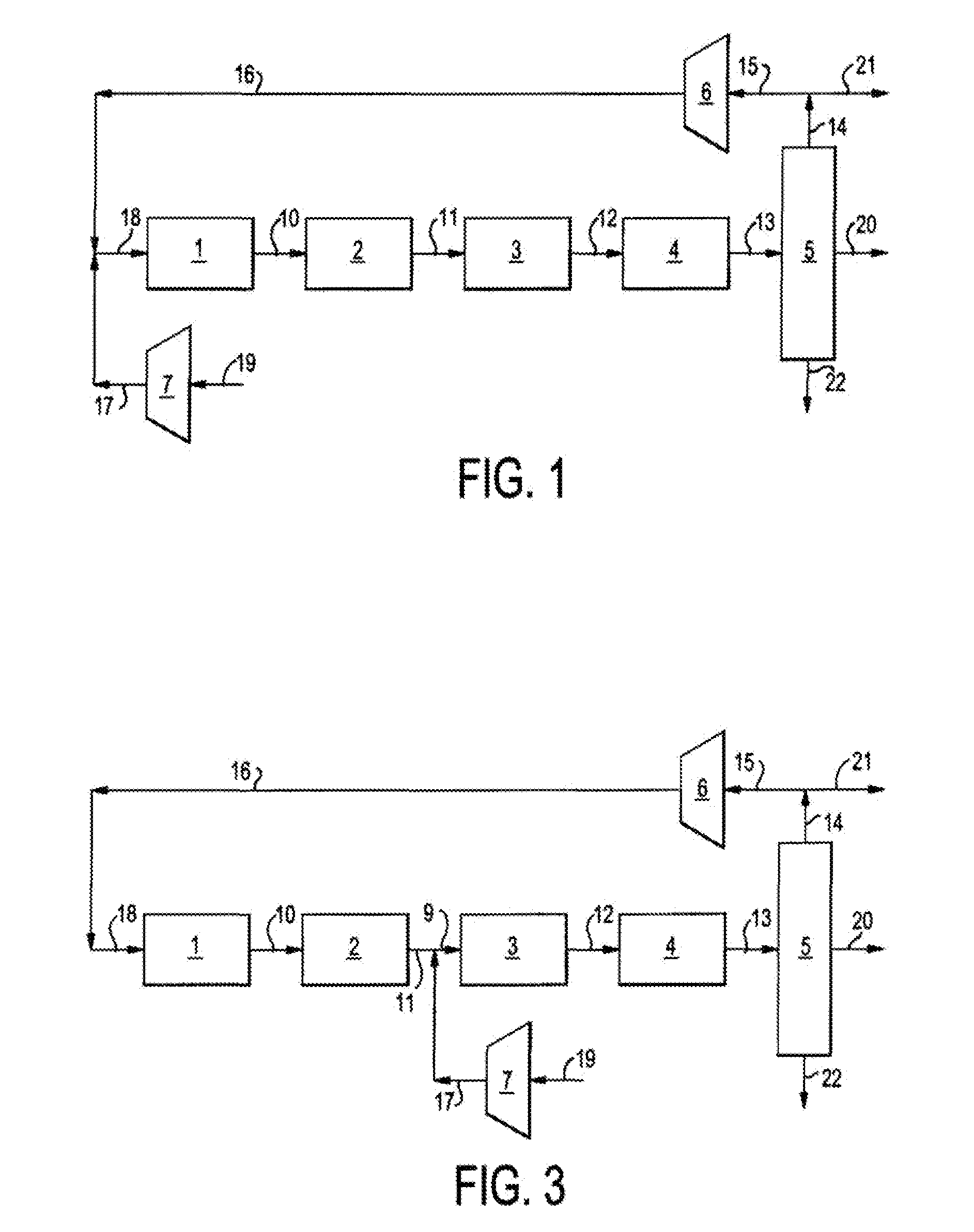
Single loop multistage fuel production
ActiveUS20120116137A1Speed up the conversion processImprove utilization efficiencyRefining to change hydrocarbon structural skeletonHydroxy compound preparationLiquid fuelHigh pressure
Synthetic fuels are produced from synthesis gas in a four-stage reactor system with a single recycle loop providing the requisite thermal capacity to moderate the high heat release of the reactions and to provide the reactants and reaction environments for the efficient operation of the process. The first stage converts a portion of the synthesis gas to methanol, the second stage converts the methanol to dimethylether, the third stage converts the methanol and dimethylether to fuel and the fourth stage converts the high melting point component, durene, and other low volatility aromatic components such as tri- andtetra-methylbenzenes to high octane branched paraffins. The four-stage catalyst used for hydrotreating is resistant to CO poisoning. The reactions i produce water as a side product that is carried through to a high pressure separator after the fourth stage. The streams from the separator are a liquid fuel stream, a water stream and a gaseous stream that contains light hydrocarbon gases and the unreacted synthesis gas. The larger part of this gas stream is recycled to the inlet of the first stage and mixed with the fresh synthesis gas stream. Alternatively, the fresh synthetic gas stream is mixed with the product of the second stage. The smaller part of the gas stream from the separator is sent to hydrocarbon recovery and to fuel gas used for providing preheat of various streams. The liquid fuel is sent for blending into fuel products, such as gasoline, jet fuel, or diesel, and the water stream can be sent, for example, to the synthesis gas producing plant for steam generation.
Owner:BLUESCAPE CLEAN FUELS LLC
Process for producing light olefins
There is provided a process for converting methanol and / or dimethyl ether to a product containing C2 to C4 olefins which comprises the step of contacting a reaction mixture which contains methanol and / or dimethyl ether and at least 10 wt % of a polymethylbenzene component selected from trimethylbenzenes, tetramethylbenzenes and mixtures thereof with a catalyst comprising a porous crystalline material. The contacting step is conducted under conversion conditions including a temperature of about 250° C. to about 500° C. and a methanol and / or dimethyl ether partial pressure of about 5 to about 250 psia (35 to 1725 kPa). The porous crystalline material used in the catalyst has a pore size greater than the critical diameter of the aromatic compound and a Diffusion Parameter for 2,2-dimethylbutane of at least 500 sec<-1 >when measured at a temperature of 120° C. and a 2,2-dimethylbutane pressure of 60 torr (8 kPa).
Owner:EXXONMOBIL CHEM PAT INC
Method for preparing low-temperature finalizing polyurethane elastic fiber
ActiveCN102899740AReduce energy consumptionGood shaping effectMonocomponent synthetic polymer artificial filamentPolyesterPolymer science
The invention relates to a method for preparing a low-temperature finalizing polyurethane elastic fiber, comprising the steps of carrying out condensation polymerization on polyether diol PTMG, polyester diol PE, diphenylmethane diisocyanate MDI and tetramethyl benzene dimethyl diisocyanate m-TMXDI to obtain a carbamic acid ester prepolymer, fully dissolving the prepolymer by taking DMAC (Dimethyl Acetamide) or DMF (Dimethyl Formamide) as the solvent, carrying out chain extension and chain termination on the prepolymer by using a chain extender and a chain terminator to obtain polyurethane urea solution, and finally adding various functional auxiliaries in the polyurethane urea solution and preparing to obtain the polyurethane elastic fiber with low-temperature finalizing performance via a dry spinning system.
Owner:ZHEJIANG HUAFENG SPANDEX
Single loop multistage fuel production
ActiveUS8686206B2Speed up the conversion processImprove efficiencyRefining to change hydrocarbon structural skeletonHydroxy compound preparationBenzeneReactor system
Synthetic fuels are produced from synthesis gas in a four-stage reactor system with a single recycle loop providing the requisite thermal capacity to moderate the high heat release of the reactions and to provide the reactants and reaction environments for the efficient operation of the process. The first stage converts a portion of the synthesis gas to methanol, the second stage converts the methanol to dimethylether, the third stage converts the methanol and dimethylether to fuel and the fourth stage converts the high melting point component, durene, and other low volatility aromatic components such as tri- and tetra-methylbenzenes to high octane branched paraffins.
Owner:BLUESCAPE CLEAN FUELS LLC
Molecular sieve based catalyst for directly preparing polymethyl benzene and preparation and usage methods thereof
ActiveCN105964293ALow costPromote environmental protectionMolecular sieve catalystsMolecular sieve catalystLiquid productMolecular sieve
The invention discloses a molecular sieve based catalyst for directly preparing polymethyl benzene and preparation and usage methods thereof. The molecular sieve based catalyst for preparing the polymethyl benzene (tetramethylbenzene- hexamethylbenzene) by using CO, methanol, dimethyl ether or C1-C8 aromatic hydrocarbons and other raw materials uses a ZSM-12 molecular sieve as a main component, can form a multi-component catalyst with a metal and / or structural strengthening agent and / or a stability additive. According to a preparation method of the ZSM-12 molecular sieve and a method for preparing the multi-component catalyst by utilizing the ZSM-12 molecular sieve, the temperature is 450-520 DEG C and the weight space velocity of raw materials is 0.2-15 g / g / h in aromatization reaction, and the polymethyl benzene yield in an obtained organic liquid product is above 80%.
Owner:TSINGHUA UNIV
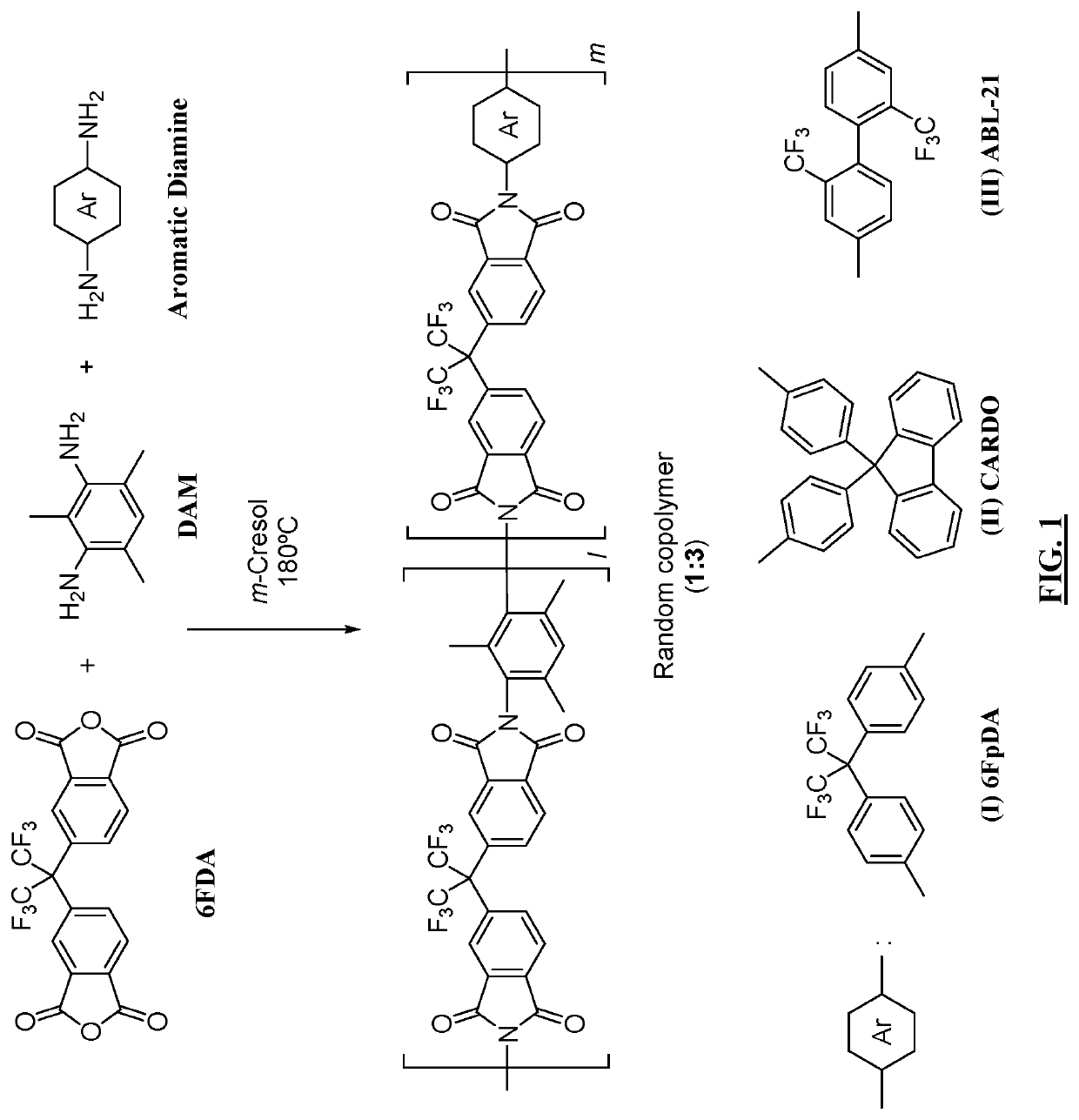
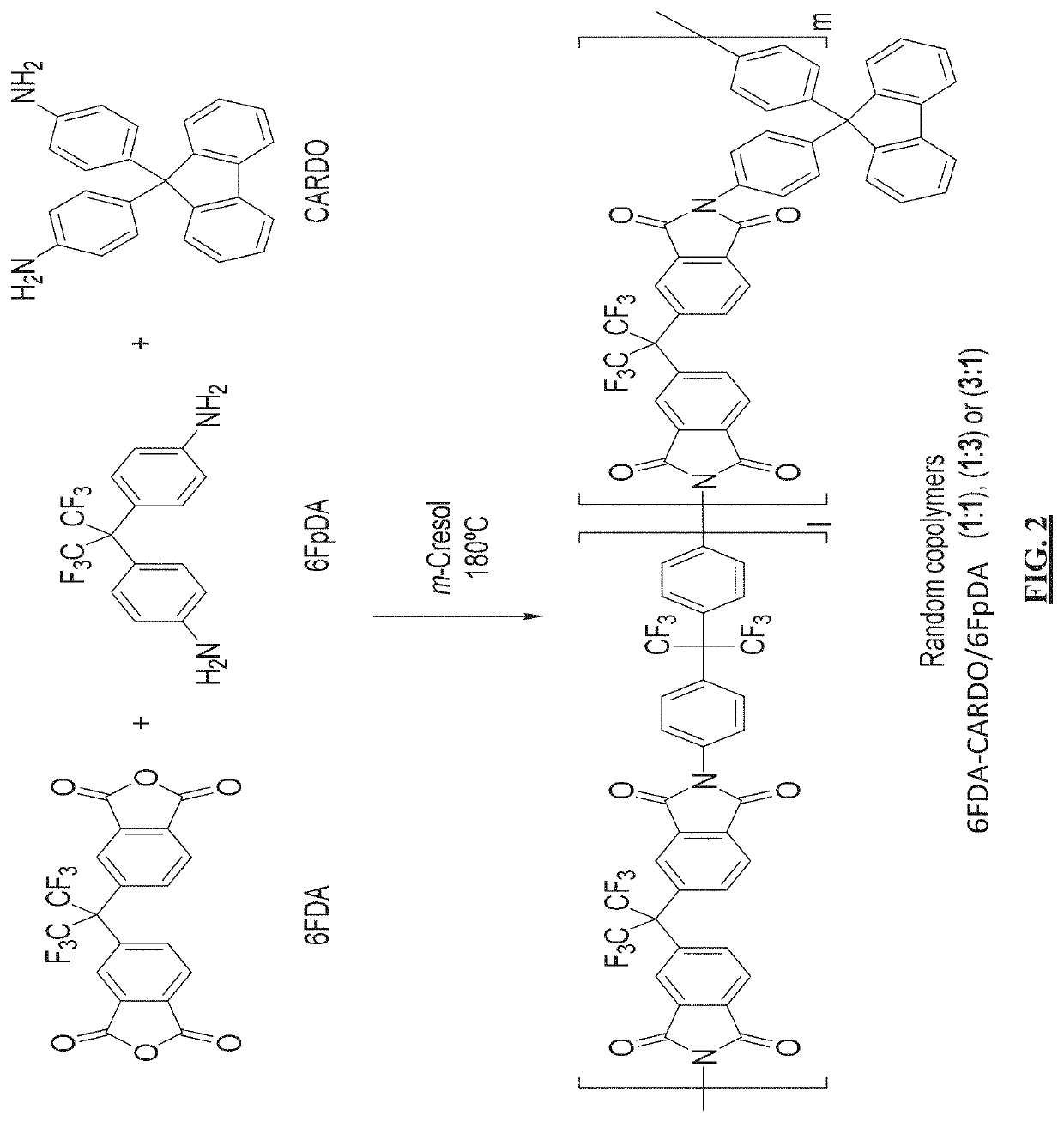
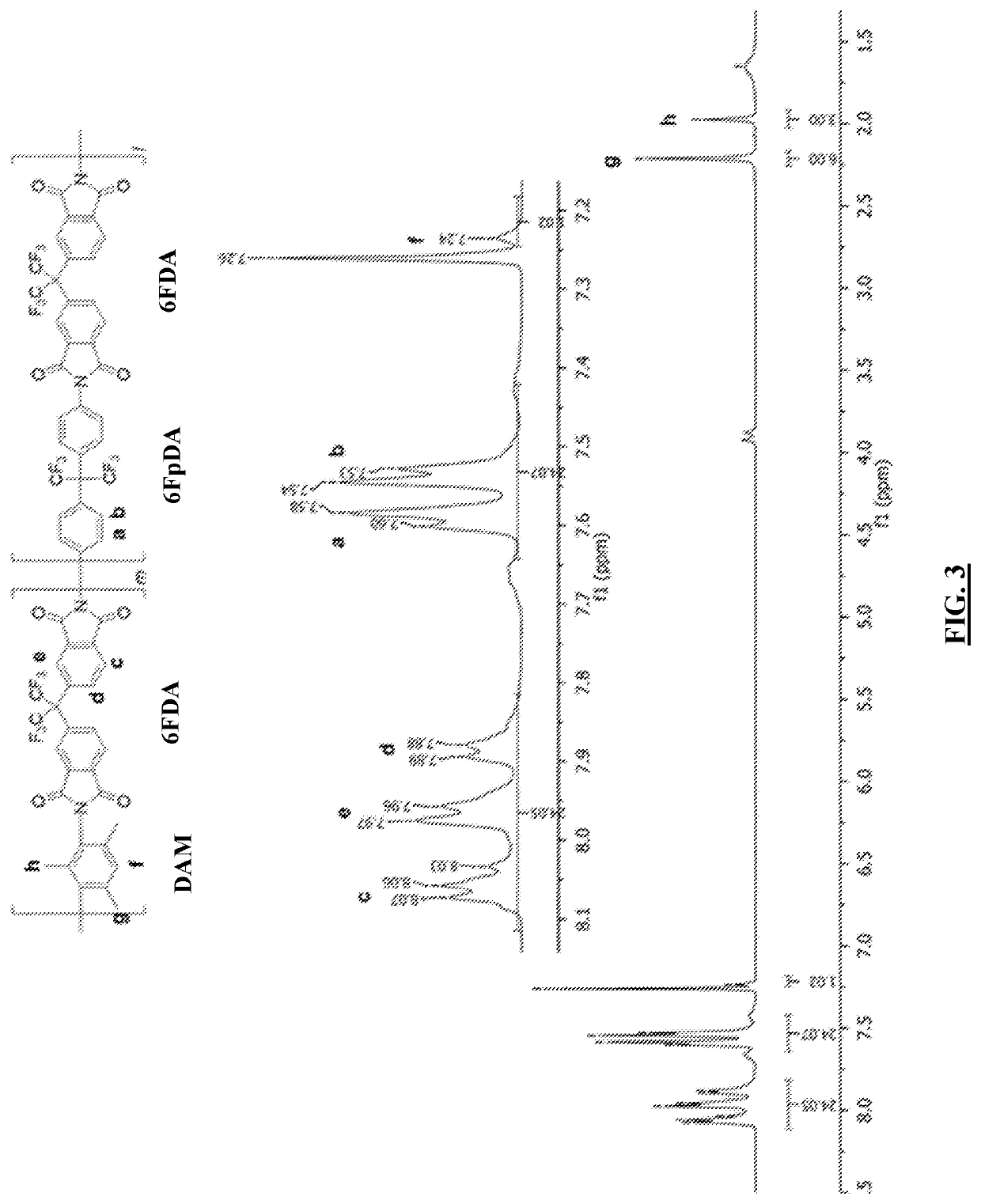
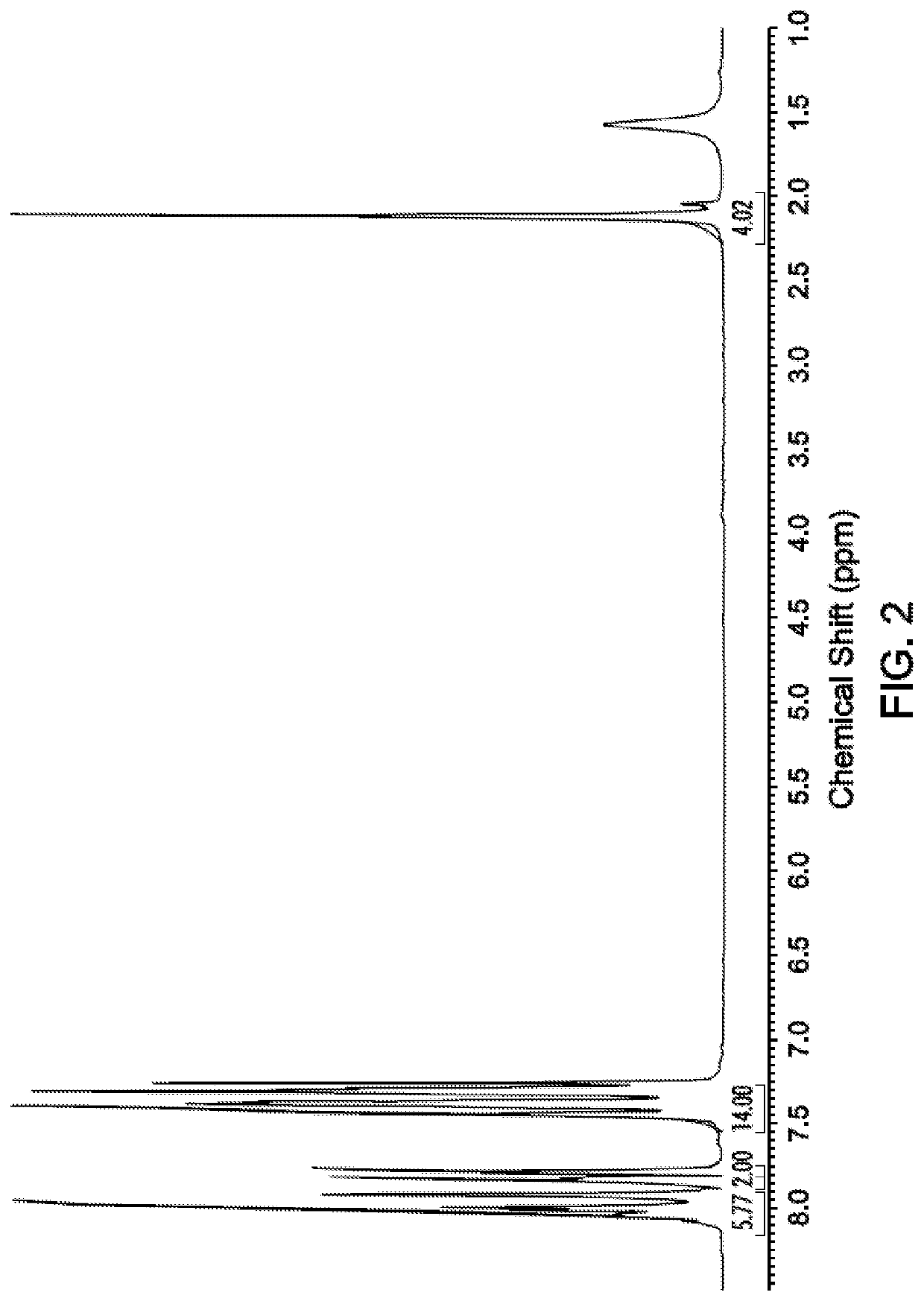
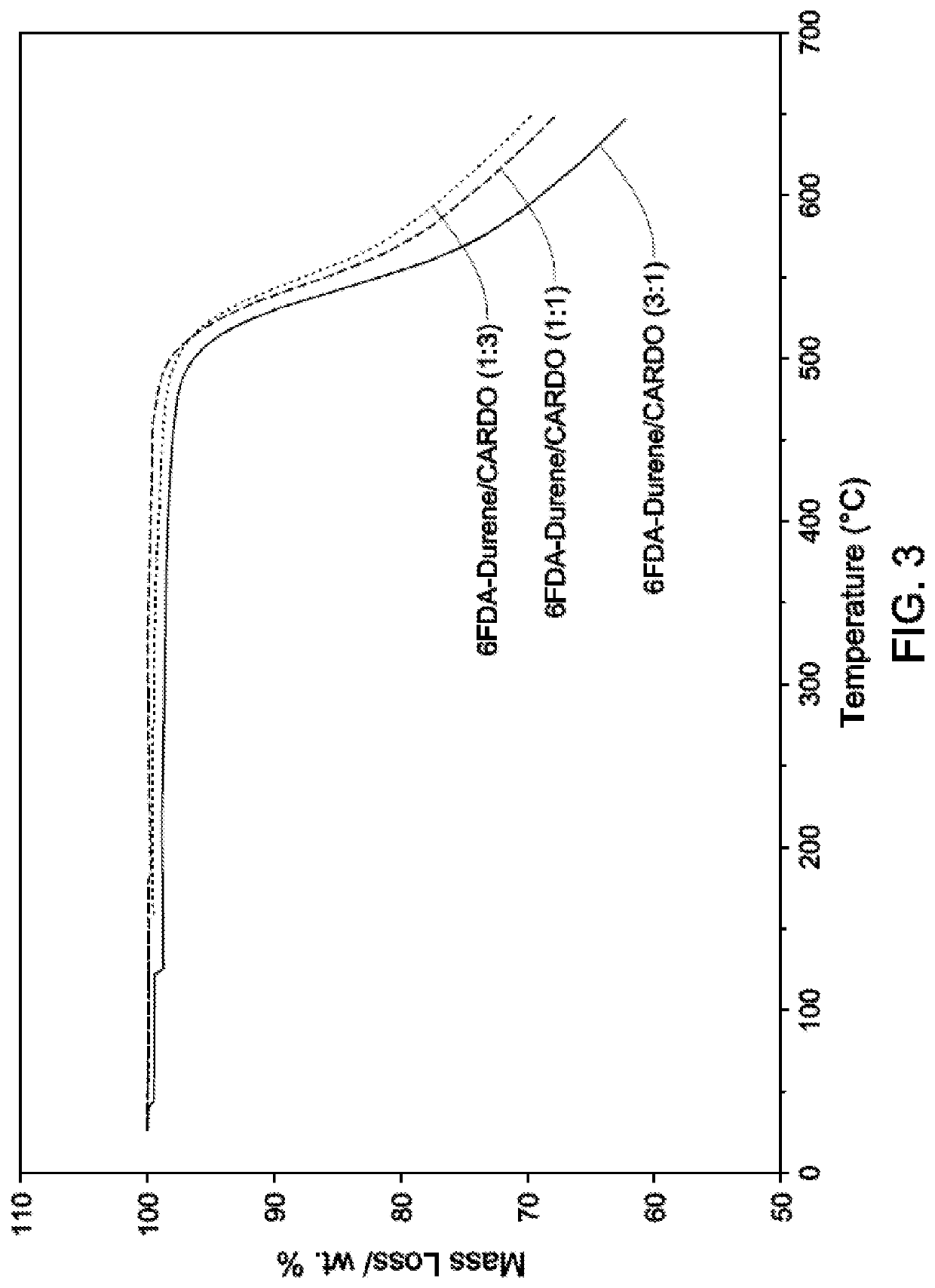
Cardo-Type Co-Polyimide Membranes For Sour Gas Feed Separations From Natural Gas
ActiveUS20180345229A1Less footprintFlexible operationSemi-permeable membranesGas treatmentPolyimide membraneMethyl group
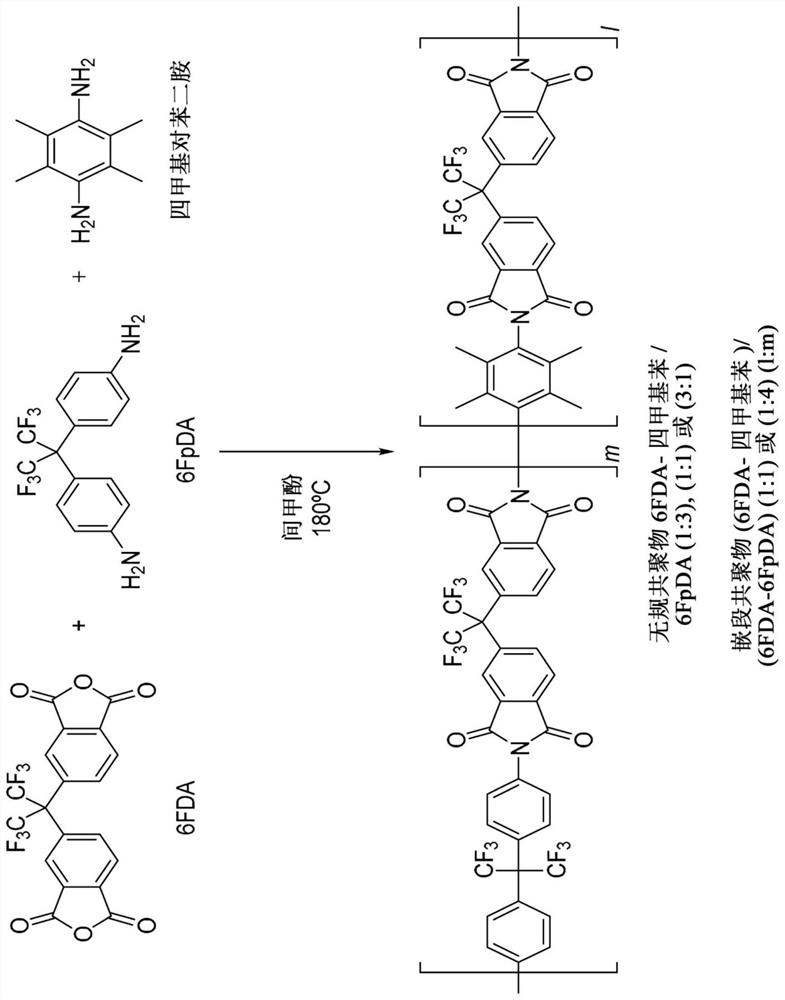
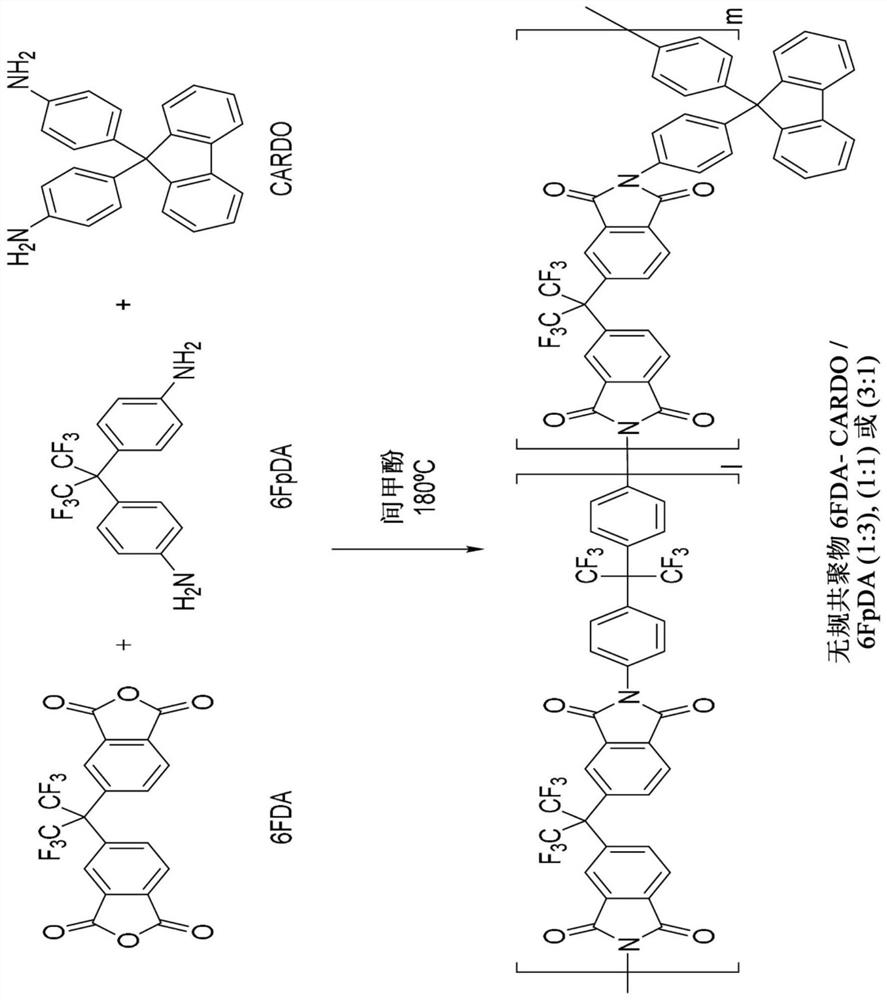
Co-polyimide membranes for separating components of sour natural gas where embodiments can include at least three distinct moieties polymerized together, the moieties including a 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) based moiety; a 9,9-bis(4-aminophenyl) fluorene (CARDO) based moiety; and 2,3,5,6-tetramethyl-1,4-phenylenediamine (durene diamine) based moiety.
Owner:SAUDI ARABIAN OIL CO
Application method of ratio-type cyanide ion fluorescent probe molecule
InactiveCN103134787ATo achieve specific identificationHigh electron deficiencyFluorescence/phosphorescenceFluoProbesFluorescence
The invention relates to an application method of a ratio-type cyanide ion fluorescent probe molecule. The invention belongs to the technical field of anion detection. The method is characterized in that the method is an application of a ratio fluorescent probe molecule, which is 1,2,2,3-tetramethyl-4,5-benzoindoline, used in cyanide ion detection. The probe molecule is obtained by a one-step process of periodic methane alkylation of 2,3,3-trimethyl-4,5-benzindole. In a system where DMF:H2O=1:1 (V / V), the probe molecule is excited by using 320nm light, and emission occurs at 461nm. When cyanide ion is added, intra-molecular charge transfer (ICT) process is broken, emission peak at 461nm is reduced, and new emission peak occurs at 425nm. Therefore, solution fluorescence is turned from bluish green to deep blue. In a system wherein cyanide ion coexists with other anions, the probe molecule shows high interference resistance, high specificity, and high sensitivity.
Owner:TAIYUAN UNIV OF TECH
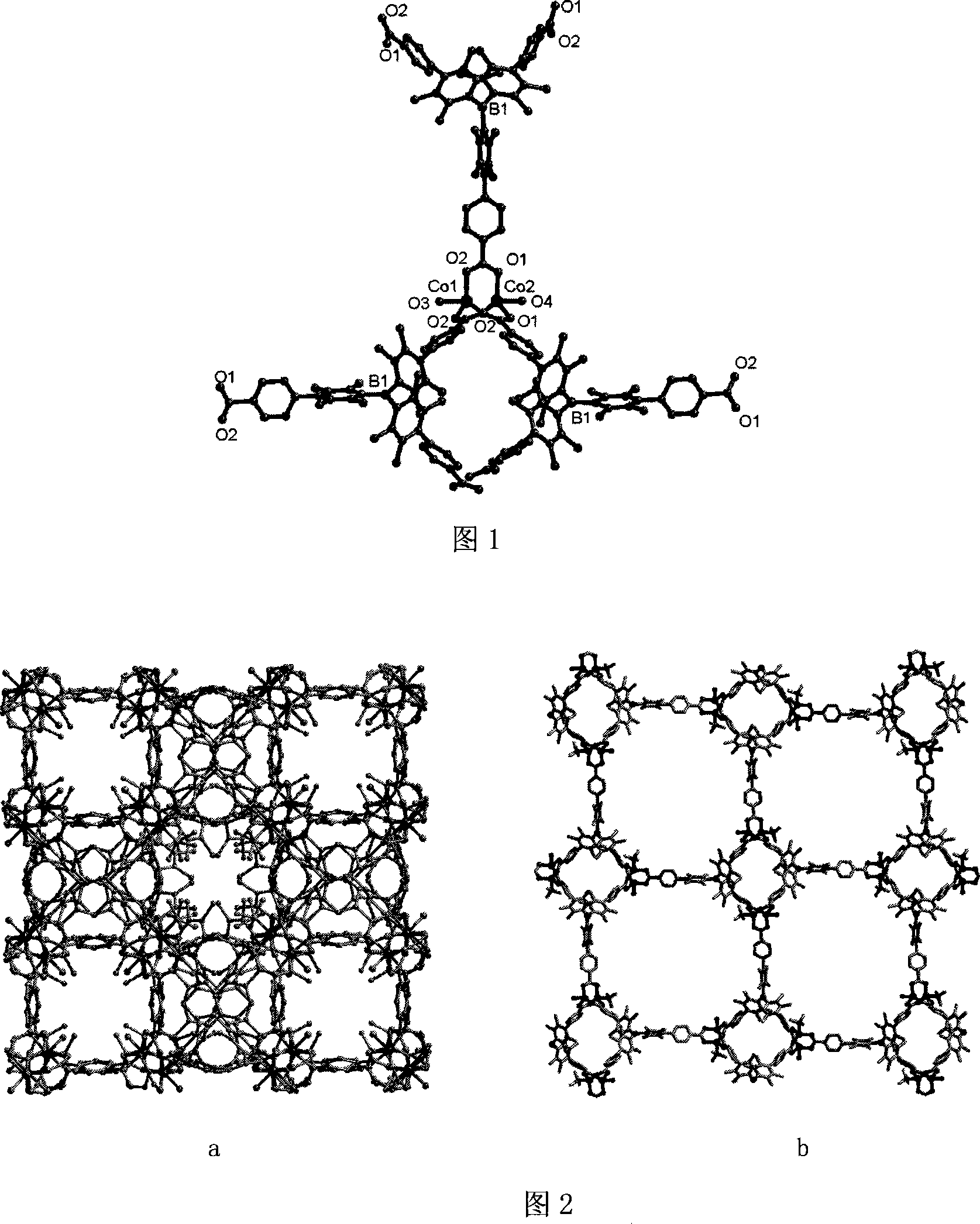
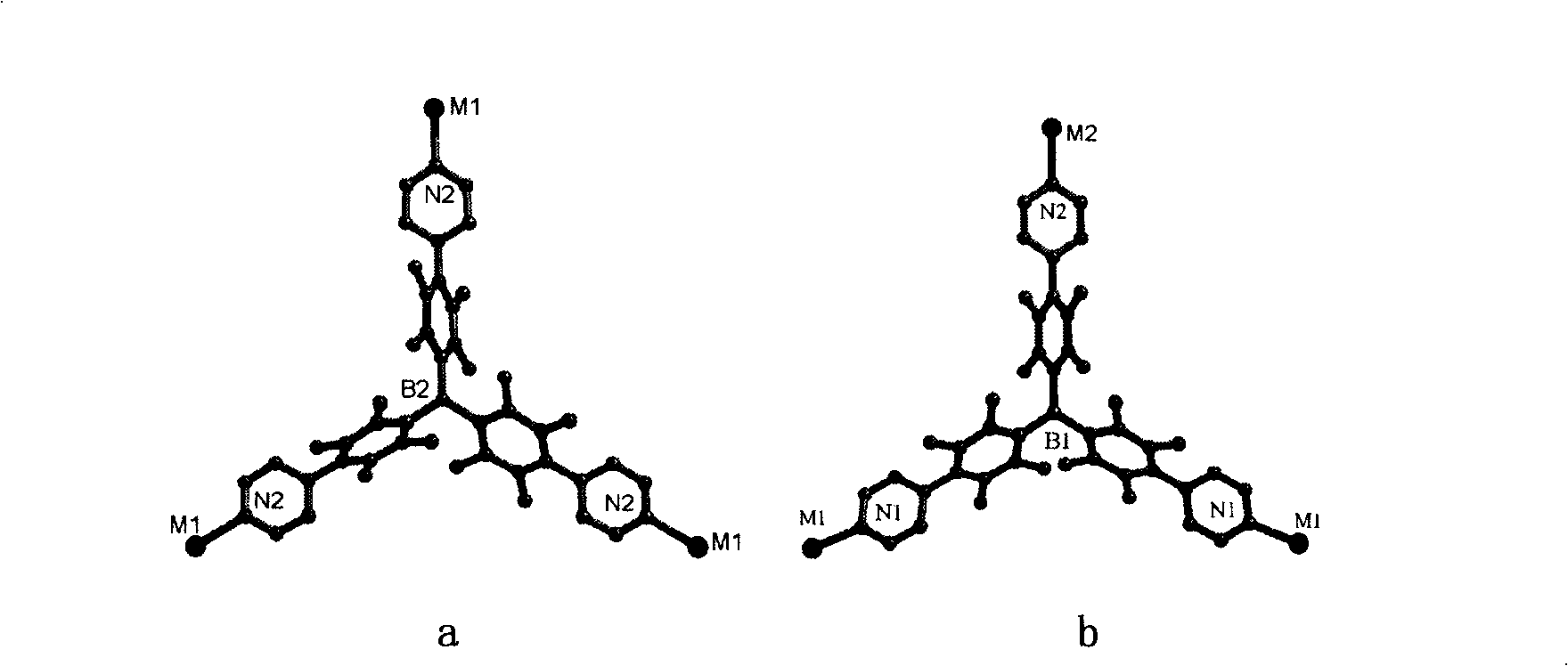
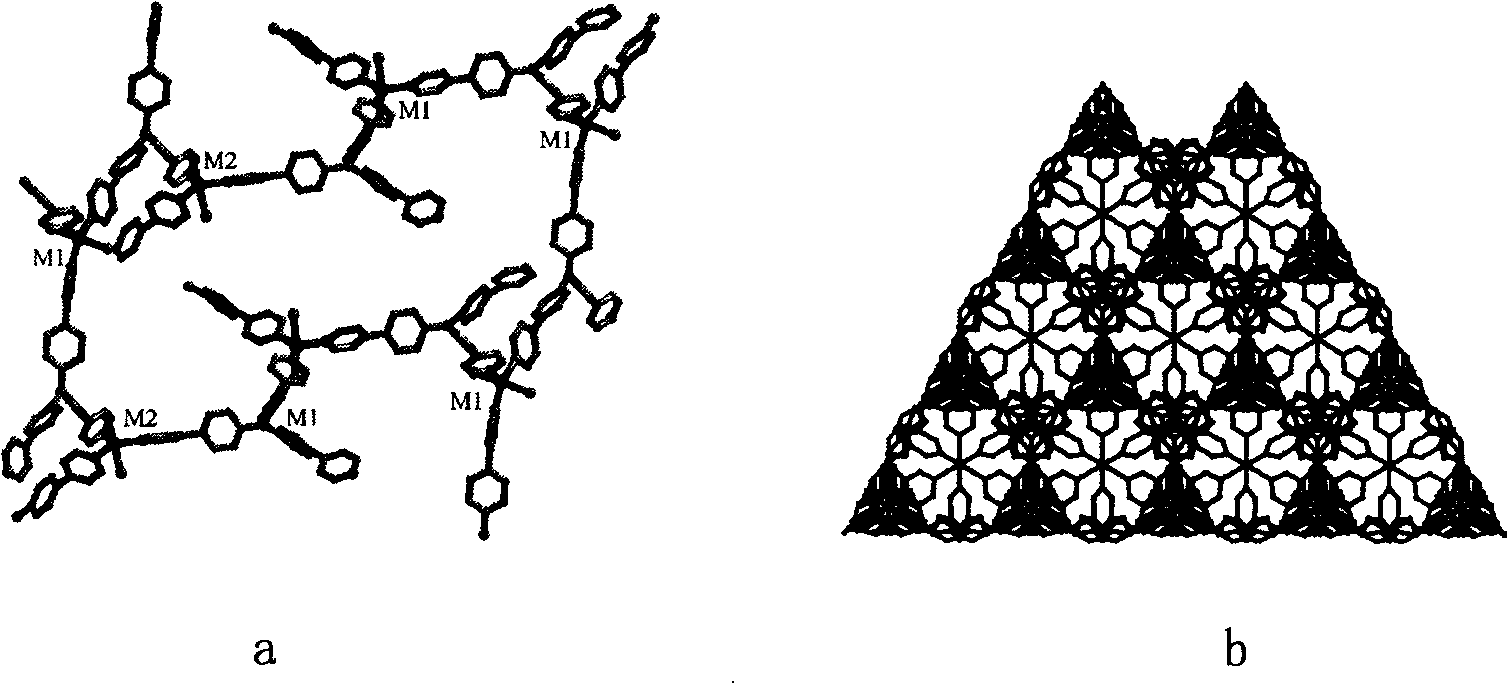
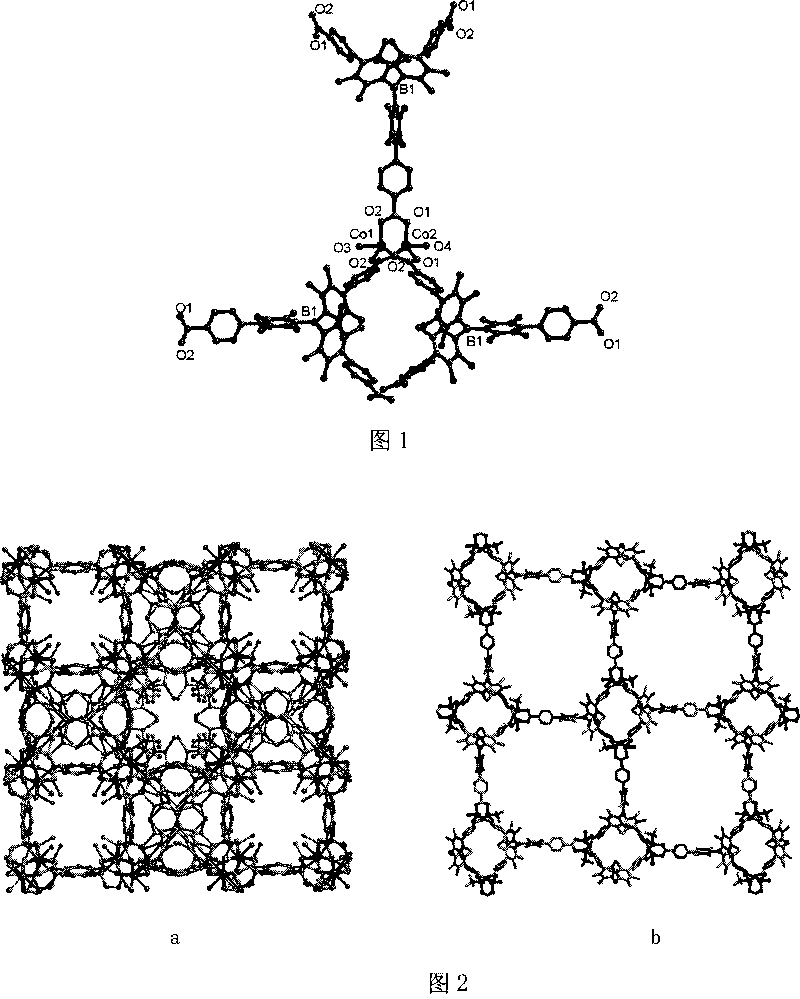
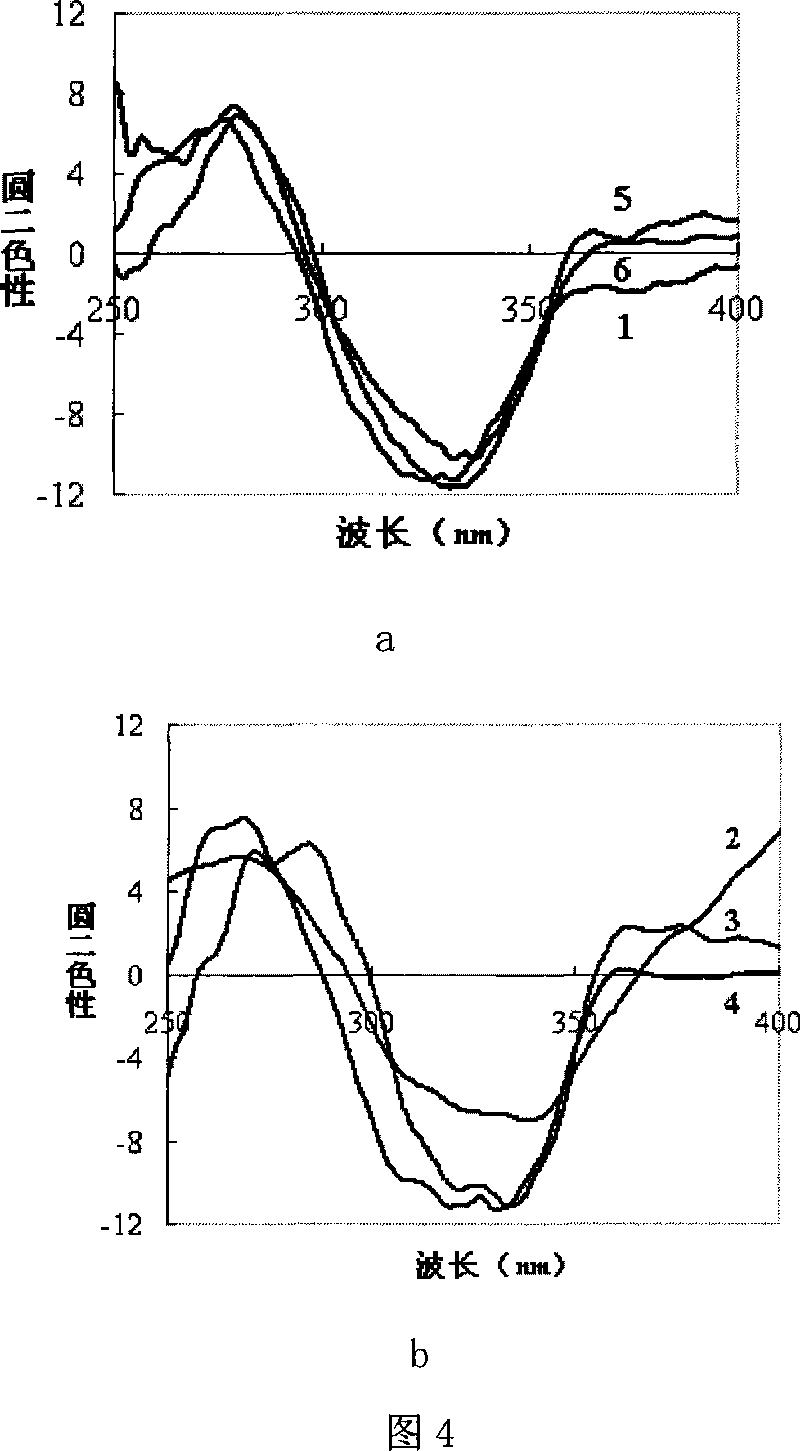
Method for preparing chirality non-linear optical metal-organic boron polymer crystal material
InactiveCN101230077AGood second-order nonlinear optical propertiesIngenious design ideasCopper organic compoundsCobalt organic compoundsBenzoic acidManganese
The invention relates to a preparation method for nonlinear optical metal-organic boron polymer crystal material provided with equal chirality. The method adopts the steps that firstly, ligand H3L, namely tri-(4-para benzoic acid-2, 3, 5, 6-tetramethylbenzene) borane is synthesized, and then nonlinear optical metal-organic boron polymer crystal material with equal chirality is prepared, namely, ligand H3L and metal salt are mixed according to 1: 2, and dissolved by using N, N-dimethyl acetamide and methyl alcohol, the reaction liquid is positioned into a sample bottle for reaction, after the reaction is finished, the sample bottle is cooled to room temperature, the generated metal-organic polymeric material is taken out, and washed with ether for multiple times and air dried to obtain the material. The structural general formula is <M2L (OH) (MeOH)>.3H2O, wherein, metal M represents cobalt, manganese, nickel, copper, zinc or cadmium. The material produced through the invention has very good second order nonlinear optical property, and compared with KDP, and the generation effect of second harmonics is 1.5 times higher.
Owner:SHANGHAI JIAO TONG UNIV
Method of producing s-(-)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid and product obtained by the method
InactiveUS20100063305A1High chemical purityHigh optical purityAsymmetric synthesesArylCarboxylic acid
The present invention provides an industrially available method for efficiently producing high-purity S-(−)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid excellent in solid-liquid separability from an S-(−)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid ester, and also provides products obtained by the method.Under a temperature condition of 50-80° C. in an aqueous solvent, (A) an S-(−)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid ester represented by the general formula (1) is hydrolyzed under a basic condition for 1-3 hours; then (B) the insoluble matters contained in the reaction solution resulting from the hydrolysis are removed; and (C) an acid is added to the resulting solution to effect crystallization; provided that R in the general formula (1) represents an alkyl or aryl group.
Owner:MITSUBISHI GAS CHEM CO INC
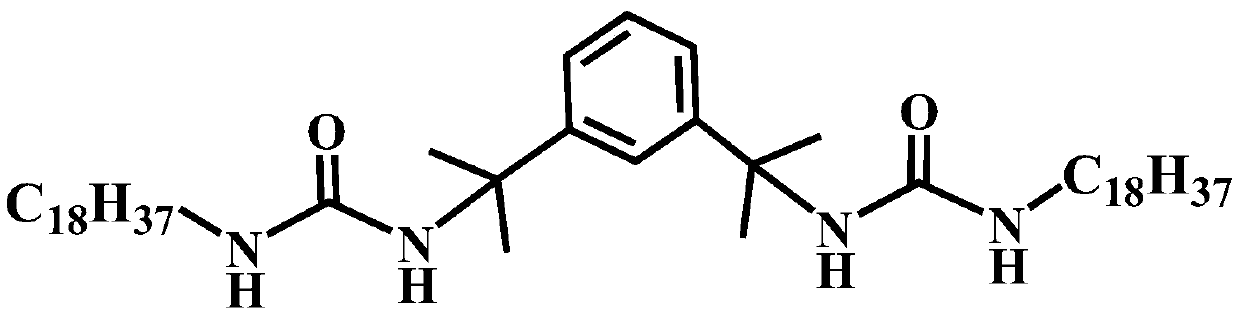
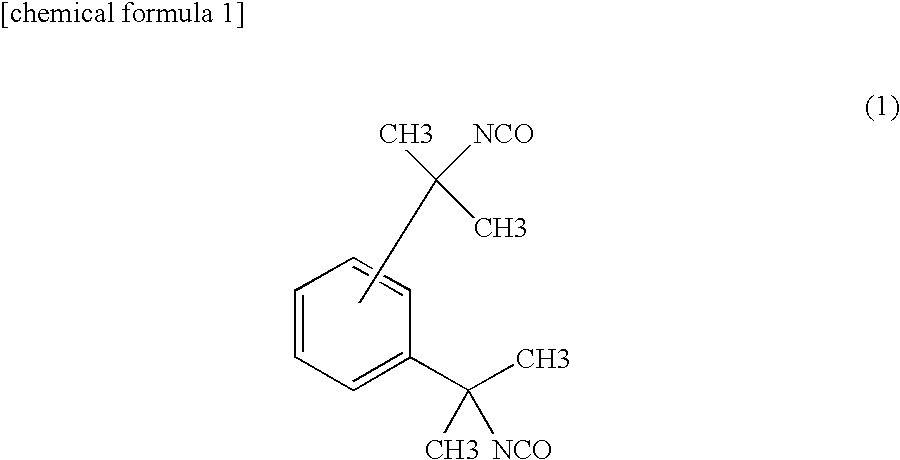
Diurea-based organic small molecular gel factor, preparation method thereof, thixotropic hydrocarbon fuel gel material and preparation method thereof
InactiveCN109796375AExcellent gel-forming propertiesThe synthesis steps are simpleUrea derivatives preparationOrganic compound preparationKeroseneMaterials science
The invention relates to a diurea-based organic small molecular gel factor, a preparation method thereof, a thixotropic hydrocarbon fuel gel material and a preparation method thereof. The preparationmethod of the diurea-based organic small molecular gel factor comprises the following steps of: dissolving octadecylamine in a reaction solvent; adding tetramethylbenzene diisocynate, reacting for 4-12h under the protection of nitrogen, filtering and removing reaction liquid after the reaction to obtain a solid powder product, wherein each mole of octadecylamine is added with 4-10L of the reactionsolvent, and the molar ratio of the octadecylamine to the tetramethylbenzene diisocynate is 2:1-3:1; the diurea-based organic gel factor can be used for preparing the thixotropic organic gel materialfrom hydrocarbon fuel hanging type tetrahydrodicyclopentadiene, aviation kerosene and the like; and the organic gel material has excellent performance and good application prospect in the field of gel propellants.
Owner:HUBEI INST OF AEROSPACE CHEMOTECHNOLOGY
Process for producing light olefins
InactiveUS20020091292A1High compressive strengthChange conversionMolecular sieve catalystCatalystsDimethyl etherTetramethylbenzenes
There is provided a process for converting methanol and / or dimethyl ether to a product containing C2 to C4 olefins which comprises the step of contacting a reaction mixture which contains methanol and / or dimethyl ether and at least 10 wt % of a polymethylbenzene component selected from trimethylbenzenes, tetramethylbenzenes and mixtures thereof with a catalyst comprising a porous crystalline material. The contacting step is conducted under conversion conditions including a temperature of about 250° C. to about 500° C. and a methanol and / or dimethyl ether partial pressure of about 5 to about 250 psia (35 to 1725 kPa). The porous crystalline material used in the catalyst has a pore size greater than the critical diameter of the aromatic compound and a Diffusion Parameter for 2,2-dimethylbutane of at least 500 sec-1 when measured at a temperature of 120° C. and a 2,2-dimethylbutane pressure of 60 torr (8 kPa).
Owner:EXXONMOBIL CHEM PAT INC
Resin powder composition for slush molding and molded articles
ActiveUS8399574B2Improve hydrolysis resistanceHigh rateVehicle componentsCoatingsThermoplastic polyurethaneChemistry
The present invention provides a resin powder composition for slush molding which can give slush molded articles with more excellent hydrolysis resistance. The present invention relates to a resin powder composition for slush molding which is characterized by comprising as the main component a thermoplastic polyurethane resin powder, preferably a thermoplastic polyurethane elastomer powder, and containing a polycarbodiimide prepared by polymerizing tetramethylxylylene diisocyanate, alkoxy terminated one having a number average molecular weight of 500 to 30,000.
Owner:SANYO CHEM IND LTD +2
Preparation method of ultralow-metal ion content pyromellitic dianhydride
The invention provides a preparation method of ultralow-metal ion content pyromellitic dianhydride, which comprises the following steps of: taking 1, 2, 4, 5-tetramethylbenzene of which the purity is 96-97 weight% as raw material, oxidizing by air, taking metal oxide which consists of vanadium, titanium, sodium and phosphorus as oxidation catalyst, reacting under the temperature of 380-420 DEG C, cooling by a heat exchanger, absorbing by adsorbent which consists of molecular sieves 3A, 4A and 5A and silicon dioxide under the temperature of 250-350 DEG C, removing harmful organic matters and metal ions, collecting crude pyromellitic dianhydride gathered by a first gathering machine, and recrystallizing, filtering and drying by low-boiling point low-toxicity solvent such as mixed solvent of acetone and butanone to obtain fine pyromellitic dianhydride, wherein the melting point of the fine pyromellitic dianhydride is 286-287.5 DEG C, the yield of the fine pyromellitic dianhydride is 40-45%, the product is crystalloid grain, the grain size of the pyromellitic dianhydride is 300-1000mm, and each content of the contained metal irons is less than 1ppm, i.e., iron, aluminum, nickel, manganese, tin, zinc, potassium, sodium, cobalt, chromium, copper and calcium.
Owner:DONGYING ZHONGJIE CHEM
Aromatic co-polyimide gas separation membranes derived from 6fda-dam-type homo-polyimides
ActiveUS20200269196A1Less footprintFlexible operationSemi-permeable membranesMembranesPolymer sciencePolyimide membrane
Co-polyimide membranes for separating components of sour natural gas including at least three distinct moieties polymerized together, the moieties including a 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) based moiety; a 2,4,6-trimethyl-m-phenylenediamine (DAM) based moiety; and at least one component selected from the group consisting of: a 4,4′-(hexafluoroisopropylidene)dianiline (6FpDA) based moiety; a 9,9-bis(4-aminophenyl) fluorene (CARDO) based moiety; a 2,3,5,6-tetramethyl-1,4-phenylenediamine (durene diamine) based moiety; a 2,2′-bis(trifluoromethyl)benzidine (ABL-21) based moiety; a 3,3′-dihydroxybenzidine based moiety; and a 3,3′-(hexafluoroisopropylidene)dianiline based moiety.
Owner:SAUDI ARABIAN OIL CO
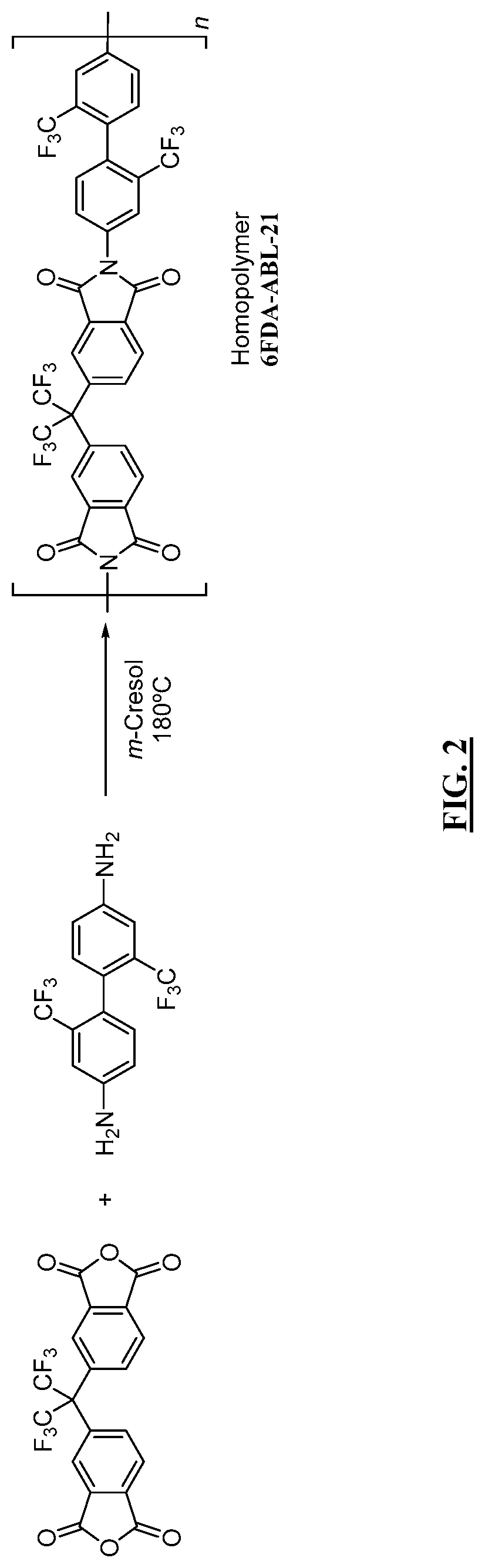
Aromatic co-polyimide gas separation membranes derived from 6FDA-6FpDA-type homo-polyimides
ActiveUS11007491B2Less footprintFlexible operationProductsSemi-permeable membranesPolymer sciencePolyimide membrane
Co-polyimide membranes for separating components of sour natural gas including at least three distinct moieties polymerized together, the moieties including a 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) based moiety; a 4,4′-(hexafluoroisopropylidene)dianiline (6FpDA) based moiety; and at least one component selected from the group consisting of: a 9,9-bis(4-aminophenyl) fluorene (CARDO) based moiety; a 2,3,5,6-tetramethyl-1,4-phenylenediamine (durene diamine) based moiety; a 2,2′-bis(trifluoromethyl)benzidine (ABL-21) based moiety; a 3,3′-dihydroxybenzidine based moiety; and a 3,3′-(hexafluoroisopropylidene)dianiline based moiety.
Owner:SAUDI ARABIAN OIL CO
Aromatic co-polyimide gas separation membranes derived from 6FDA-DAM-type homo-polyimides
ActiveUS11007492B2Less footprintFlexible operationProductsSemi-permeable membranesPolymer sciencePolyimide membrane
Co-polyimide membranes for separating components of sour natural gas including at least three distinct moieties polymerized together, the moieties including a 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) based moiety; a 2,4,6-trimethyl-m-phenylenediamine (DAM) based moiety; and at least one component selected from the group consisting of: a 4,4′-(hexafluoroisopropylidene)dianiline (6FpDA) based moiety; a 9,9-bis(4-aminophenyl) fluorene (CARDO) based moiety; a 2,3,5,6-tetramethyl-1,4-phenylenediamine (durene diamine) based moiety; a 2,2′-bis(trifluoromethyl)benzidine (ABL-21) based moiety; a 3,3′-dihydroxybenzidine based moiety; and a 3,3′-(hexafluoroisopropylidene)dianiline based moiety.
Owner:SAUDI ARABIAN OIL CO
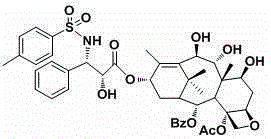
Side chain C-3 position-modified taxane analogs and preparation method thereof
InactiveCN105294611AHas anticancer biological activitySmall toxicityOrganic chemistryAntineoplastic agentsFuranSide chain
The invention relates to side chain C-3 position-modified taxane analogs and a preparation method thereof. The structural formula of the analogs can be found in the specification, wherein R is methyl phenyl, phenyl, bromophenyl, thienyl, benzene and tetrahydrofuran, methoxyphenyl, fluoro-phenyl, four methyl phenyl, naphthyl, p-nitrophenyl, furyl, trifluoromethyl, quinolyl, methyl quinoline, pyridyl and the like; R1 is hydrogen or hydrogen. According to the side chain C-3 position-modified taxane analogs and the preparation method thereof, various types of substituent groups are introduced to the 13th position of 1-dehydroxybaccatin VI taxane, a ring skeleton and a necessary functional group of taxanes are maintained, the compound of the type is enriched, the obtained compound has anticancer biological activity of natural taxol, and the side chain C-3 position-modified taxane analogs have certain application prospect on the aspect of lowering the toxic and side effect of the natural taxol; meanwhile, a quite significant foundation is laid for research of the active structure-activity relationship of the compound of the type.
Owner:SHANGHAI UNIV
AROMATIC CO-POLYIMIDE GAS SEPARATION MEMBRANES DERIVED FROM 6FDA-6FpDA-TYPE HOMO-POLYIMIDES
ActiveUS20200269195A1Less footprintFlexible operationProductsSemi-permeable membranesPolymer sciencePolyimide membrane
Co-polyimide membranes for separating components of sour natural gas including at least three distinct moieties polymerized together, the moieties including a 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) based moiety; a 4,4′-(hexafluoroisopropylidene)dianiline (6FpDA) based moiety; and at least one component selected from the group consisting of: a 9,9-bis(4-aminophenyl) fluorene (CARDO) based moiety; a 2,3,5,6-tetramethyl-1,4-phenylenediamine (durene diamine) based moiety; a 2,2′-bis(trifluoromethyl)benzidine (ABL-21) based moiety; a 3,3′-dihydroxybenzidine based moiety; and a 3,3′-(hexafluoroisopropylidene)dianiline based moiety.
Owner:SAUDI ARABIAN OIL CO
Method for Monofluoromethylation of Organic Substrates to Prepare Biologically Active Organic Compounds
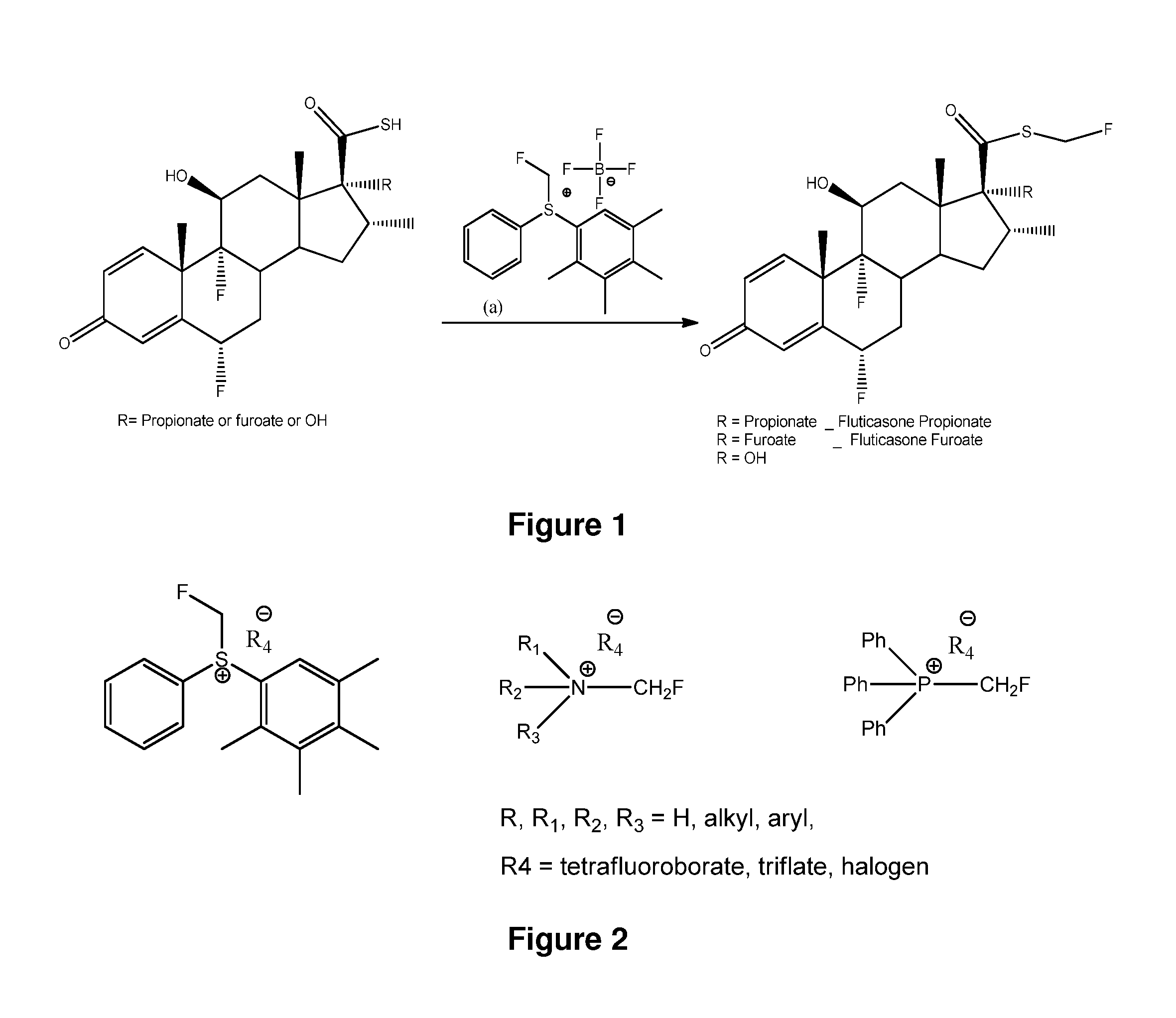
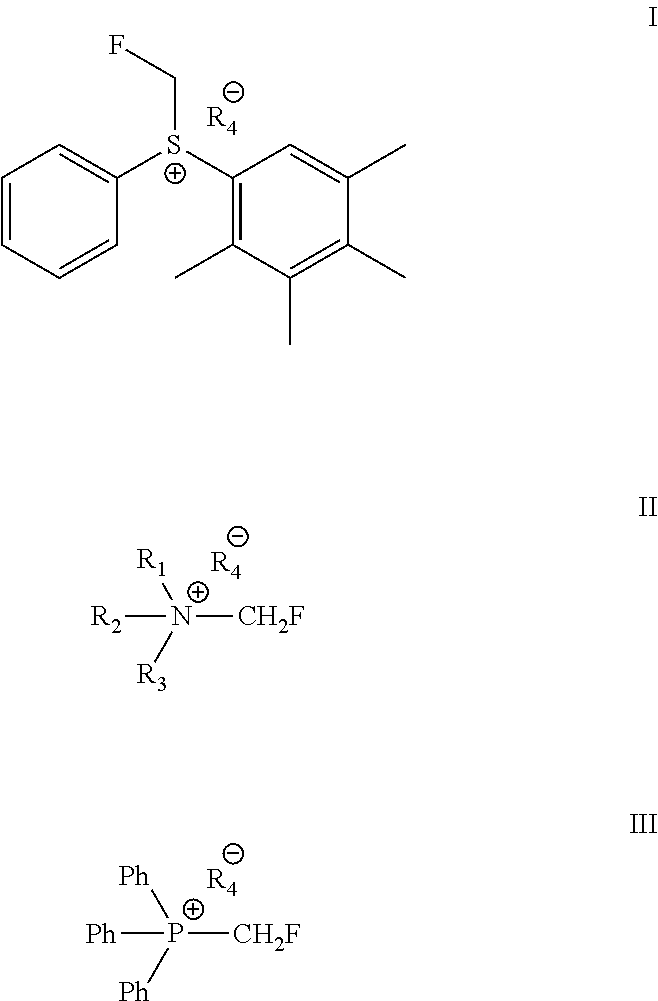
Described is a process for the preparation of monofluoromethylated organic biologically active compounds using monofluoromethylated reagents. Fluticasone Propionate and Fluticasone Furoate can be prepared using, for example, S-monofluoromethyl-S-phenyl-2,3,4,5-tetramethylphenylsulfonium tetrafluoroborate as monofluoromethylating reagent instead of bromofluoromethane.
Owner:HOVIONE INTER
Method for extracting naphthalene, 1-methylnaphthalene and 2-methylnaphthalene from ethylene tar
ActiveCN102134500BIncrease contentImprove protectionTar working-up by distillationTowerMethylated naphthalene
The invention discloses a method for extracting naphthalene, 1-methylnaphthalene and 2-methylnaphthalene from ethylene tar. The method comprises the following steps of: adding ethylene cracking tar with the total content of naphthalene and methylnaphthalene of not less than 30 percent from the middle of a first rectifying tower (5) under certain conditions; recovering materials of which light components are removed by the first rectifying tower at the bottom of the tower; adding from the middle of a second rectifying tower (10), and obtaining the naphthalene with the purity of not less than 95 percent on the top of the tower; adding the materials of which the naphthalene is removed at the bottom of the second rectifying tower from the middle of a third rectifying tower (15), and removing tetramethyl benzene and other components of which the boiling point is lower than that of the 2-methylnaphthalene at the top of the tower; recovering the materials of which the light fraction is removed by the third rectifying tower at the bottom of the tower, adding from the middle of a fourth rectifying tower (20), and obtaining the 2-methylnaphthalene with the purity of not less than 98 percenton the top of the tower; and recovering the materials of which the 2-methylnaphthalene is removed by the fourth rectifying tower at the bottom of the tower, adding from the middle of a fifth rectifying tower (25), and obtaining the 1-methylnaphthalene with the purity of not less than 98 percent. By the method, wastewater and waste residue are not generated and extraction efficiency is high.
Owner:广东新华粤石化集团股份公司 +2
Aromatic co-polyimide gas separation membranes derived from 6fda-6fpda-type homo-polyimides
Co-polyimide membranes for separating components of sour natural gas including at least three distinct moieties polymerized together, the moieties including a 2,2'-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) based moiety; a 4,4'-(hexafluoroisopropylidene)dianiline (6FpDA) based moiety; and at least one component selected from the group consisting of: a 9,9-bis(4-aminophenyl) fluorene (CARDO) based moiety; a 2,3,5,6-tetramethyl-1,4-phenylenediamine (durene diamine) based moiety; a 2,2'-bis(trifluoromethyl)benzidine (ABL-21) based moiety; a 3,3 '-dihydroxybenzidine based moiety; and a 3,3'-(hexafluoroisopropylidene)dianiline based moiety.
Owner:SAUDI ARABIAN OIL CO
Waterborne polyurethane grouting material and preparation method thereof
InactiveCN102093537BConvenient sourceAvoid poisoningOther chemical processesIsophorone diisocyanatePolymer chemistry
The invention discloses a preparation method of a waterborne polyurethane grouting material. The method is as follows: isocyanate, retarding agent and hydrophilic polyether polyol are mixed to react and obtain the finished product, wherein isocyanate is selected from diphenylmethane diisocyanate, liquefied diphenylmethane diisocyanate, polymethylene polyphenyl isocyanate isophorone diisocyanate, dicyclohexylmethylmethane-4,4'-diisocyanate, hexamethylene diisocyanate and polymethylene polyphenyl isocyanate or the mixture of toluene diisocynate and the isocyanates; and the degree of functionality of hydrophilic polyether polyol is 2-6, the ratio of ethylene oxide to propylene oxide is 99 / 1-60 / 40 and the molecular weight is 1000-20000. The invention also relates to the hydrophilic polyurethane grouting material prepared by the method. The preparation method of the invention is simple and convenient and has wide raw material sources; the prepared hydrophilic polyurethane grouting materialdoes not contain organic solvent, thus avoiding the damage on the health of the constructor and promoting environmental protection; and the grouting material can have lower viscosity and good permeability and waterproof and leaking stoppage effect.
Owner:SHANGHAI DONGDA CHEM
In Situ Moisture Generation and Use of Polyfunctional Alcohols for Crosslinking of Silane-Functionalized Resins
InactiveUS20100297374A1Improve propertiesReduce rateSynthetic resin layered productsInsulatorsPolymer sciencePoly ethylene
Polymer compositions comprise a (i) silane-functionalized polymer, e.g., a polyethylene grafted with vinyl triethoxy silane, (H) polyfunctional alcohol e.g., α,α,α′,α′˜tetramethyl-I,3-benzenediethanol and, optionally, (iii) acid, e.g., an alkylated aryl disulfonic acid. The use of polyfunctional alcohols in the absence of a strong acid yields a light-crosslinking of the silane-functionalized polymer and this, in turn, provides a polymer melt with improved extensional properties such as elongational viscosity and melt strength. The use of a polyfunctional alcohol in combination with a blocked strong acid provides a slow rate of crosslinking during melt processing and a high degree of ultimate crosslinking after the polymer composition has been shaped or molded.
Owner:DOW GLOBAL TECH LLC
Method for preparing non-linear optical metal-organic boron polymer crystal material
InactiveCN101230071BMild reaction conditionsSimple and fast operationGroup 3/13 element organic compoundsCadmium organic compoundsChemical industryPolymer crystals
The invention relates to a method for preparing nonlinear optical metal-organic boron polymer crystal material, and belongs to the chemical industry technical field. Firstly, ligand H3L, namely, tri-(4-pyridine-2,3,5,6-tetramethylbenzene) borane is synthesized, then second order nonlinear optical metal-organic boron polymer crystal material with equal chirality is prepared, namely, ligand H3L andmetal cadmium salt are mixed according to 2: 1 mole ratio, the mixed solvent of ethyl alcohol and toluene is added, the reaction liquid is positioned into a sample bottle for reaction, and the crystal is taken out, washed and air dried after the reaction is finished, to obtain the second order nonlinear optical metal-organic boron polymer crystal material. The structural general formula is [MX2L].2H2O, wherein, MX2 represents CdCl2, Br2, I2, (NO3)2, (OAc)2, or (ClO4)2. Compared with the prior art, not only the reaction time of the invention is short, and the condition is temperate, but also both the purity and the production rate of the obtained crystal material are high.
Owner:SHANGHAI JIAO TONG UNIV
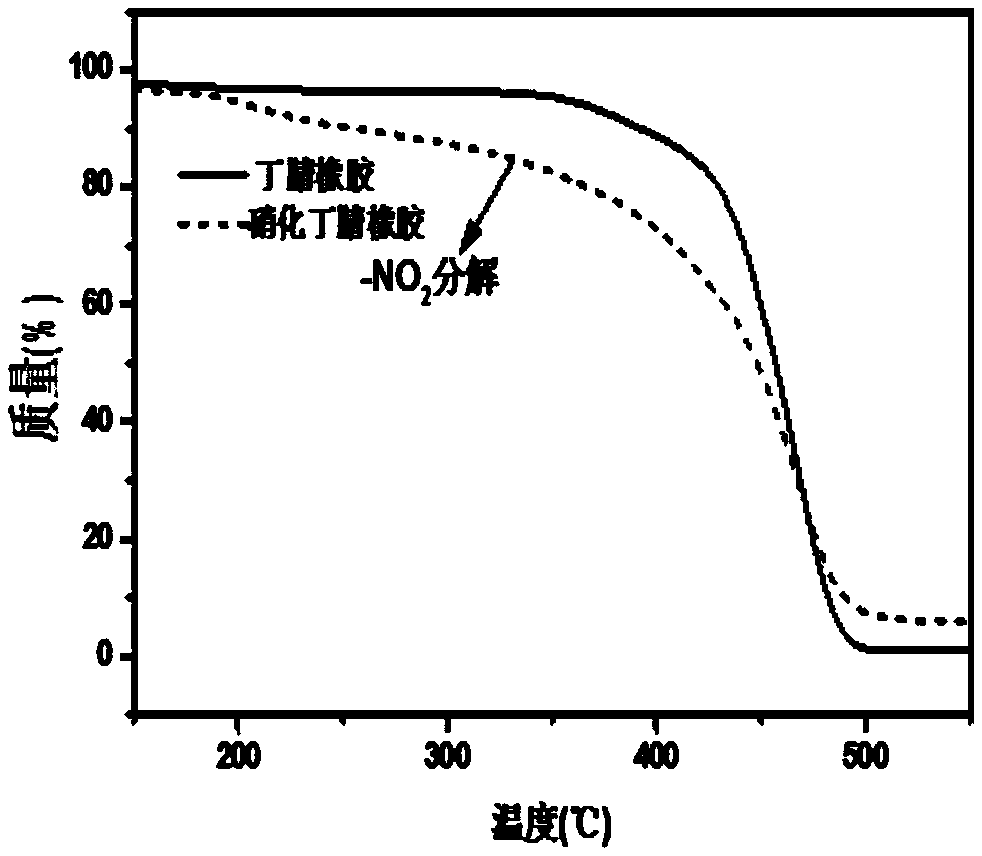
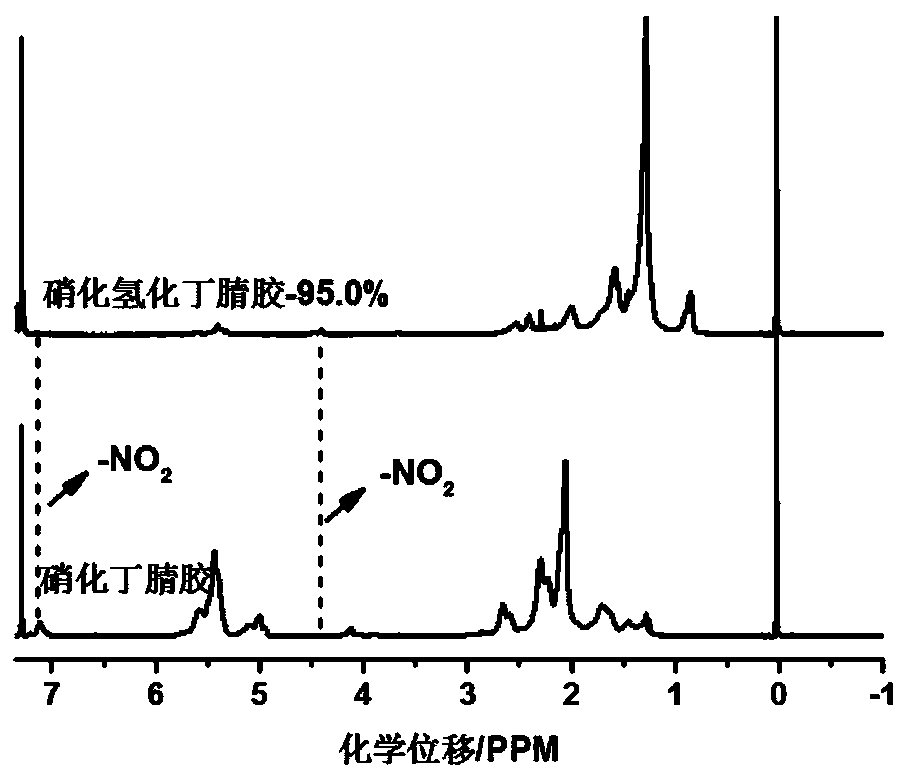
Nitrated butadiene-acrylonitrile rubber as well as preparation method and application thereof
The invention relates to modification of rubber materials, in particular to nitrated butadiene-acrylonitrile rubber as well as a preparation method and application thereof. The preparation method comprises the following step that nitration reaction is carried out on butadiene-acrylonitrile rubber by taking AgNO2 as a nitration agent so as to obtain the nitrated butadiene-acrylonitrile rubber, wherein in the nitration reaction, one of TEMPO, DPPH or p-benzoquinone and tetramethyl-benzoquinone serves as a catalyst. According to the nitrated butadiene-acrylonitrile rubber as well as the preparation method and application thereof, the butadiene-acrylonitrile rubber is nitrated by adopting the AgNO2 as the nitration reagent, the epoxidation step can be avoided, the nitrated butadiene-acrylonitrile rubber is obtained in one step, no gel is generated in the reaction process, the reaction time is short, the process is simple, and the operation is convenient; the liquid butadiene-acrylonitrilerubber is changed into an energetic bonding material from a common liquid rubber material, and the nitro group-containing nitrated liquid butadiene-acrylonitrile rubber; and meanwhile, a certain amount of nitro groups are introduced into molecular chains of conventional hydrogenated butadiene-acrylonitrile rubber to serve as active reactive groups, and a new research idea is provided for further modification.
Owner:BEIJING UNIV OF CHEM TECH
A kind of nitrated nitrile rubber and its preparation method and application
The invention relates to modification of rubber materials, in particular to nitrated butadiene-acrylonitrile rubber as well as a preparation method and application thereof. The preparation method comprises the following step that nitration reaction is carried out on butadiene-acrylonitrile rubber by taking AgNO2 as a nitration agent so as to obtain the nitrated butadiene-acrylonitrile rubber, wherein in the nitration reaction, one of TEMPO, DPPH or p-benzoquinone and tetramethyl-benzoquinone serves as a catalyst. According to the nitrated butadiene-acrylonitrile rubber as well as the preparation method and application thereof, the butadiene-acrylonitrile rubber is nitrated by adopting the AgNO2 as the nitration reagent, the epoxidation step can be avoided, the nitrated butadiene-acrylonitrile rubber is obtained in one step, no gel is generated in the reaction process, the reaction time is short, the process is simple, and the operation is convenient; the liquid butadiene-acrylonitrilerubber is changed into an energetic bonding material from a common liquid rubber material, and the nitro group-containing nitrated liquid butadiene-acrylonitrile rubber; and meanwhile, a certain amount of nitro groups are introduced into molecular chains of conventional hydrogenated butadiene-acrylonitrile rubber to serve as active reactive groups, and a new research idea is provided for further modification.
Owner:BEIJING UNIV OF CHEM TECH
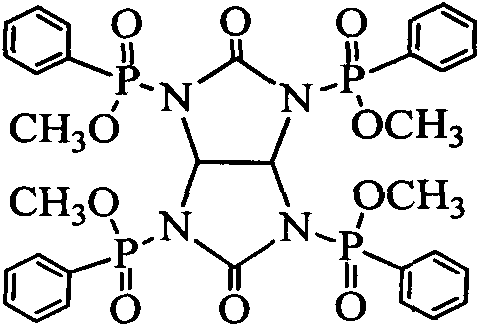
Tetra(0-methyl-phenylphosphinyl)glycoluril (TMPG) flame retardant composition and application method thereof
ActiveCN104194041AImprove flame retardant performanceNo reduction in mechanical strengthGlycolurilPolyester
The invention relates to a tetra(0-methyl-phenylphosphinyl)glycoluril (TMPG) flame retardant composition. The flame retardant composition is prepared from TMPG and any one or two or three of melamine cyanurate (MCA), melamine polyphosphate (MPP) and diethyl aluminum phosphinate (1240) through compounding according to any weight ratio and uniformly mixing, wherein the weight fraction of TMPG in the flame retardant composition is greater than 0. The flame retardant composition disclosed by the invention has the advantages that multiple elements are synergic, the material compatibility is good, the flame retardance is high, the flame retardant composition is halogen-free and environment-friendly, the application cost can be reduced and the like, so that the flame retardant composition can be applied to flame retardants of polyester, polyamide and the like.
Owner:苏州艾科迪新材料科技有限公司
Cardo-type co-polyimide membranes for sour gas feed separations from natural gas
ActiveUS10913036B2Improve breathabilityGood choiceSemi-permeable membranesGas treatmentPolymer scienceMoiety
Co-polyimide membranes for separating components of sour natural gas where embodiments can include at least three distinct moieties polymerized together, the moieties including a 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) based moiety; a 9,9-bis(4-aminophenyl) fluorene (CARDO) based moiety; and 2,3,5,6-tetramethyl-1,4-phenylenediamine (durene diamine) based moiety.
Owner:SAUDI ARABIAN OIL CO
Method for preparing chirality non-linear optical metal-organic boron polymer crystal material
InactiveCN101230077BGood second-order nonlinear optical propertiesIngenious design ideasCopper organic compoundsCobalt organic compoundsBenzoic acidManganese
The invention relates to a preparation method for nonlinear optical metal-organic boron polymer crystal material provided with equal chirality. The method adopts the steps that firstly, ligand H3L, namely tri-(4-para benzoic acid-2, 3, 5, 6-tetramethylbenzene) borane is synthesized, and then nonlinear optical metal-organic boron polymer crystal material with equal chirality is prepared, namely, ligand H3L and metal salt are mixed according to 1: 2, and dissolved by using N, N-dimethyl acetamide and methyl alcohol, the reaction liquid is positioned into a sample bottle for reaction, after the reaction is finished, the sample bottle is cooled to room temperature, the generated metal-organic polymeric material is taken out, and washed with ether for multiple times and air dried to obtain thematerial. The structural general formula is <M2L (OH) (MeOH)>.3H2O, wherein, metal M represents cobalt, manganese, nickel, copper, zinc or cadmium. The material produced through the invention has verygood second order nonlinear optical property, and compared with KDP, and the generation effect of second harmonics is 1.5 times higher.
Owner:SHANGHAI JIAOTONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com