Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
220 results about "Methylated naphthalene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
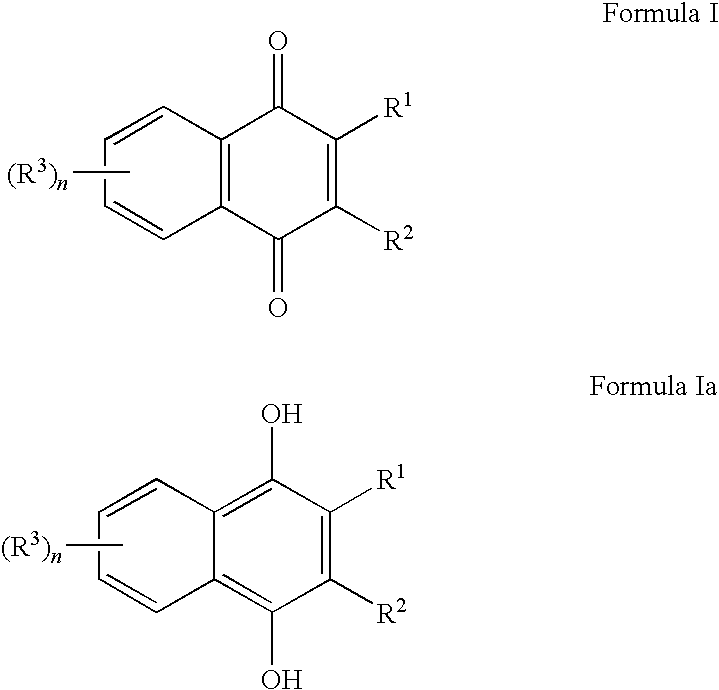
Naphthoquinone compositions with Anti-aging, Anti-inflammatory and skin even-toning properties
InactiveUS20100029784A1Reduce and treat and prevent signEffective protectionCosmetic preparationsBiocideOxidative stressNaphthoquinone
The present invention relates to methods and compositions comprising naphthoquinones such as 2,3-dimethylnaphthalene-1,4-dione, for the use of treating, regulating or preventing a skin condition characterized by oxidative stress or a degenerative process. Methods of preventing, lightening or reducing the appearance of visible and / or tactile discontinuities of the skin resulting from skin pigmentation or skin aging are also disclosed.
Owner:AMPERE LIFE SCI
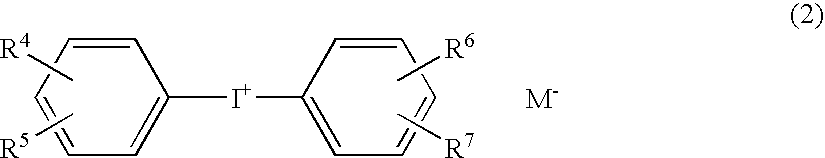
Photopolymerization initiator and photopolymerizable composition
InactiveUS20040186195A1High polymerization activityEasy to getImpression capsPhotomechanical apparatusDiketonePolycyclic compound
A photopolymerization initiator comprising (A) a photo acid-generating compound such as diaryliodonium salt (e.g., diphenyl iodonium, bis(p-chlorophenyl)iodonium, etc.), (B) a photo oxidation radical-generating compound such as diarylketone compound, alpha-diketone compound or ketocoumarin compound, and (C) a fused polycyclic aromatic compound such as 1,4-dimethylnaphthalene, 1-methylanthracene, 9-methylanthracene, 9,10-dimethylanthracene or 9,10-diethylanthracene. The photopolymerization initiator makes it possible to efficiently polymerize the cationically polymerizable monomer by the irradiation with visible light.
Owner:TOKUYAMA CORP +1
Marine concrete corrosion resistant additive and preparation thereof
The invention relates to the technical field of building material, in particular to a maritime work concrete corrosion-proof additive. The maritime work concrete corrosion-proof additive consists of a calcium sulphoaluminate expansion agent, a methyl naphthalene sulphonic acid water reducing agent, organosilicon hydrophobic powder, neopentylene glycol, milled slag powder, polypropylene fibers, silicon ash, superfine fly ash and arenaceous quartz. The maritime work concrete corrosion-proof additive can reduce the water consumption for mixing concrete, improve the water retention and cohesiveness, and improve the resistance of the concrete to seawater erosion and the durability.
Owner:TONGJI UNIV
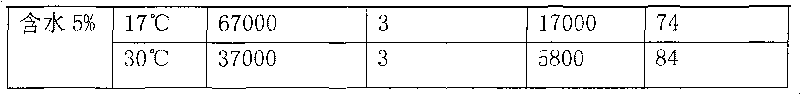
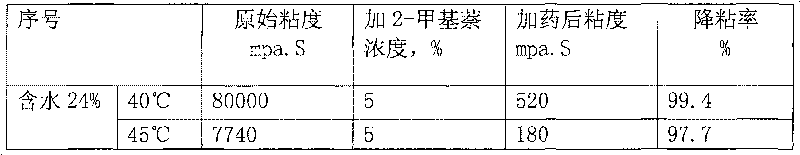
Application of methylnaphthalene in lowering viscosity of thickened oil
ActiveCN101717626ALow viscositySpeed up the flowFluid dynamicsFluid removalOil viscosityHigh surface
The invention discloses application of methylnaphthalene in lowering viscosity of thickened oil, which comprises application of 1-methylnaphthalene in lowering viscosity of thickened oil and the specific implementation steps, 2-methylnaphthalene in lowering viscosity of thickened oil and the specific implementation steps and mixed methylnaphthalene in lowering viscosity of thickened oil and the specific implementation steps. The invention enlarges the application method of methylnaphthalene, clearly demonstrates the specific application method of using methylnaphthalene to lower viscosity of thickened oil, solves the problems of great load and power consumption, frequent machinery accidents, high surface line return pressure, and the like of an oil extractor during extracting and outwards transporting thickened oil in the background art, and can effectively lower viscosity of thickened oil and reduce extraction and outward transportation cost of thickened oil.
Owner:盘锦河升大地石油科技有限公司
Isoprenyl derivatives and their use in the title treatment and prevention of osteoporosis and cardiovascular calcification
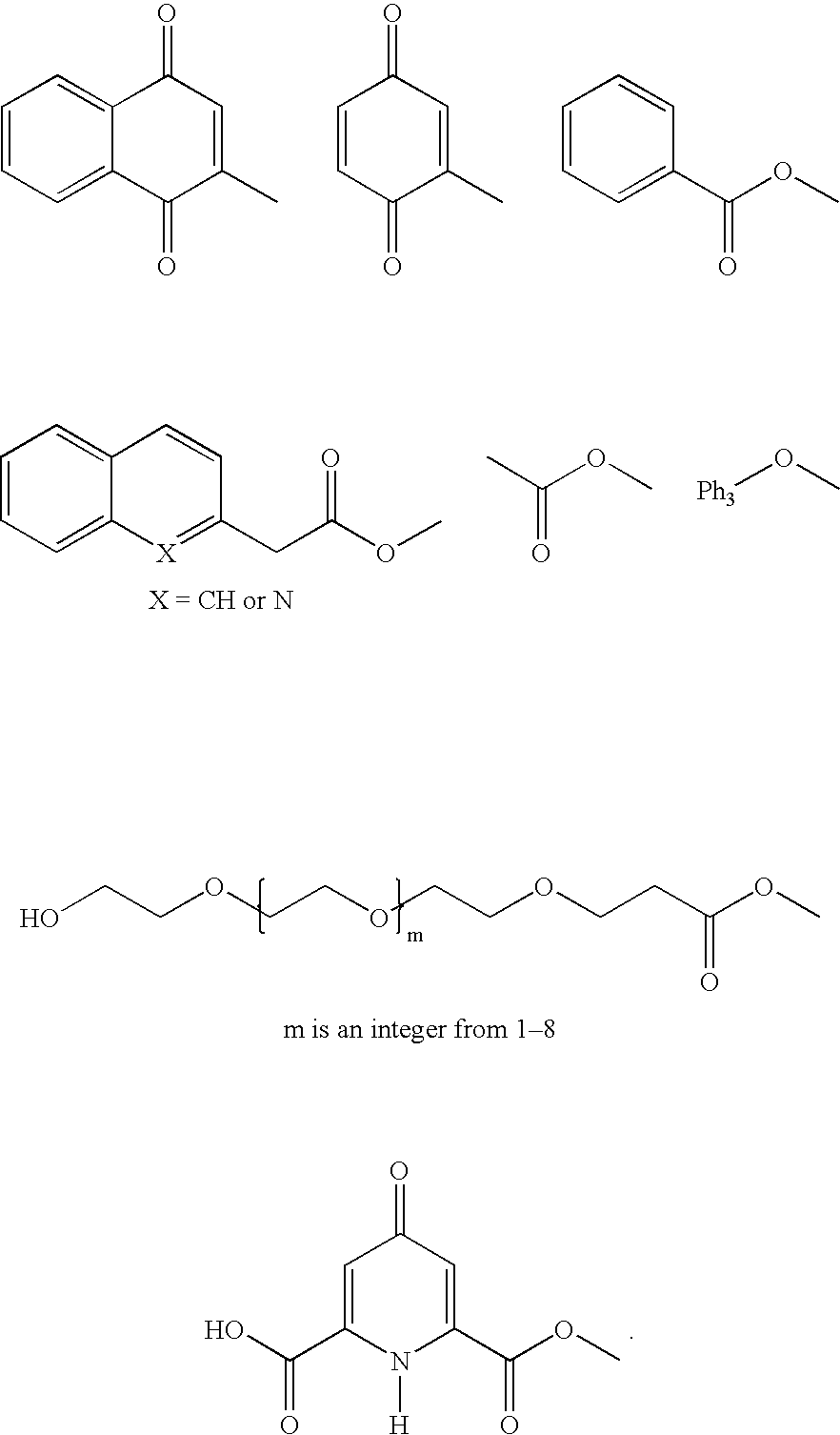
A non-toxic biologically active compound is provided having the following general formula (I): wherein n is an integer from 1 to 14, preferably from 2 to 4, and R is an organic moiety, preferably a group different from but structurally substantially similar to 2-methyl naphthoquinone, or a group P—C(R1)—P, where each P stands for a—PO(OH)2 group and R1 is a (poly)isoprenyl group, hydroxy, halogen (preferably chloro or bromo), or hydrogen, or a pharmaceutically acceptable derivative thereof. These compounds are useful for the treatment or prevention of certain disorders in a mammal, especially a human being, for example postmenopausal loss of bone in women, juvenile or senile osteoporosis in men and women, cardiovascular calcification and other ectopic calcifications.
Owner:NATTOPHARMA
Process for producing dialklylnaphthalenes
InactiveUS6204422B1Various problemIncrease profitMolecular sieve catalystsOrganic chemistry methodsProduct gasPetroleum
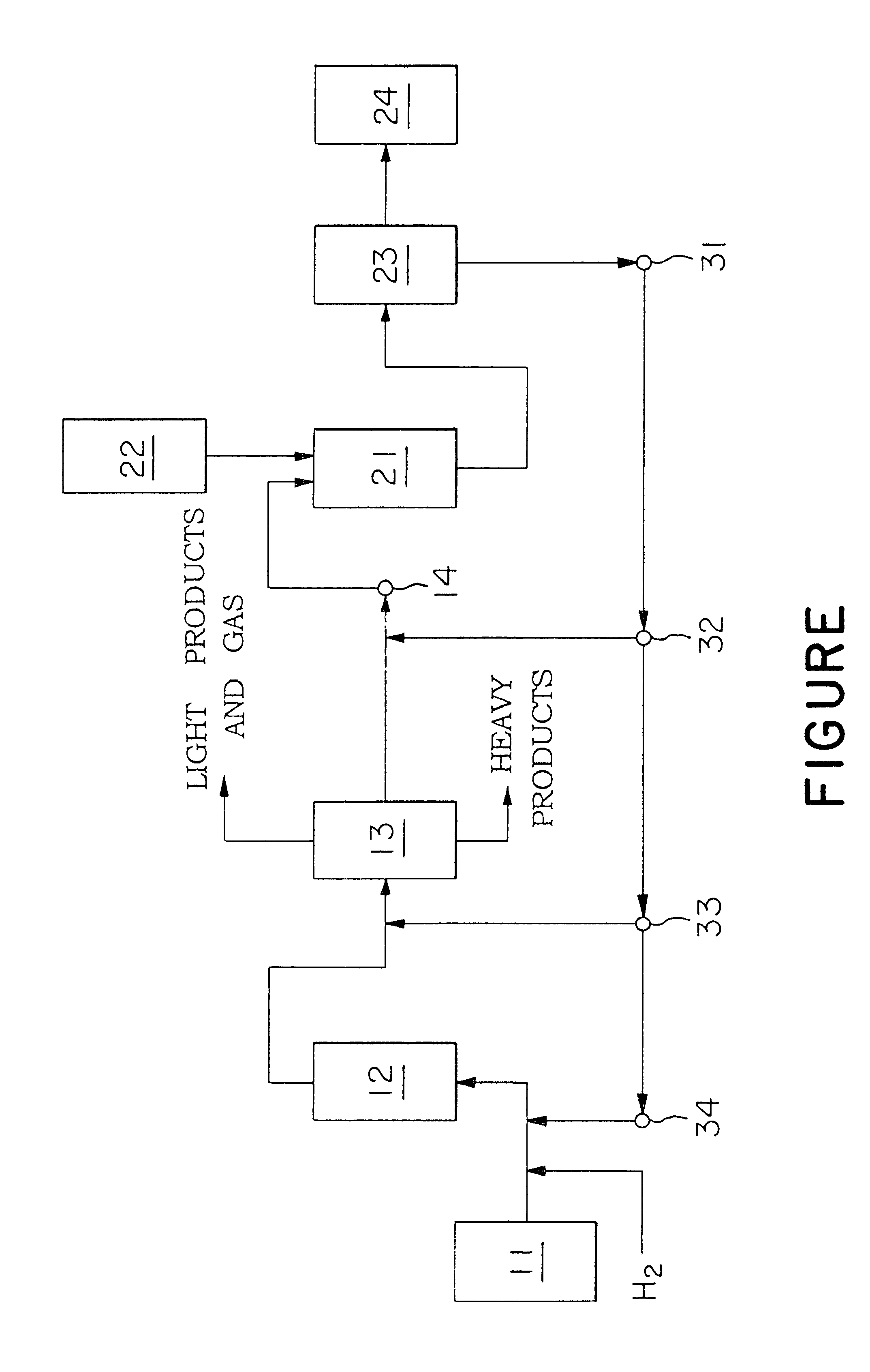
A rational technical process for producing dialkylnaphthalenes from petroleum-derived starting materials which exist in abundance is established. The process for producing dialkylnaphthalenes contains the steps (1), (2), (3), and (4): (1) 1st step which is for catalytically hydrodealkylating a stock oil containing a substantial amount of naphthalene and / or alkylnapthalenes, separating a product oil form a product gas, and separating and recovering a fraction composed mainly of naphthalene and / or methylnaphthalene from the product oil; (2) 2nd step which is for catalytically alkylating or catalytically transalkylating the fraction, recovered in the 1st step, composed mainly of naphthalene and / or methylnaphthalene directly without pretreatment for purification, with an alkylating agent or transalkylating agent to prepare alkylnaphthalenes; (3) 3rd step which is for separating and recovering dialkylnaphthalenes from the alkylnaphthalenes obtained in the 2nd step; and (4) 4th step which is for returning at least a part of the residual fraction, after the separation and recovery of dialkylnaphthalenes in the 3rd step, as at least a part of the stock oil in the 1st step and / or at least part of the alkylating agent or transalkylating agent in the 2nd step to the corresponding step.
Owner:FUJI OIL CO LTD
Method for synthesizing 2-methyl-1, 4-naphthoquinone
InactiveCN102516054AAvoid pollutionMild conditionsQuinone preparation by oxidationIce waterPtru catalyst
The invention relates to a method for synthesizing 2-methyl-1, 4-naphthoquinone through taking 2-methylnaphthalene as a raw material, which comprises the following steps of: taking the 2-methylnaphthalene as the raw material, taking glacial acetic acid as a solvent, taking hydrogen peroxide as an oxidizing agent and catalyzing with a catalyst to synthesize the 2-methyl-1, 4-naphthoquinone. The reaction temperature is 20-80 DEG C, and the dripping rate is 0.02-1 mL / min. After the end of dripping, the temperature is preserved for 0.5-4 hours. After the reaction is ended, and feed liquid is concentrated to 1 / 2-1 / 6 of the original volume, the feed liquid is poured into ice water with the volume being 3-6 times that of the feed liquid and is then stewed, filtered and dried to obtain the 2-methyl-1, 4-naphthoquinone. The method has the beneficial effects that a mixed acid catalytic system is used; compared with a traditional chromate oxidation method, the method has the advantages of good selectivity and high yield; and the pollution to a large quantity of chromium metal ions generated in the traditional industrial production can be avoided, and therefore, the industrial application value is higher.
Owner:SOUTHEAST UNIV
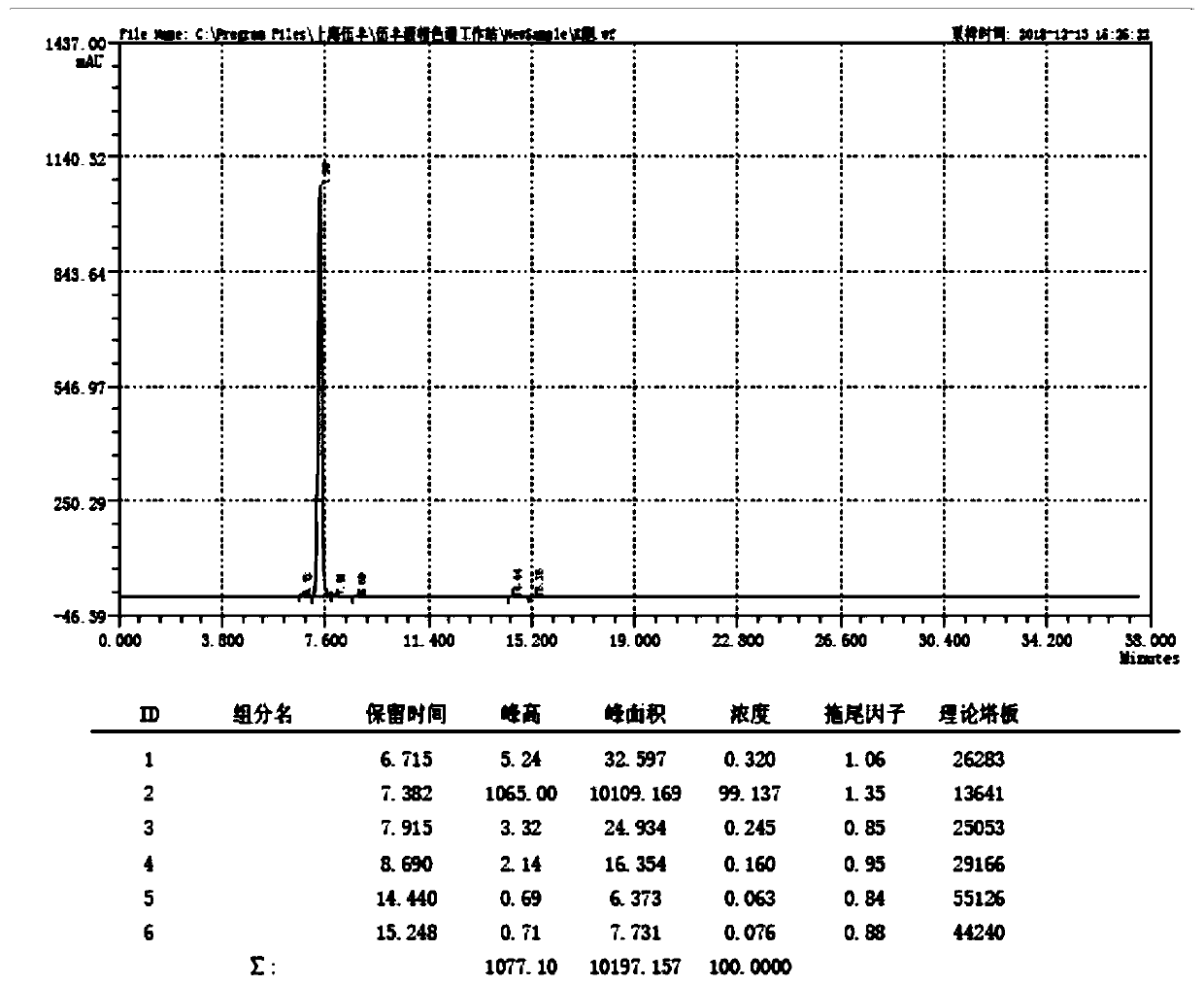
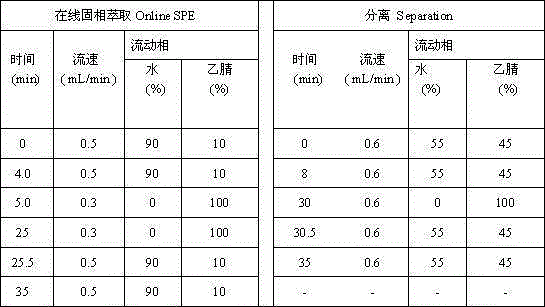
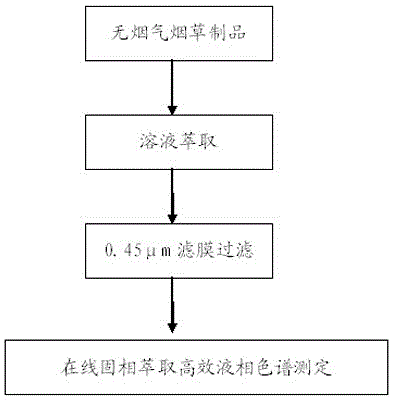
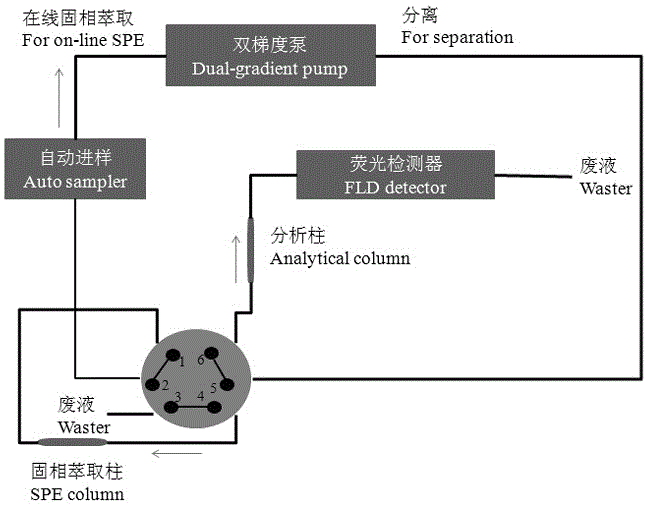
Method for determining polyaromatic hydrocarbons in smokeless tobacco product by utilization of on-line solid phase extraction high performance liquid chromatography
InactiveCN105136931AEasy to handleMany detection targetsComponent separationBiotechnologyFluoranthene
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
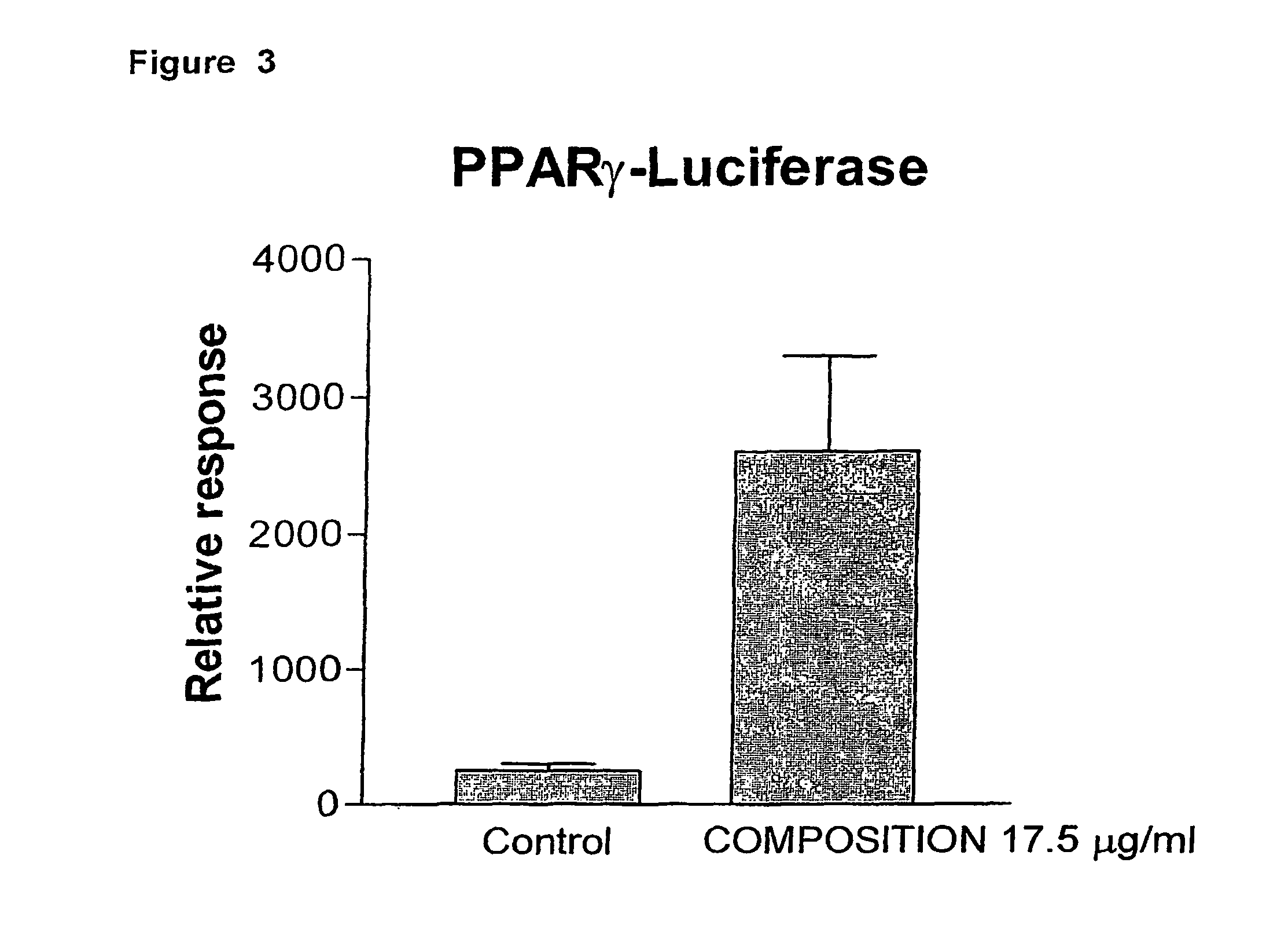
Composition of labdane diterpenes extracted from andrographis paniculata, useful for the treatment of autoimmune diseases, and alzheimer disease by activation for PPR-gamma receptors
ActiveUS8084495B2Alleviating symptom and courseReduced responseBiocideNervous disorderImmunologic disordersAutoimmune thyroid disease
The diterpenic labdane 3-[2-[decahydro-6-hydroxy-5-(hydroxymethyl)-5,ha-dimethyl-2-methylene-1-naphthalenyl]ethylidene]-dihydro-4-hydroxy-2(3h)-furanone, chemically diagrammed asinhibits the synthesis of pro-inflammatory cytokines, activates the PPAR-gamma receptor and diminishes nuclear factor kappa B.The compound is useful to treat auto-immune diseases, for organ and tissue transplantation, and to treat immunodeficiency (e.g., AIDS).
Owner:INNOBIOSCI
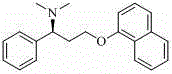
Method for preparing (S)-N, N-dimethyl-3-(naphthol-1-oxygroup)-1- phenyl propyl group-1-amine
InactiveCN102942496ALow priceSimple and fast operationOrganic compound preparationAmino-hyroxy compound preparationAlpha-naphtholEthyl acetate
The invention discloses a method for preparing (S)-N, N-dimethyl-3-(naphthol-1-oxygroup)-1- phenyl propyl group-1-amine. (S)-3-(naphthol-1-oxygroup)-1- phenyl propyl group-1-amine serves as the raw materials, and dimethyl carbonate replaces formaldehyde in the prior art to serve as a methylation reagent to prepare the (S)-N, N-dimethyl-3-(naphthol-1-oxygroup)-1- phenyl propyl group-1-amine. On the basis, the (S)-N, N-dimethyl-3-(naphthol-1-oxygroup)-1- phenyl propyl group-1-amine is dissolved in ethyl acetate to further prepare (S)-N, N-dimethyl-3-(naphthol-1-oxygroup)-1- phenyl propyl group-1-amine hydrochloride. The method reduces environment pollution without increasing the cost, simplifies reaction operations, reduces requirements on equipment and can be used in industrial production easily.
Owner:YANGZHOU POLYTECHNIC INST
Preparation method of 1,4-naphthalenedicarboxylic acid
ActiveCN103739484AReduce pollutionReduce discardingOrganic compound preparationCarboxylic compound preparationMANGANESE ACETATESodium acetate
The invention discloses a preparation method of 1,4-naphthalenedicarboxylic acid. The preparation method comprises the following steps of dissolving 1-methyl-4-naphthoic acid into glacial acetic acid, adding cobalt acetate, manganese acetate and sodium acetate in a mass ratio of 1: 50 to the mixed liquor, heating to 90 DEG C until all the materials are dissolved, then adding a catalyst, heating to 130 DEG C, uniformly pumping air, and cooling and filtering to obtain a crude product of 1,4-naphthalenedicarboxylic acid; adding acetic anhydride and replenishing the catalyst to the mother liquor to react again with 1-methyl-4-naphthoic acid; dissolving the crude product of 1,4-naphthalenedicarboxylic acid into water, adding caustic soda flakes, heating to 70-90 DEG C, regulating the PH value to 7-8, adding activated carbon, after stirring and suction filtration, heating to 70-90 DEG C, regulating the PH value below 2, cooling to 20-30 DEG C to carry out centrifugal separation, washing the solid material until the solid material is neutral, and drying the material, thus obtaining a finished product. The preparation method adopts air and oxygen for oxidation, is abundant in resources and low in production cost, is free from waste residues, and is environment-friendly and energy-saving.
Owner:黄石市利福达医药化工有限公司
Method for producing naphthalenedicarboxylic acid
ActiveCN101077857ASafeReduce manufacturing costsOrganic compound preparationCarboxylic acid esters preparationAcetic acidPhysical chemistry
A method for producing naphthalenedicarboxylic acid comprising the steps of: dissolving 2,6-dimethylnaphthalene in acetic acid solvent; oxidizing the product from the dissolution process using oxygen and a diluent gas; crystallizing naphthalenedicarboxylic acid that has been produced from the oxidation process; and separating the crystallized naphthalenedicarboxylic acid, wherein the amount of the diluent gas being discharged from and recycled to the oxidation process is controlled during the oxidation process, and the amount of mother liquor being recycled to the dissolution process after crystallization is controlled during the separation process, is provided.
Owner:HYOSUNG CHEM CORP
Flame-retardant microsphere electrolyte and preparation method thereof
InactiveCN110994019AAvoid burnsSecondary cells servicing/maintenanceOrganic electrolytesElectrochemistryMethylated naphthalene
The invention discloses a flame-retardant microsphere electrolyte and a preparation method thereof. The electrolyte comprises core-shell microspheres, a dispersing agent and an electrolyte, the core-shell microspheres are composed of an inner core flame retardant TPP and a packaging shell PVDF-HFP, wherein the mass ratio of the core-shell microspheres in the electrolyte is 2%-10%, the dispersing agent is one or more of sodium methylene dimethyl naphthalene sulfonate, 2-naphthalene sulfonic acid formaldehyde polymer sodium salt and a polyacrylic acid copolymer, and the mass ratio of the dispersing agent in the electrolyte is 0.1%-3.0%. According to the flame-retardant microsphere electrolyte disclosed in the invention, the core-shell structure TPP(at)PVDF-HFP flame-retardant microspheres are added into the electrolyte in combination with a microcapsule technology and a flame-retardant technology, so that the battery combustion can be prevented fundamentally, the adverse effect on the electrochemical performance of the battery due to direct addition of a flame retardant is avoided, and the core-shell structure TPP(at)PVDF-HFP flame-retardant microspheres have a wide application prospect.
Owner:SHANGHAI AEROSPACE POWER TECH +1
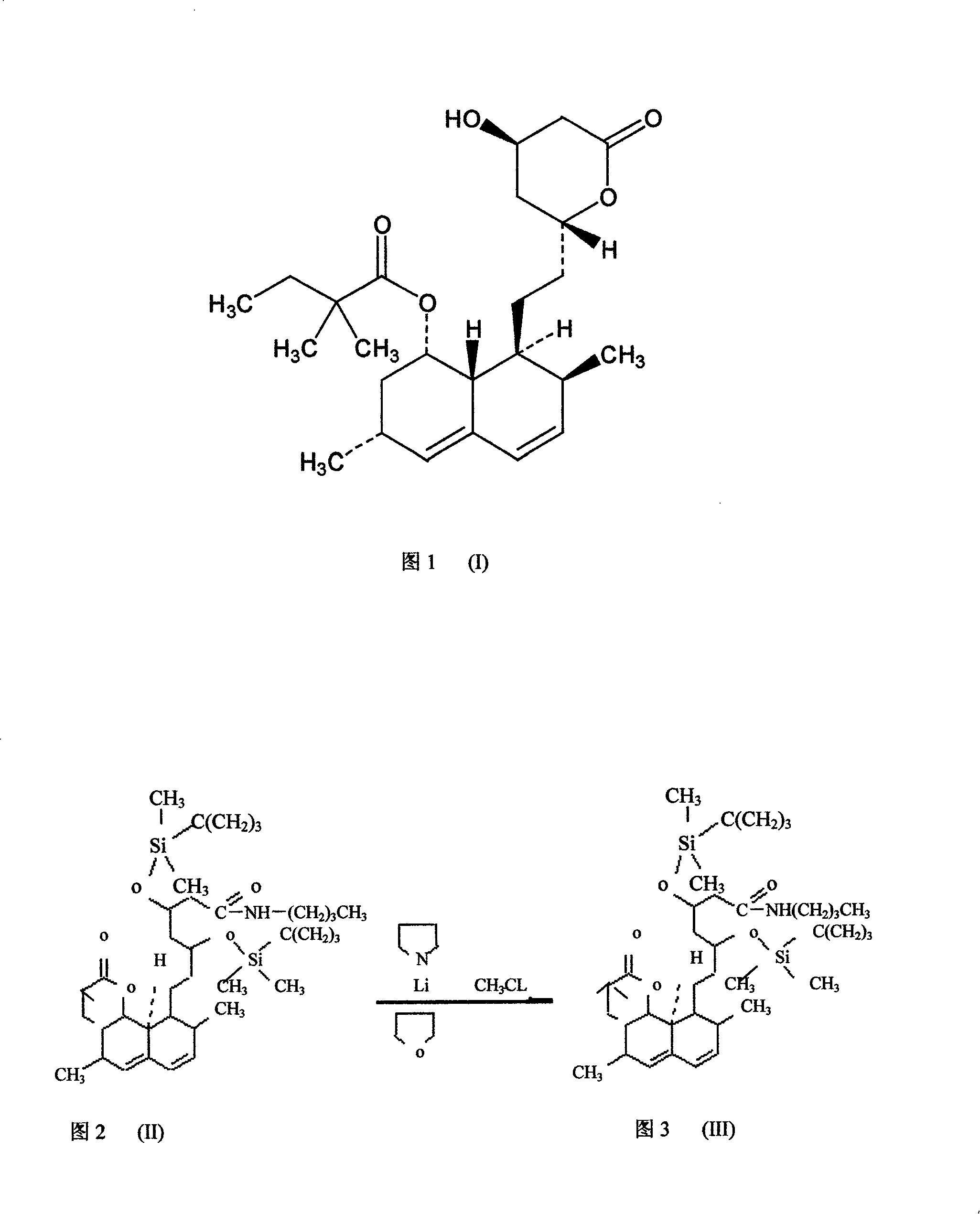
Method for synthesizing statins compounds
InactiveCN101190907ASimple processReduce manufacturing costOrganic chemistryDisiloxaneMethylating Agent
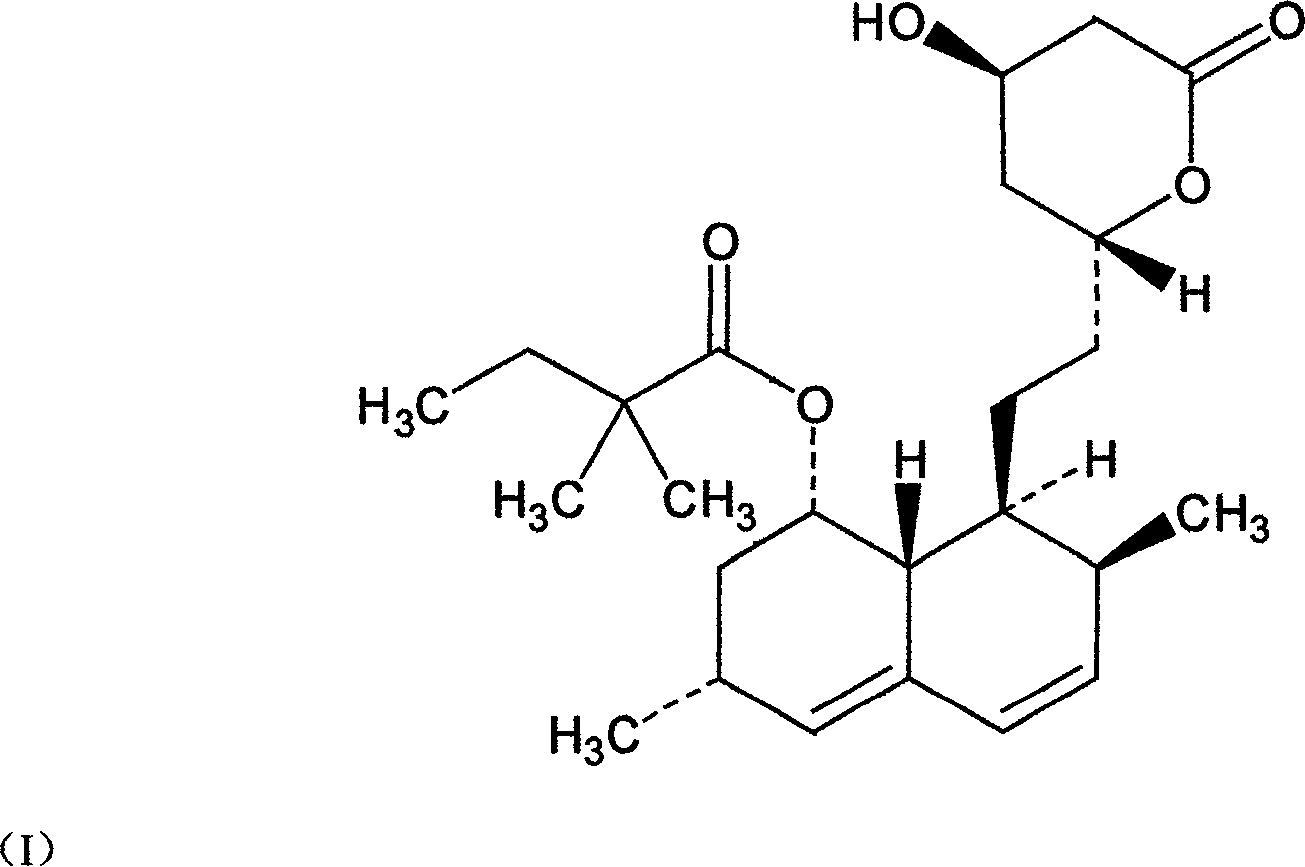
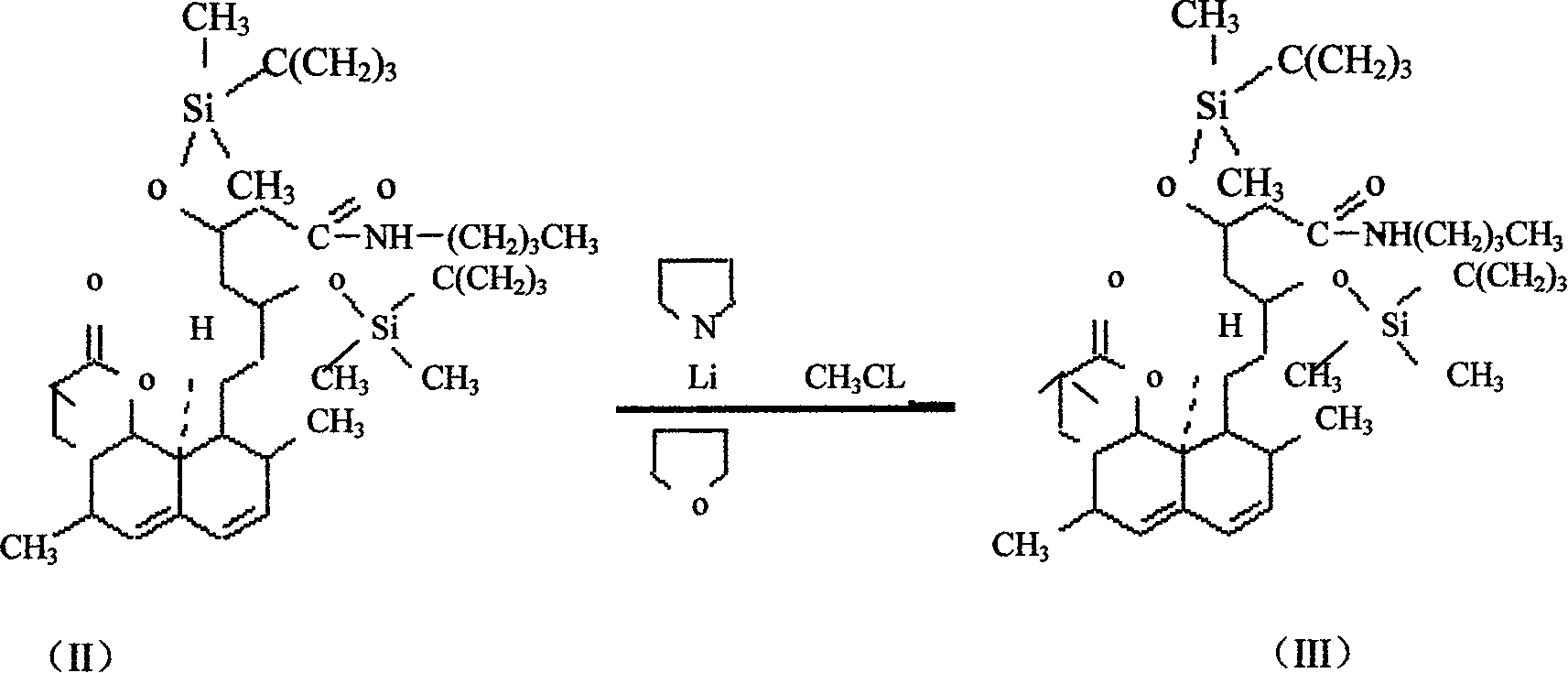
The invention relates to a novel synthesis method of statin compounds, in particular to a preparation method of methylation reaction of Simvastatin (2,2-dimethylbutyric acid-8-((4R, 6R)-6-(2-((1s, 2s, 6R, 8S, 8aR)-1,2,6,7,8,8-a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl))tetrahydrochysene-4-hydroxy-2H-pyran-2-ketone ester) and analogues thereof. The method consists of the reaction of Lovastatin amido disiloxane and methylating agent.
Owner:马群力 +1
Method for determining migration quantity of volatile organic compounds in adhesive sticker for food label in water-based food simulating object
ActiveCN103575848AOvercome the disadvantage of not being able to directly enter water samplesHigh detection sensitivityComponent separationWater basedMass spectrometry detector
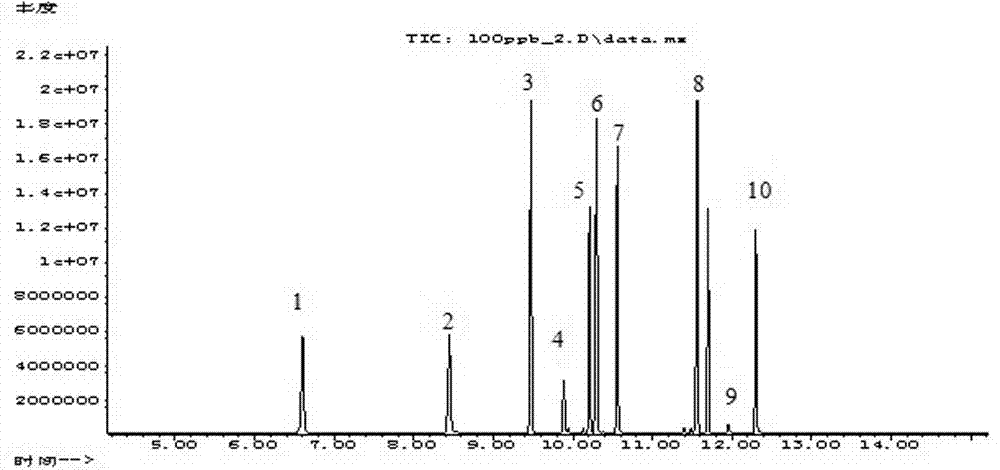
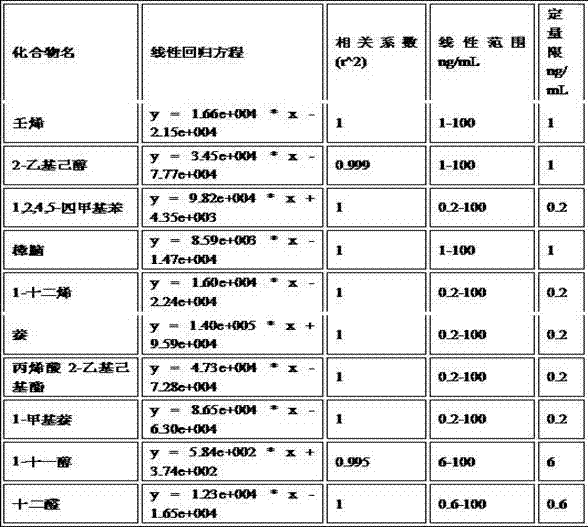
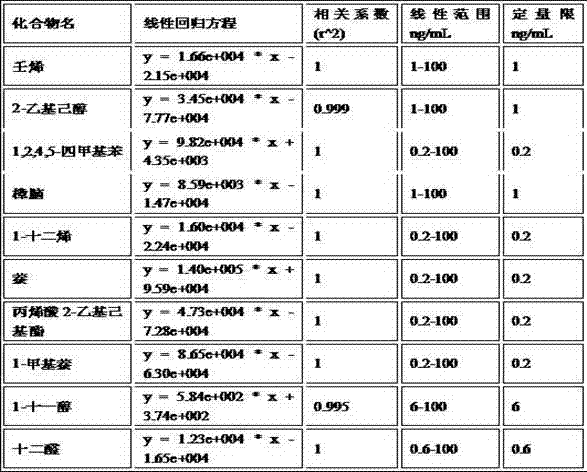
The invention discloses a method for determining the migration quantity of volatile organic compounds in an adhesive sticker for a food label in a water-based food simulating object. The method comprises the following steps: performing a direct or indirect migration test on the adhesive sticker for the food label through a water-based food simulating solution; sampling the water-based food simulating solution through purging and trapping; separating 10 volatile organic compounds through HP-5ms or chromatographic columns with similar properties; performing detection through a mass spectrometry detector; performing quantification through an external standard method, wherein the 10 volatile organic compounds include nonene, 2-ethylhexanol, 1,2,4,5-tetramethylbenzene, camphor, 1-dodecene, naphthalene, 2-ethylhexyl acrylate, 1-methylnaphthalene, 1-undecanol and dodecyl aldehyde. The method is accurate in test, easy to operate, high in efficiency, quick, high in sensitivity and high in practicality, is applicable to qualitative and quantitative determination on the volatile organic compounds in the adhesive sticker for the food label through a manufacturing enterprise of the adhesive sticker for the food label and relevant government supervision departments, and can be used for avoiding harm to the physical health of a consumer caused by over-large migration quantity of the volatile organic compounds.
Owner:厦门佰能检验技术服务有限公司
Device and method for extracting acenaphthene and acenaphthylene in LCO bicyclic aromatic hydrocarbon
ActiveCN109438164AEffective purityEffective yieldDistillation purification/separationHydrocarbonsAcenaphthyleneSolvent
The invention discloses a device and method for extracting acenaphthene and acenaphthylene in LCO bicyclic aromatic hydrocarbon. The device comprises an extraction rectifying tower, a regeneration tower, a crystallizing device, a washer and a sidestream rectifying tower. The method comprises the following steps: during preparation, firstly, adding a mixed liquid containing acenaphthene and acenaphthylene and an extractant from a raw material feed inlet and an extractant feed inlet of the extraction rectifying tower for extraction and rectification; feeding a fraction containing the extractantand dimethylnaphthalene into the regeneration tower for regeneration; recycling the regenerated extractant fraction to the extraction rectifying tower; feeding the fraction containing acenaphthene andacenaphthylene at the bottom of the extraction rectifying tower into the crystallizing device; adding a crystallizing solution for crystallization at the same time; feeding the obtained coarse acenaphthene into the washer; washing the coarse acenaphthene to obtain crystal acenaphthene; feeding the crystallizing mother liquor and washing agent mother liquor into the sidestream rectifying tower tobe rectified to obtain an acenaphthylene fraction; and recycling the crystallizing solvent to the crystallizing device and the washer. According to the device, the purity and the yield of acenaphtheneseparately reach 99.57% and 80.60% and the purity and the yield of acenaphthylene separately reach 98.53% and 77.59%.
Owner:NANJING NORMAL UNIVERSITY
Reboiler cleaning agent and cleaning method thereof
ActiveCN101255387AEfficient removalThe descaling process is simple but not complicatedOrganic non-surface-active detergent compositionsNon-surface-active detergent solventsProcess engineeringEnvironmental engineering
A detergent for reboiler and cleaning method thereof are disclosed, which pertains to the detergent field. The detergent comprises raw materials with weight proportion as follows: alpha-methylnaphthanlene 5-10 parts; beta-methylnaphthanlene 50-70 parts; naphthalin 2-15 parts; heavy aromatic hydrocarbon solvent 10-35 parts. Advantages are that organic scale formation formed in the reboiler because of decomposition of heat conductive oil due to long-term heating or condensation is effectively removed; contaminant release process is simple; detergent after contaminant release can be recycled and reused, which is economic and environmental protective.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
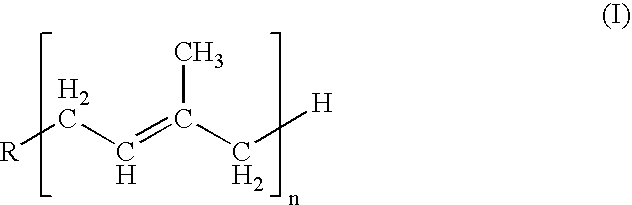
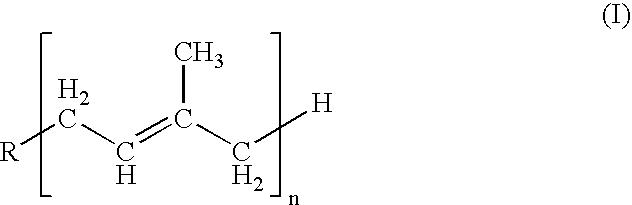
2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative as well as preparation method and application thereof
InactiveCN102942568AImprove thermal stabilityImprove solubilityOrganic chemistryLuminescent compositionsTwo-photon absorptionCarbazole
The invention relates to a 2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative as well as a preparation method and an application thereof, which relates to the 1,8-naphthyridine derivative and a preparation method and an application thereof, and solves the problems in the existing bi-photon absorption material that a molecular synthesis route of a large yoke system is long, the efficiency is low, the heat stability is poor and the photochemical stability is poor. The structural formula of the 2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative is as follows: the preparation method of the 2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative is characterized in that 2- methyl-1, 8-naphthyridine is linked with N-alkyl carbazole to have Knoevenagel reaction under the condition by adopting zinc chloride (ZnCl2) as condensing agent and dimethyl formamide (DMF) as solvent. The 2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative has strong fluorescent absorption performance and bi-photon absorption performance and good photochemical stability and heat stability. The 2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative is applied to the field of the laser three-dimensional micro machining and the conversion of bi-photon fluorescent.
Owner:HEILONGJIANG UNIV
Resin type waterproof coating material
InactiveCN103602122ANo pollution in the processGood adhesionCoatingsPolymer scienceO-Phosphoric Acid
The present invention discloses a resin type waterproof coating material, which is prepared from the following raw materials by weight: 3.5-8 parts of dihydroxypolydimethylsiloxane, 1.2-2.4 parts of 8-hydroxyquinoline, 1.3-3.7 parts of alkyl dimethyl benzyl ammonium salt type dodecyl dimethyl benzyl ammonium chloride, 0.8-2.2 parts of phosphoric acid, 1.2-3 parts of silicone oil, 1.3-3.4 parts of a sodium methyl naphthalene sulfonate formaldehyde condensation substance, 2.3-3.5 parts of secondary alcohol sodium sulfate, and 1.3-2.6 parts of alpha-cyanoacrylate adhesive. Compared with the existing waterproof coating material, the resin type waterproof coating material of the present invention has characteristics of corrosion resistance, aging resistance, strong adhesion, high temperature resistance, low temperature resistance, good ductility and high elongation at break, and integrates a plurality of excellent performances. In addition, the aqueous coating material has characteristics of no environment pollution, no harm on human body, safety and environmental protection.
Owner:QINGDAO HAIBAN PLASTIC IND & TRADE
Method for catalytically preparing 2,6-dimethylnaphthalene
ActiveCN108341734AHigh acid densityImprove reaction stabilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalystsMesitylene2,6-Dimethylnaphthalene
The invention discloses a method for catalytically preparing 2,6-dimethylnaphthalene through using magnetic ionic liquid. The method comprises the following steps of adding a mixed solution of methylnaphthalene and mesitylene in an ultrasonic reaction kettle provided with a stirrer, stirring to react for 35-45min in inert atmosphere, then adding a magnetic ionic liquid catalyst, reacting for 15-80min under the condition that temperature is 25-40 DEG C, the inert atmosphere exists, the stirring speed is 400-600r / min and the power of ultrasonic wave is 150-300W, and standing for 30min after completing reaction; a lower-layer catalyst can be directly recycled after subjected to magnetic filed separation; the mixed solution with an upper layer containing the 2,6-dimethylnaphthalene is furtherseparated and purified to obtain a 2,6-dimethylnaphthalene product and incompletely reacted raw materials. The method has the advantages of high catalytic activity, precise process, high reaction transformation rate, high 2,6-dimethylnaphthalene yield and the like.
Owner:广东和汇新材料有限公司
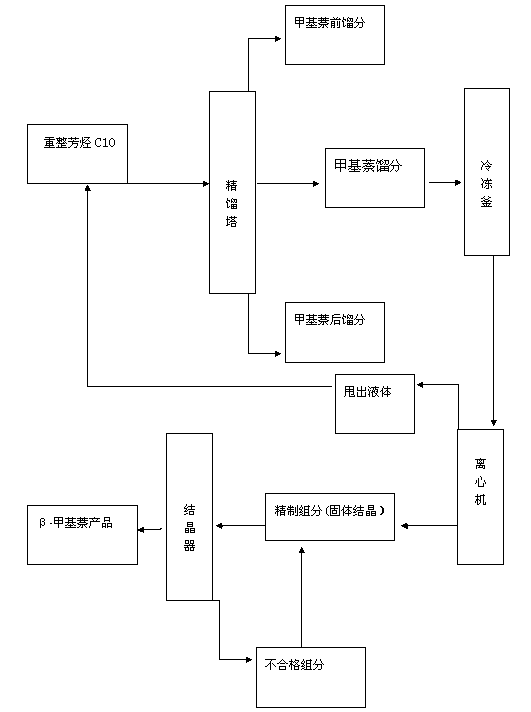
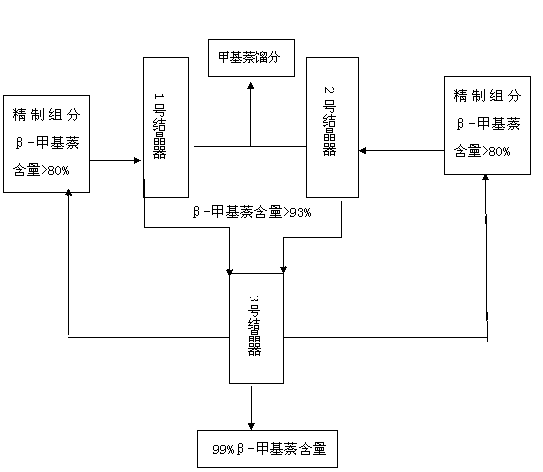
Process method for extracting high-purity beta-methylnaphthalene from reformed aromatic hydrocarbon C10
InactiveCN103288584ALow yieldLow costHydrocarbon purification/separationHydrocarbonsCatalytic reformingSocial benefits
The invention relates to a process method for extracting high-purity beta-methylnaphthalene from reformed aromatic hydrocarbon C10. The process method comprises the following steps of: by taking catalytically reformed by-product hydrocarbon C10 as a raw material and refined components as raw materials after carrying out rectification, freezing crystallization and refining on the catalytically reformed by-product hydrocarbon C10, putting the raw materials into a multistage countercurrent fractional crystallizer, and refining and extracting the beta-methylnaphthalene, so as to obtain the beta-methylnaphthalene with the purity high than 99%. The process method has the advantages of simple process, relatively low equipment investment and production cost, no polluted by-product and relatively high economic benefit and social benefit.
Owner:天津市兴源化工有限公司
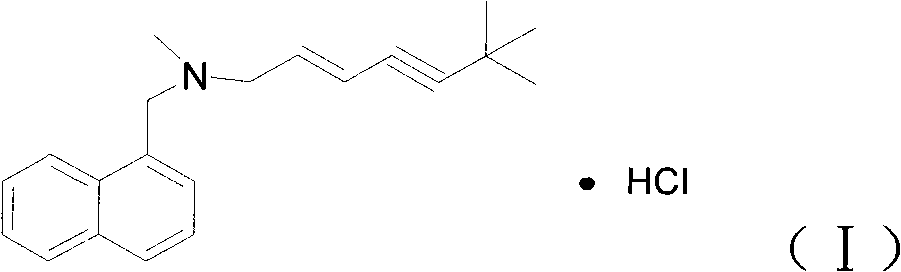
Preparation method of Terbinafine hydrochloride
InactiveCN101870655ARaw materials are cheap and easy to getThorough responseAntimycoticsPhysical/chemical process catalystsSodium iodideAlkyne
The invention relates to a preparation method of Terbinafine hydrochloride, which comprises the following steps: dissolving a certain amount of catalyst, i.e. sodium iodide (or potassium iodide) in an organic solvent, adding 1-chloro-6,6-dimethyl-2-heptylene-4-alkyne, sequentially adding alkali and N-methyl-1-naphthalene methylamine hydrochloride, carrying out condensation at 10-40 DEG C, salifying the obtained product in an alcohol system with hydrogen chloride to obtain the Terbinafine hydrochloride, and carrying out recrystallization in an alcohol / water mixed solution to obtain the qualified product. In the method, the raw materials are cheap and easily-acquired, the reaction is complete, and the reaction time is greatly shortened. Therefore, the method is completely suitable for industrialized mass production.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA +1
Method for purifying 4-acetyl-1-methyl naphthalene in acetyl methyl naphthalene mixture
ActiveCN109761775AHigh purityHigh yieldCarbonyl compound separation/purificationNaphthaleneCrystallization
The invention relates to a method for purifying 4-acetyl-1-methyl naphthalene in an acetyl methyl naphthalene mixture. The melting point difference between 4-acetyl-1-methyl naphthalene and other acetyl methyl naphthalene is adopted to obtain the 4-acetyl-1-methyl naphthalene with high purity. The acetyl methyl naphthalene mixture is put into a melting crystallization device, and the temperature is reduced from normal temperature to 10-15 DEG C, a mother solution without crystallization is released, and then the crystallized solid is started to heat up and sweat from 10-15 DEG C, the sweatingtemperature is 15-37 DEG C, after the end point of sweating is reached, sweating is stopped, and sweat is discharged; and finally, the crystalline solid in the melting crystallization device is heatedand melted to obtain the 4-acetyl-1-methyl naphthalene. According to the method for purifying the 4-acetyl-1-methyl naphthalene in the acetyl methyl naphthalene mixture, under the condition of not reducing the productive rate, the 4-acetyl-1-methyl naphthalene with the purity larger than 99.0% is obtained, and the operation is easy to operate.
Owner:湖北海力环保科技股份有限公司
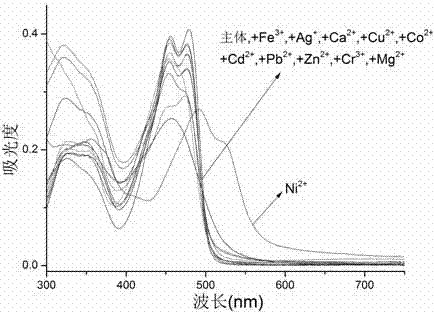
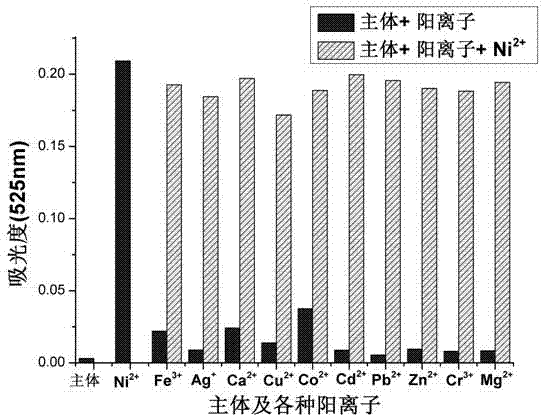
A nickel ion acceptor compound, synthesis thereof and applications of the compound
InactiveCN104193678AOrganic chemistryMaterial analysis by observing effect on chemical indicatorChemical compoundQuinoline
The invention provides a nickel ion acceptor compound that is 1-(quinoline-8-imino)methylenedinaphthol and a synthesis method thereof and belongs to the technical field of kation detection. Recognition effects of the acceptor compound on eleven kations comprising Fe<3+>, Ag<+>, Ca<2+>, Cu<2+>, Co<2+>, Ni<2+>, Cd<2+>, Pb<2+>, Zn<2+>, Cr<3+>, Mg<2+>, and the like are researched by a colorimetric method and an ultraviolet-visible absorption spectrometry method. The results show that: the acceptor compound can achieve single selective colorimetric recognition of the Ni<2+> in a DMSO-H2O-HEPES system, the lowest limit of detection achieves 2.2*10<-7> mol*L<-1>, detection sensitivity for the Ni<2+> is high, and the compound has good anti-interference capability. In addition, nickel ion detection paper based on the acceptor compound is also prepared and can conveniently and rapidly detect nickel ions in water.
Owner:NORTHWEST NORMAL UNIVERSITY
PVC (polyvinyl chloride) elastomer and method for preparing same
The invention discloses a PVC (polyvinyl chloride) elastomer and a method for preparing the same. The PVC elastomer comprises PVC, nanometer calcium carbonate, calcium stearate, zinc stearate, epoxidized soybean oil, 1, 1-methylene-2-naphthol, hydrogenated nitrile butadiene rubber, divinyl benzene, magnesium oxide, dibutyltin maleate, polypropylene glycol adipate and SMA (styrene maleic anhydride). The PVC elastomer and the method have the advantages that the PVC elastomer is high in strength, the tensile strength of the PVC elastomer is 20-60 MPa, and the Shore hardness of the PVC elastomer is 80-100 A; the PVC elastomer is good in appearance, the impact strength of the PVC elastomer is 15-35 kJ / m<2>, and the Vicat softening point of the PVC elastomer is 115-155 DEG C; the permanent compression deformation of the PVC elastomer is 15-35%, the PVC elastomer is high in elasticity, the bending strength of the PVC elastomer is 15-55 MPa, and the tear strength of the PVC elastomer is 35-55 N / mm; the PVC elastomer is excellent in weather fastness and creep resistance, low in cost and easy to operate and can be widely produced, the breaking elongation of the PVC elastomer is 450-650%, the melt flow index of the PVC elastomer is 6-10 g / 10min, the water absorption of the PVC elastomer is 0.001-0.02%, and accordingly exiting materials can be continuously replaced.
Owner:陈逸君
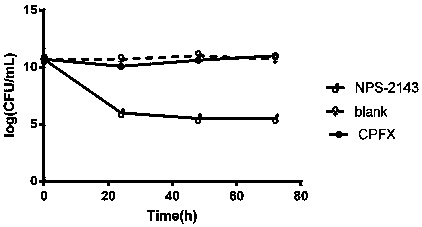
Novel antibacterial application of NPS-2143
InactiveCN108420814AGood inhibition rateAntibacterial agentsNitrile/isonitrile active ingredientsSystematic nameMicrobiology
The invention discloses novel antibacterial application of NPS-2143. The systematic name of NPS-2143 is 2-chloro-6-[(2R)-3-[[1,1-dimethyl-2-(2-naphthyl)ethyl]amino]-2-hydroxypropoxy]benzonitrile. Theinvention provides application of NPS-2143 in inhibition of Gram-positive bacteria and persistent bacteria thereof. According to the results of determination of inhibitory activity against the growthof a plurality of Gram-positive bacteria, NPS-2143 has good inhibitory activity against common Gram-positive bacteria such as MSSA strains (ATCC 25923), MSSA strains (ATCC 6538) and MRSA strains (ATCC33591). NPS-2143 has superiority in inhibition of sensitive Gram-positive bacteria and drug-resistant Gram-positive bacteria and in resistance of persistent bacteria compared with ciprofloxacin (CPFX), and shows good application prospects in the development of novel antibacterial drugs.
Owner:SICHUAN UNIV
Preparation method of 1, 4-naphthalene dicarboxylic acid
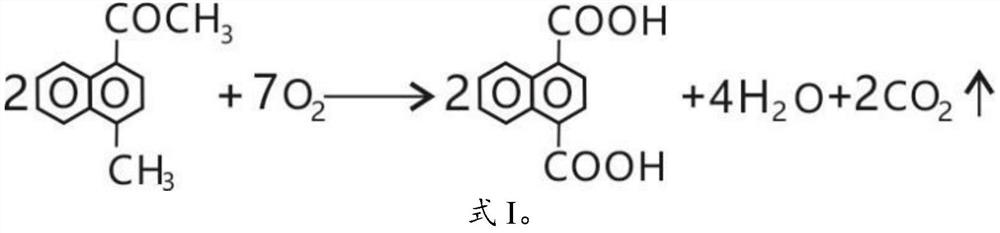
PendingCN111747840ASolve supply problemsSimple preparation stepsOrganic compound preparationCarboxylic compound preparationOrganic synthesisMethyl palmoxirate
The invention belongs to the technical field of organic synthesis and provides a preparation method of 1, 4-naphthalene dicarboxylic acid. According to the preparation method of the invention, with the 1-methyl-4-acetonaphthone adopted as an initial raw material, oxygen oxidation is directly performed to prepare the 1, 4-naphthalene dicarboxylic acid. The preparation method is simple in preparation steps and is easy in operation. With the preparation method adopted, the problem of raw material supply is solved; the price of 1-methyl-4-naphthalene dicarboxylic acid is 50,000 yuan lower than that of 1-methyl-4-naphthoic acid per ton; the total cost of the 1, 4-naphthalene dicarboxylic acid can be reduced by 40,000 yuan, and therefore, the preparation method has good economic benefits.
Owner:黄石市利福达医药化工有限公司
Catalyst for one-step preparation of 2,6-dimethylnaphthalene from synthesis gas and 2-methylnaphthalene
InactiveCN110694680AImprove catalytic conversion efficiencyExtended service lifeMolecular sieve catalystsMolecular sieve catalystMolecular sievePtru catalyst
The invention relates to a catalyst for one-step preparation of 2,6-dimethylnaphthalene from synthesis gas and 2-methylnaphthalene, wherein the catalyst comprises 70-95 wt% of an EU-1 / ZSM-23 mixed crystal molecular sieve of a hydrogen type nanometer needle-like crystal, 5-30 wt% of a binder and an oxide auxiliary agent, and the content of the oxide auxiliary agent is 1-20 wt% of the total weight of the molecular sieve and the binder. Compared with the existing 2,6-dimethylnaphthalene catalytic synthesis technology, the application of the catalyst in the catalytic reaction for one-step preparation of 2,6-dimethylnaphthalene from synthesis gas and 2-methylnaphthalene has the following characteristics that the reaction process is short, the catalytic reaction efficiency is high, and the service life of the catalyst is long, so that the yield of 2,6-dimethylnaphthalene is effectively increased, and the synthesis cost of 2,6-dimethylnaphthalene is reduced.
Owner:XIANGTAN UNIV
Method for measuring polycyclic aromatic hydrocarbons in cigarette cut tobacco by on-line solid phase extraction and high performance liquid chromatography
InactiveCN105181857AEasy to handleMany detection targetsComponent separationPolycyclic aromatic hydrocarbonPerylene
The invention discloses a method for measuring polycyclic aromatic hydrocarbons in cigarette cut tobacco by on-line solid phase extraction and high performance liquid chromatography. The polycyclic aromatic hydrocarbons refer to the following thirteen compounds: naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, fluorene, phenanthrene, anthracene, fluoranthene, benzo (a) anthracene, benzo (b) fluoranthene, benzo (k) fluoranthene, benzo (a) pyrene, dibenzo (a,h) anthracene and benzo (g,h,i) perylene. The method is characterized in that the polycyclic aromatic hydrocarbons in the cigarette cut tobacco are extracted by adopting a triangular flask holding cyclohexane, and the content of the polycyclic aromatic hydrocarbons in the cigarette cut tobacco is measured by utilizing the on-line solid phase extraction and the high performance liquid chromatography. The method has the advantages that the content of the polycyclic aromatic hydrocarbons in the cigarette cut tobacco can be rapidly and efficiently detected; pretreatment is simple; average relative standard deviation is less than 5 percent, and the average recovery rate of all indexes is between 83.6 percent to 104.1 percent; the method has the characteristics of being rapid, accurate, high in sensitivity and high in repeatability.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Preparation method of naftifine hydrochloride
InactiveCN103664631AReduce manufacturing costImprove responseOrganic compound preparationCarboxylic acid amides preparationChemical synthesisPtru catalyst
The invention provides a preparation method of naftifine hydrochloride, and belongs to the technical field of drug chemical synthesis. The preparation method comprises the following steps: taking 1-naphthoic acid as a starting raw material, in the presence of SOCl2 (Sulfoxide Chloride), reacting with a methylamine water solution to prepare N-methyl-1-naphthamide, enabling the N-methyl-1-naphthamide to react with formaldehyde and styrene in catalysis of Lewis acid in a multicomponent reaction manner to prepare carbonylated naftifine, performing hydrazine hydrate-KOH (Potassium Hydroxide) reduction, acidizing with hydrochloric acid, and separating the compound to obtain the naftifine hydrochloride. Through the cheap Lewis acid catalyzed amine, formaldehyde and styrene three-component one-pot method, the allyl structure unit is constructed in a single step, so that the reaction is greatly simplified, and the yield is improved; the preparation method is free from a noble metal catalyst, so that the production cost of the naftifine hydrochloride is reduced; the raw materials and various reagents are cheap and easy to get, the operation process is simple, the postprocessing procedures are simple, and the preparation method creates good conditions for industrial scale production and commercialization.
Owner:NORTHWEST NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com












![2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative as well as preparation method and application thereof 2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c9639949-e8f4-4f82-a356-03feea275b13/HDA00002427317500011.PNG)
![2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative as well as preparation method and application thereof 2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c9639949-e8f4-4f82-a356-03feea275b13/HDA00002427317500012.PNG)
![2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative as well as preparation method and application thereof 2-[(N- alkyl carbazolyl) vinyl]-1, 8-naphthyridine derivative as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c9639949-e8f4-4f82-a356-03feea275b13/HDA00002427317500021.PNG)