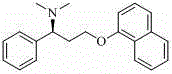
Method for preparing (S)-N, N-dimethyl-3-(naphthol-1-oxygroup)-1- phenyl propyl group-1-amine
A technology of phenylpropyl and dimethyl, which is applied in the field of preparation of -N,N-dimethyl-3--1-phenylpropyl-1-amine, which can solve the problem of unfavorable environmental protection and high toxicity of formaldehyde , formaldehyde overflow and other problems, to achieve the effect of easy recycling, low price and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of (S)-3-(naphthol-1-oxyl)-1-phenylpropyl-1-amine
[0020] The racemate 3-(naphthol-1-oxyl)-1-phenylpropyl-1-amine was prepared by the method in the literature (Chinese Journal of New Drugs, 2008, 17(24), 2119-2121).
[0021] Add racemate 3-(naphthol-1-oxyl)-1-phenylpropyl-1-amine (0.1mol), L-(+)-tartaric acid (0.25mol) into 150mL water and heat to reflux until clear transparent, add 20mL of n-butanol, continue to reflux for 20min, let cool to room temperature, precipitate a white solid, filter, and recrystallize the filter cake once with 50mL of water + 6mL of n-butanol, and add the obtained white crystals to sodium hydroxide solution (150mL, 5 %), refluxed for 2h, allowed to cool to room temperature, and an oily substance was precipitated, extracted with toluene until the aqueous layer had no product, and the resulting (S)-3-(naphthol-1-oxyl)-1-phenylpropyl-1- The toluene solution of the amine was directly used in the next reaction after be...
Embodiment 2
[0022] Example 2 Preparation of (S)-N,N-dimethyl-3-(naphthol-1-oxyl)-1-phenylpropyl-1-amine
[0023] The toluene solution of (S)-3-(naphthol-1-oxyl group)-1-phenylpropyl-1-amine obtained in Example 1 was added in a 500mL three-necked flask, and dimethyl carbonate (0.15mol ), anhydrous potassium carbonate (0.1mol), heat preservation at 60-80°C until the raw material (S)-3-(naphthol-1-oxyl group)-1-phenylpropyl-1-amine completely reacts, filter, filter The cake was washed with toluene (15mL×2), the filtrates were combined, and evaporated to dryness under reduced pressure (excess dimethyl carbonate was also evaporated and used for recycling), and the obtained oily substance was (S)-N,N- Dimethyl-3-(naphthol-1-oxyl)-1-phenylpropyl-1-amine (8.07 g), its chemical structure and absolute configuration were confirmed by hydrochloride formation.
[0024] Using different solvents, acid-binding agents, and reaction temperatures, the results are shown in Table 1
[0025] Table 1...
Embodiment 3
[0027] Example 3 Preparation of (S)-N,N-dimethyl-3-(naphthol-1-oxyl)-1-phenylpropyl-1-amine hydrochloride (dapoxetine hydrochloride)
[0028] (S)-N,N-dimethyl-3-(naphthol-1-oxyl)-1-phenylpropyl-1-amine (3.05g, 10mmol) obtained in Example 2 was dissolved in ethyl acetate Ester (16mL), slowly drop 0.1mol.L -1 Ethyl acetate solution of anhydrous hydrogen chloride until the pH value of the system is 2~3, after adding, stir for 1 hour, a white solid is precipitated, after centrifugation, pour off the supernatant, continue to add ethyl acetate (15mL), filter after stirring, the filter cake Wash with ethyl acetate (10 mL). M.p.=175.5~176.8℃, [ɑ] 20 D =+127.2°(C=1g·mL -1 ,CH 3 OH). 1 H NMR (DMSO- d 6 ): 2.567(d,3H,J=4Hz,CH 3 ) , 2.845(d,3H,J=4Hz, CH 3 ), 2.683~21757 (m,1H,2-position CH 2 ), 2.916~2.971 (m,1H,2-position CH 2 ), 3.655~3.712(m, 1H, 3-position CH), 4.101~4.149(m, 1H, 3-position CH 2 ), 4.713~4.742(m,1H,1-position CH), 6.73(d,J=8 Hz,1H,naphthalene ring-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com