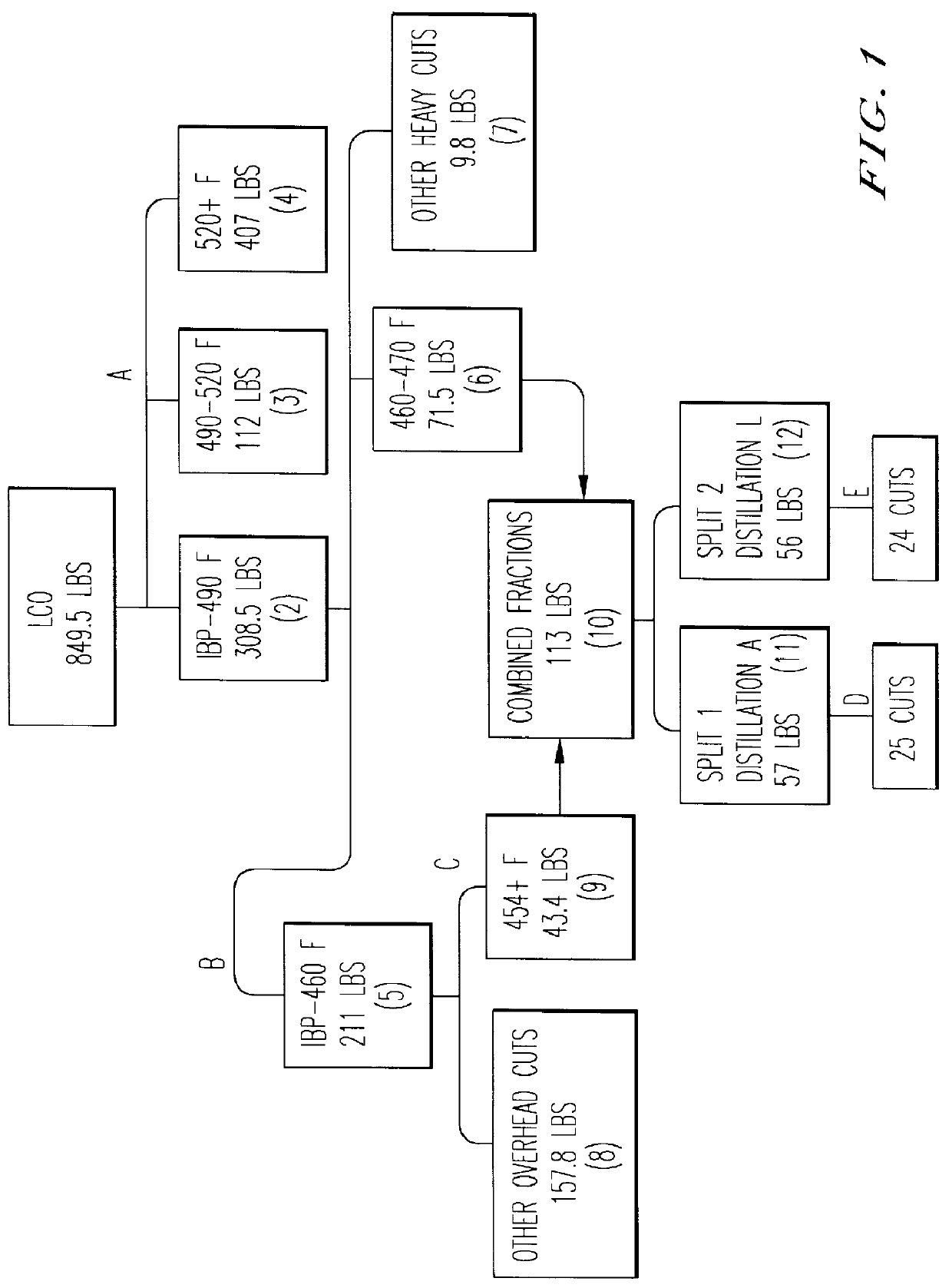
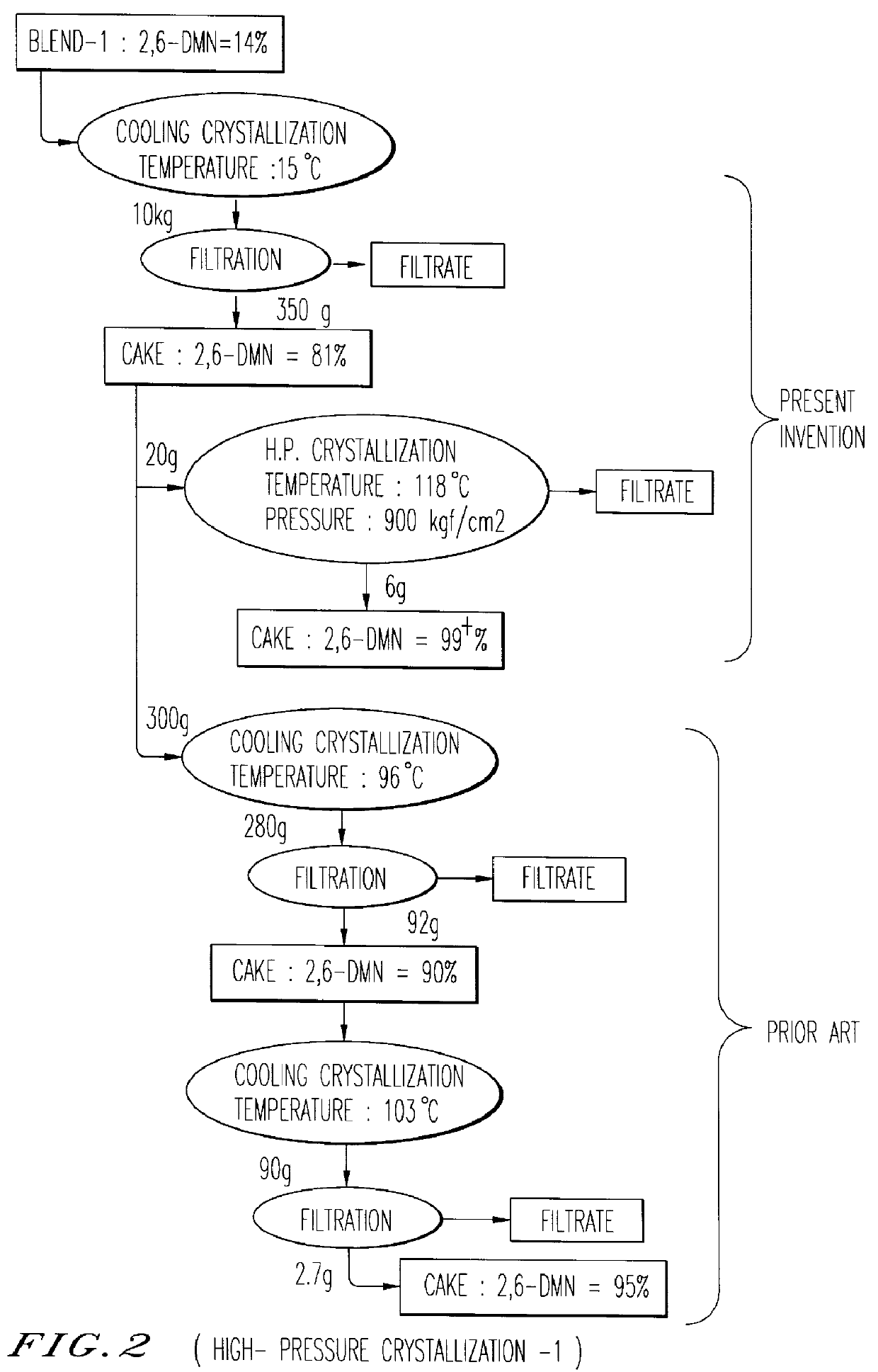
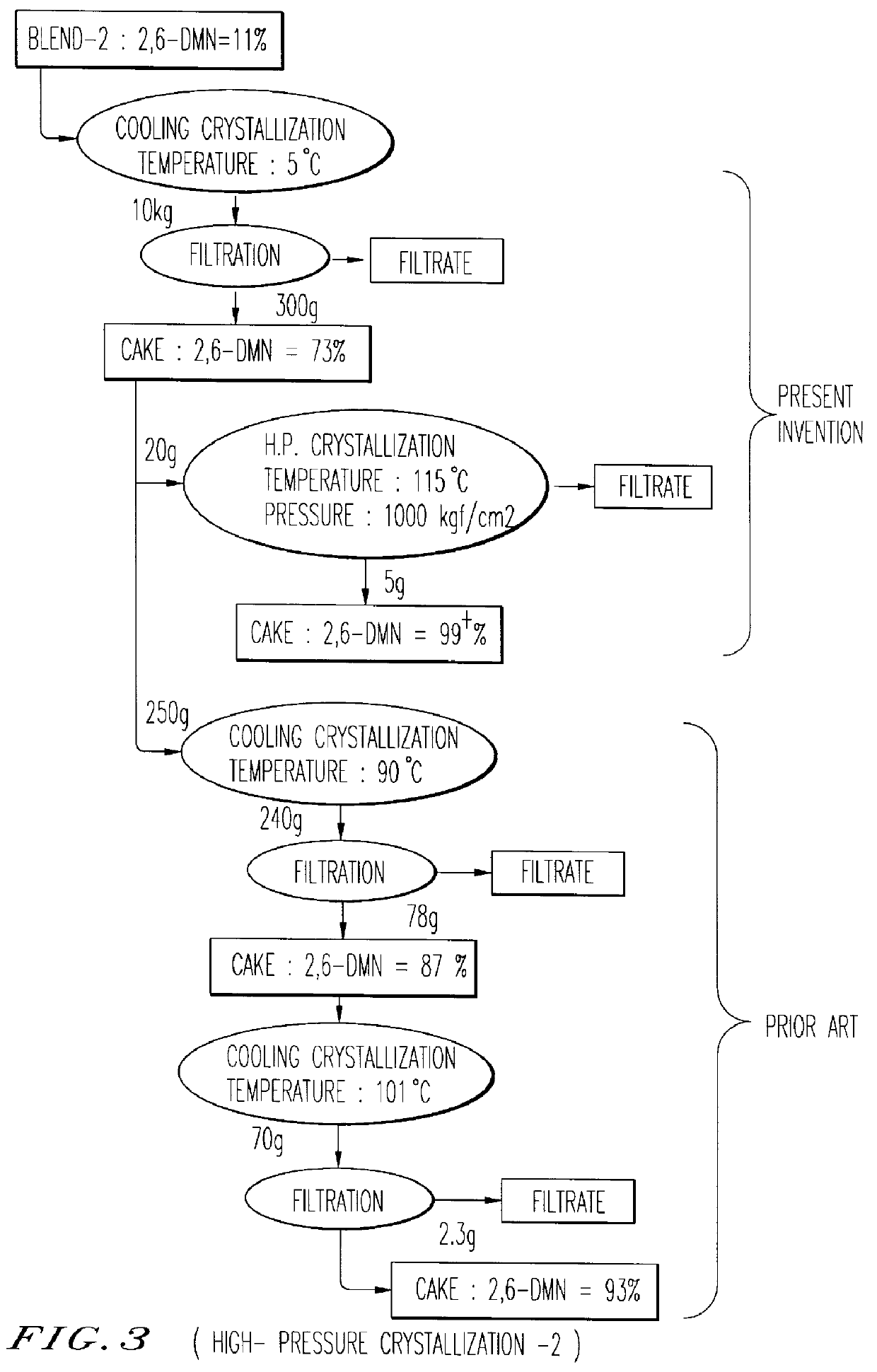
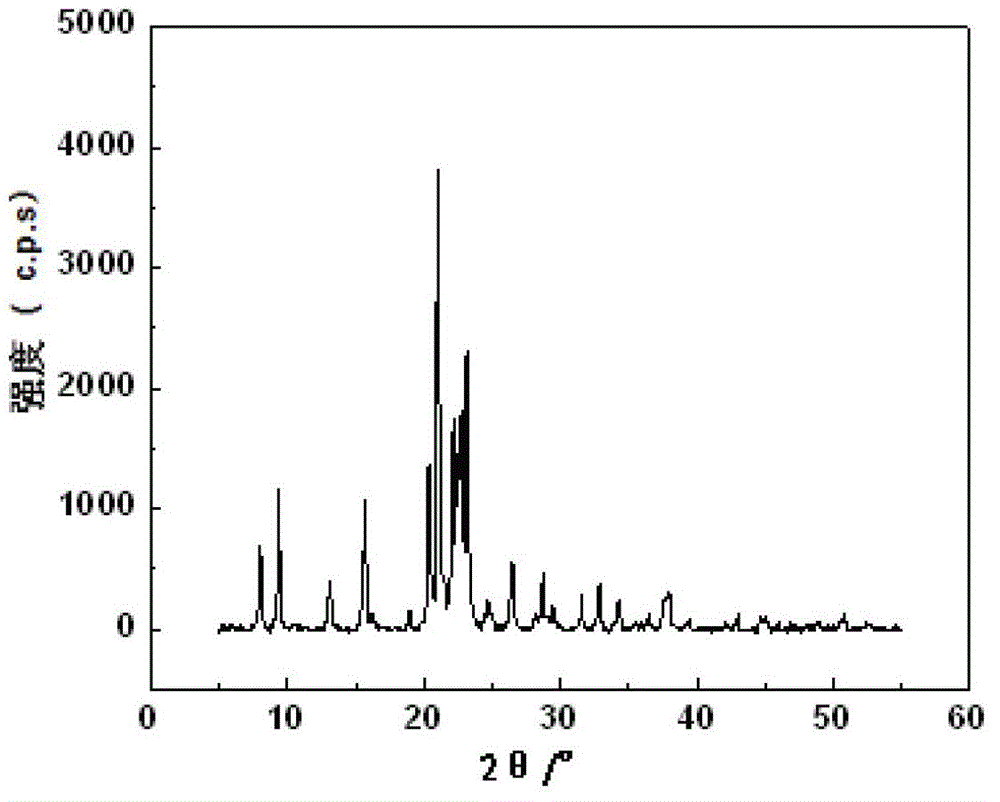
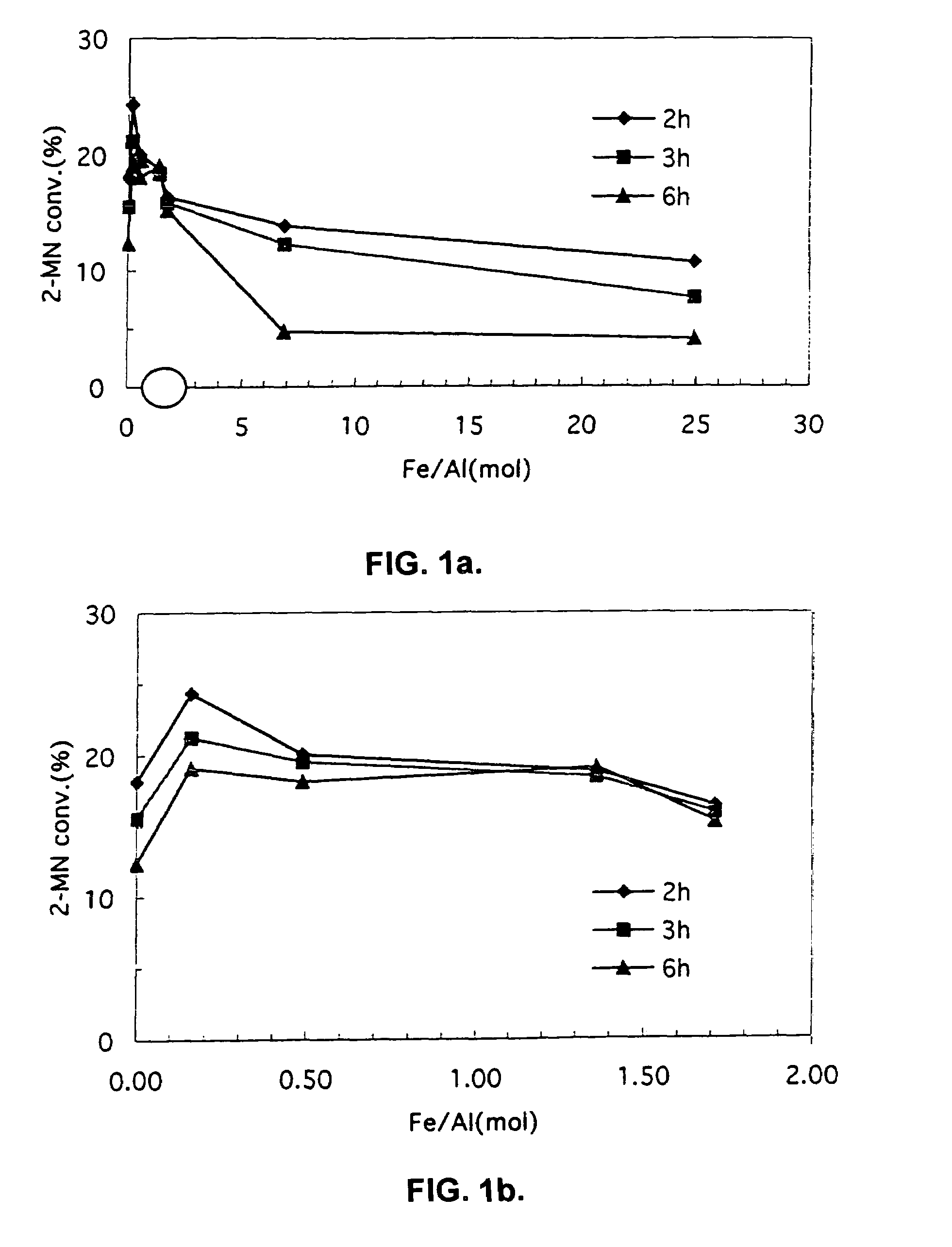
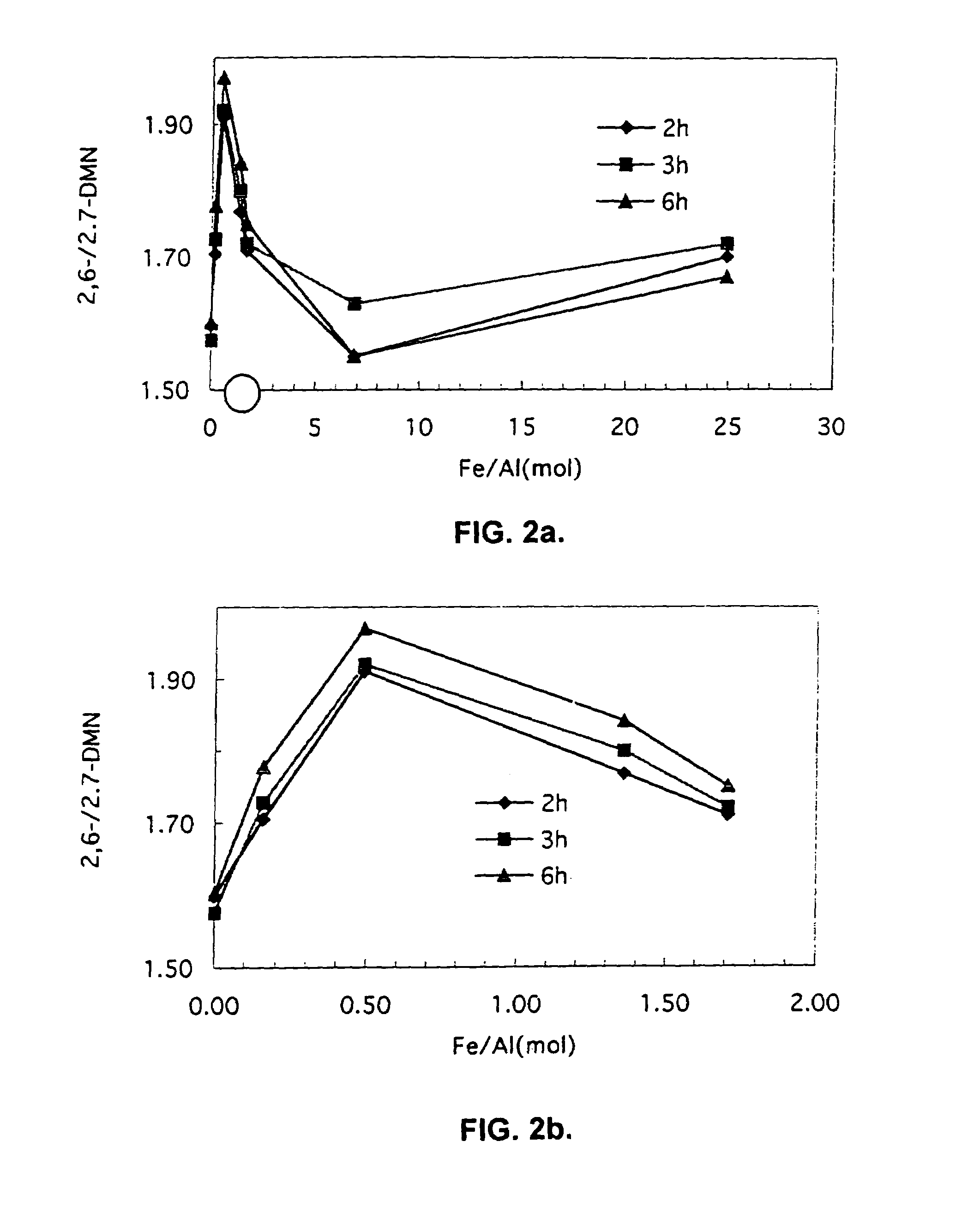
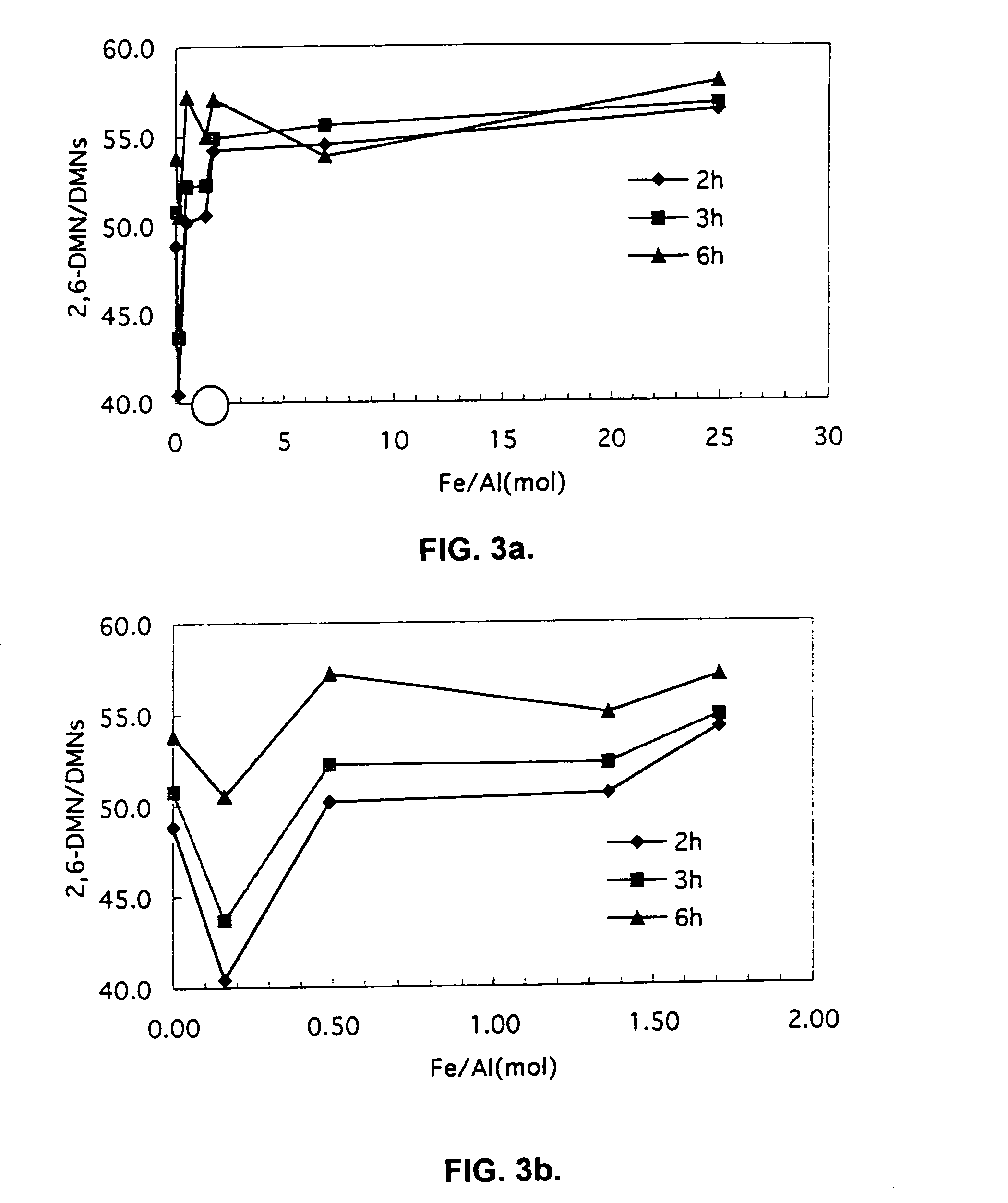
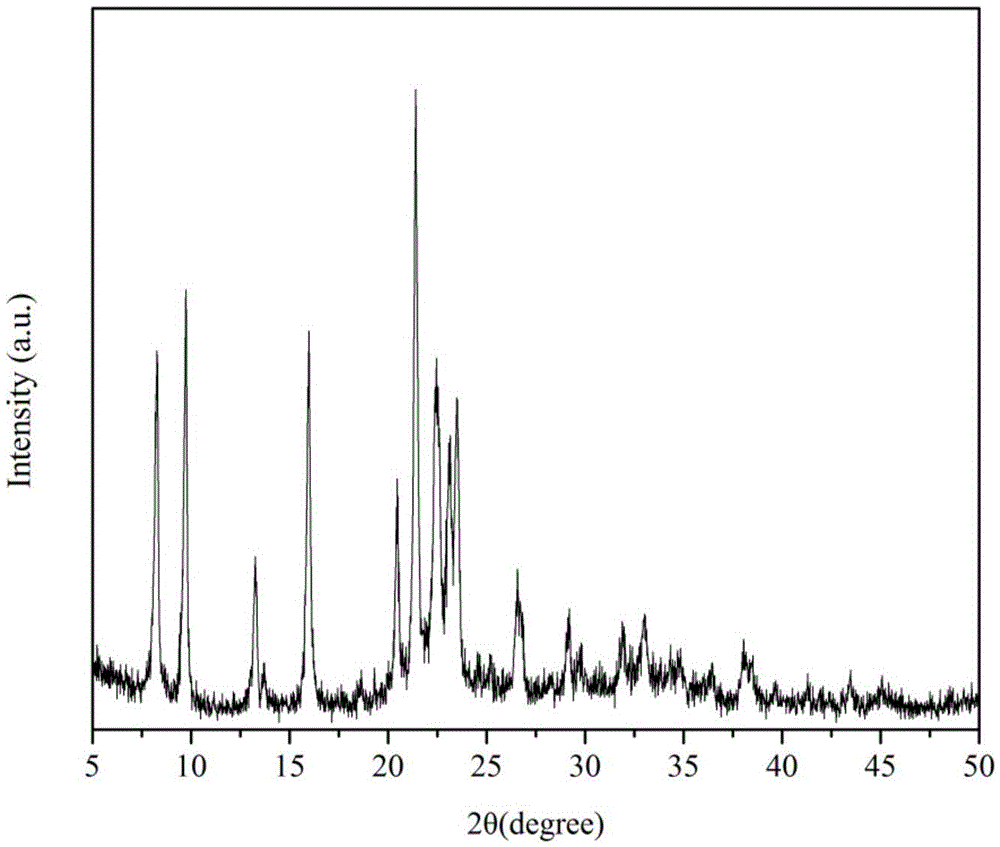
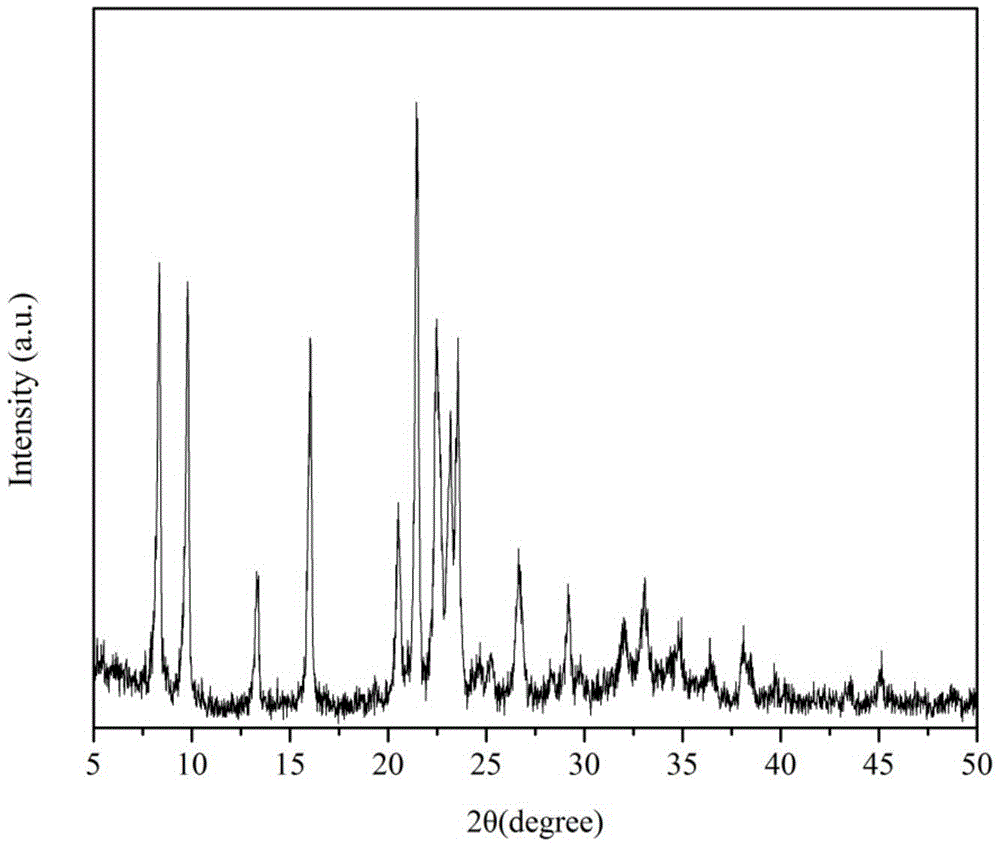
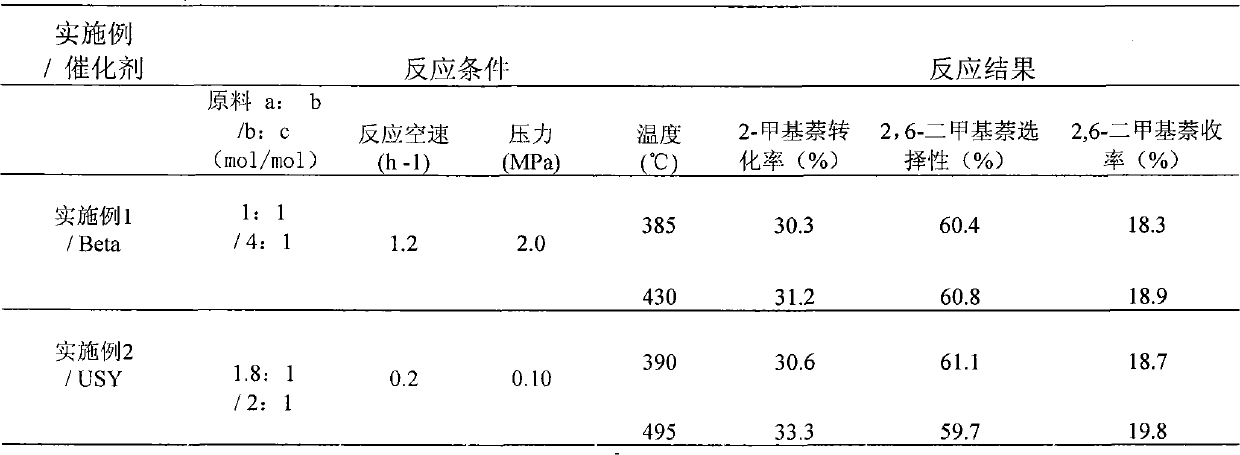
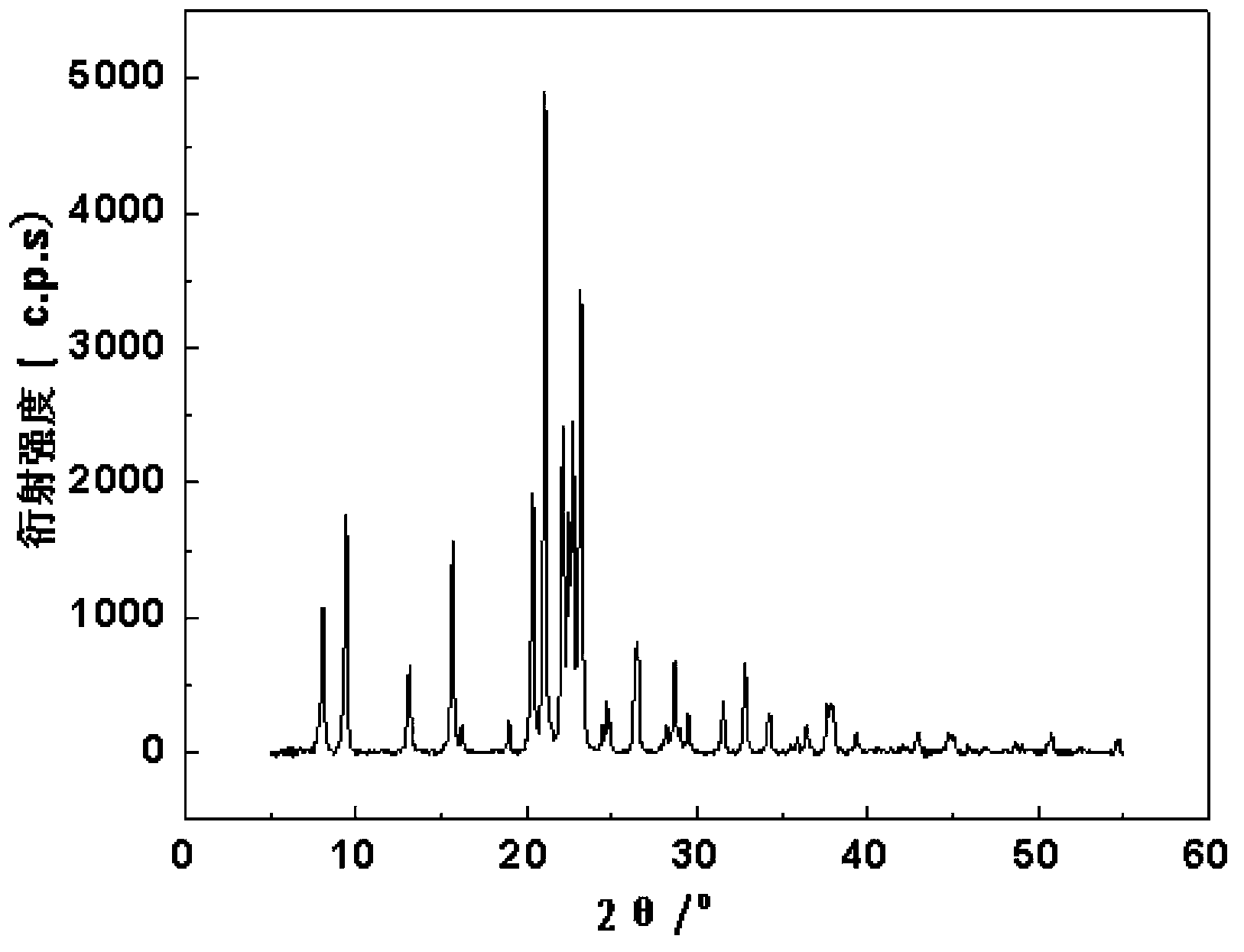
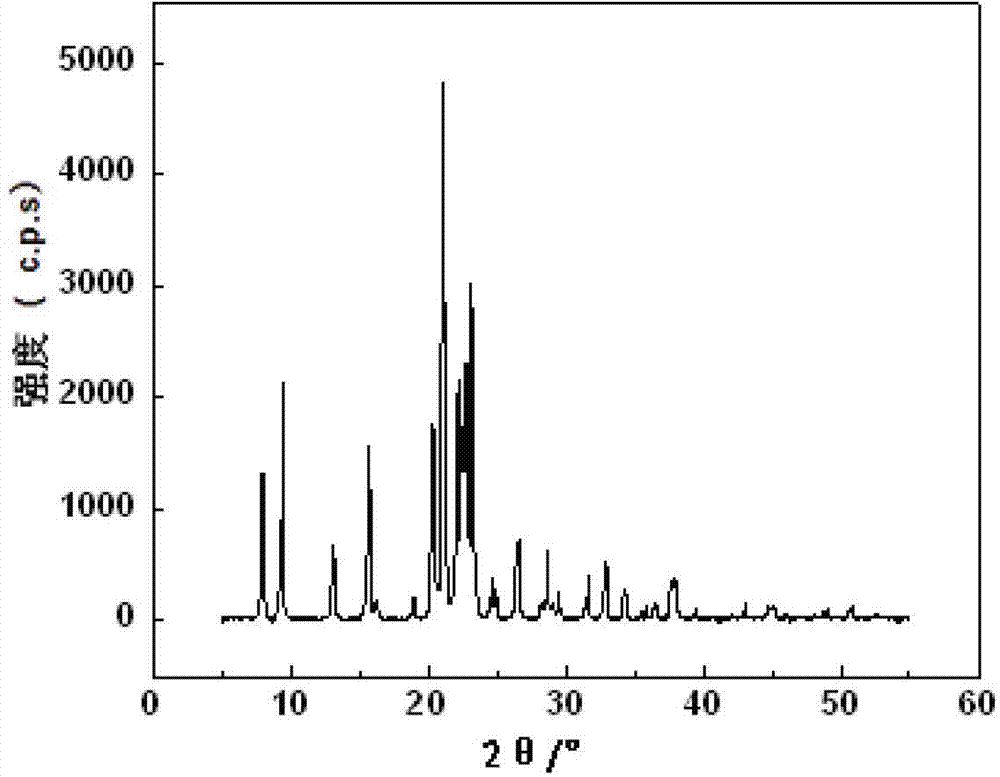
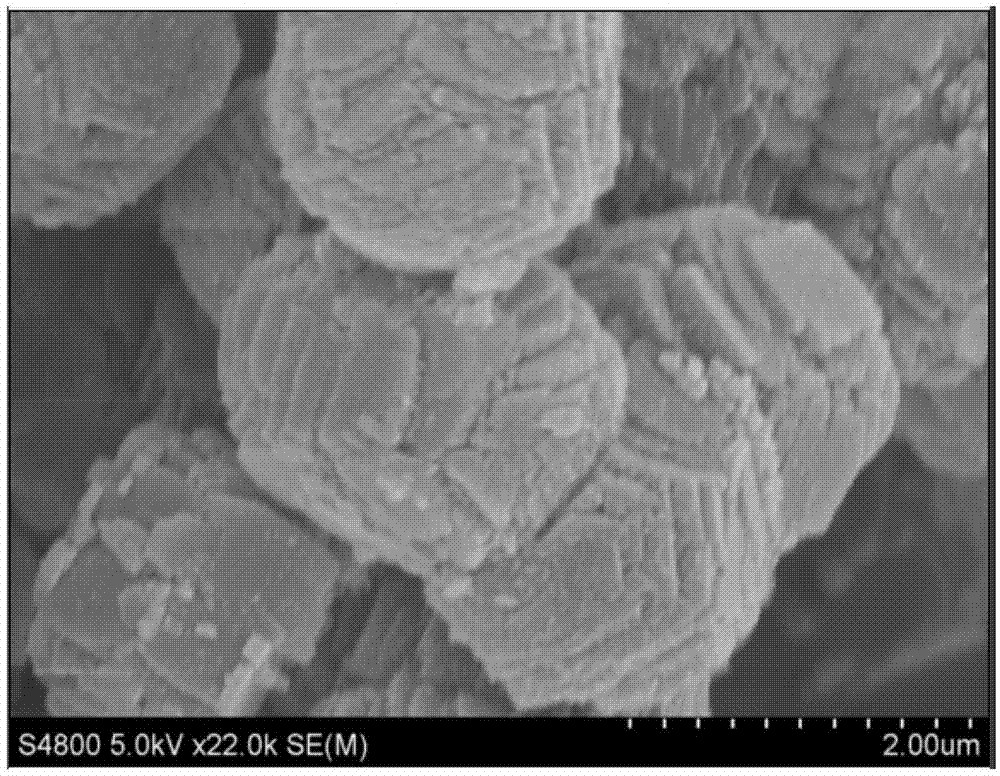
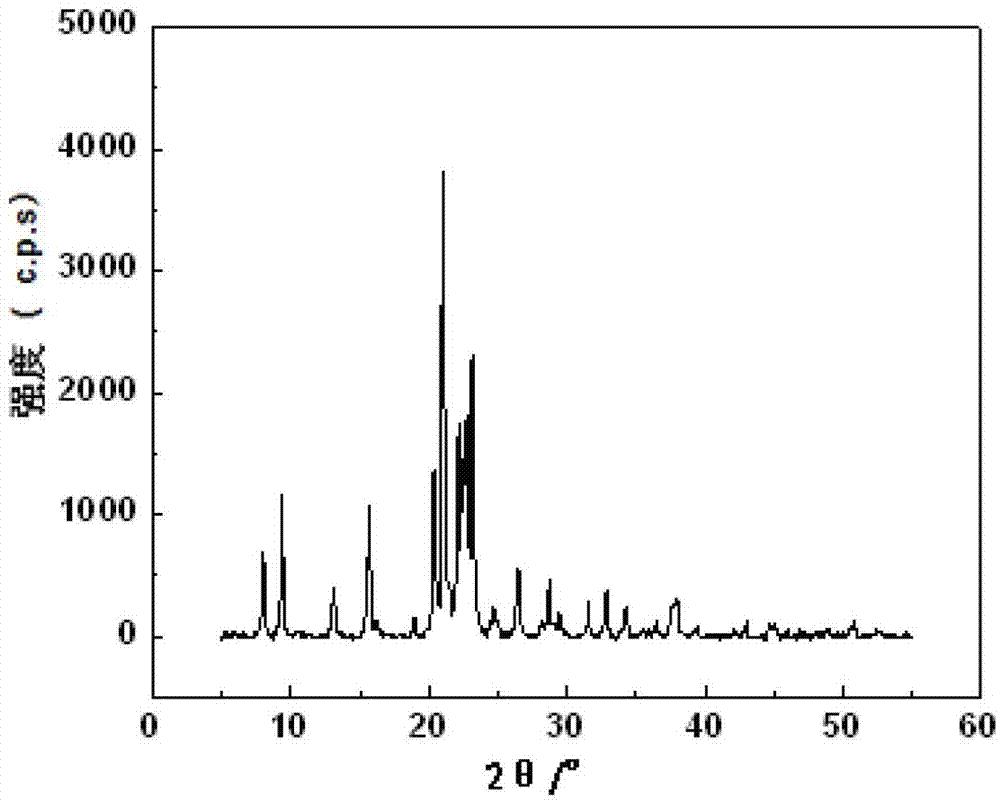
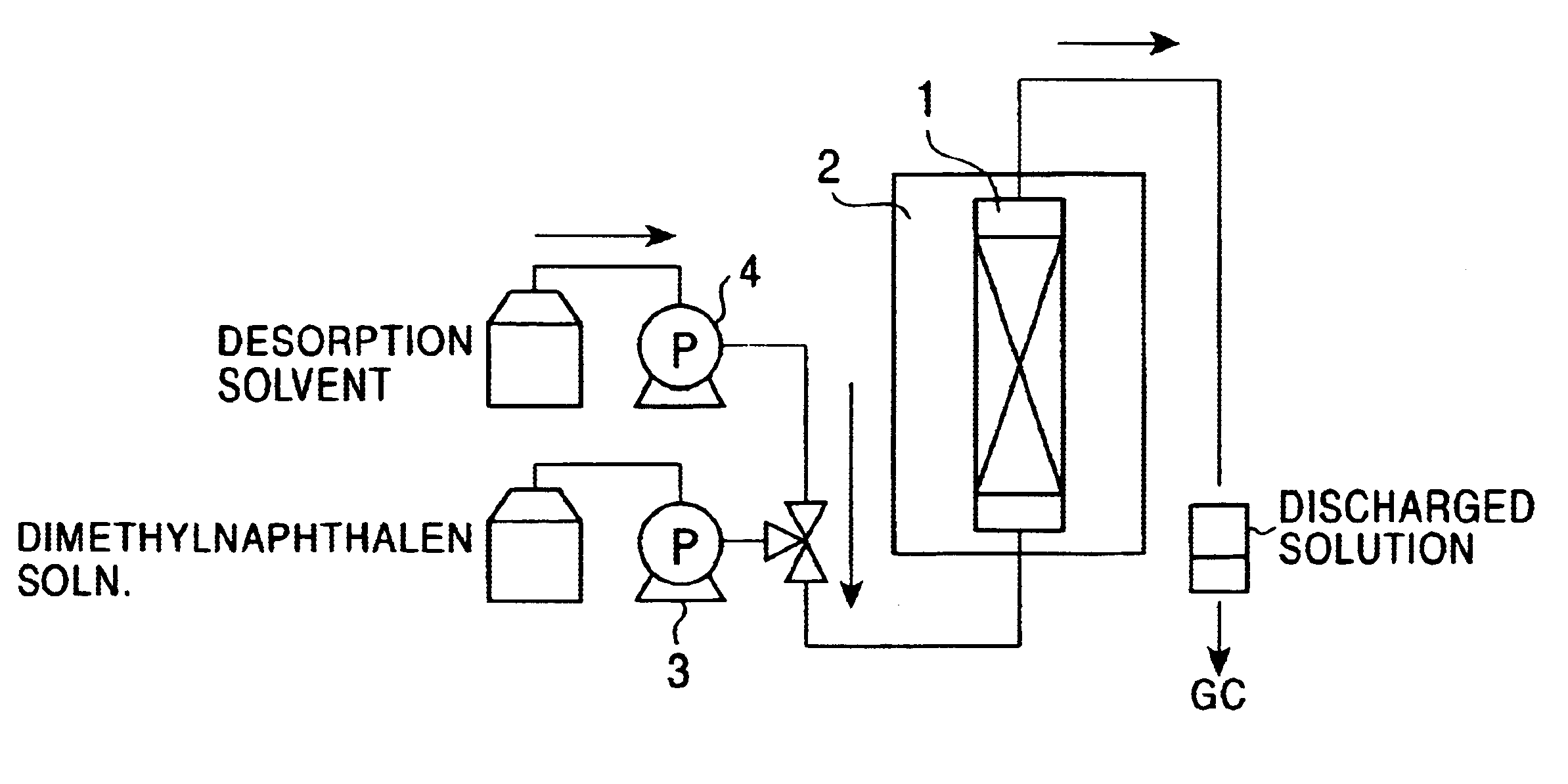
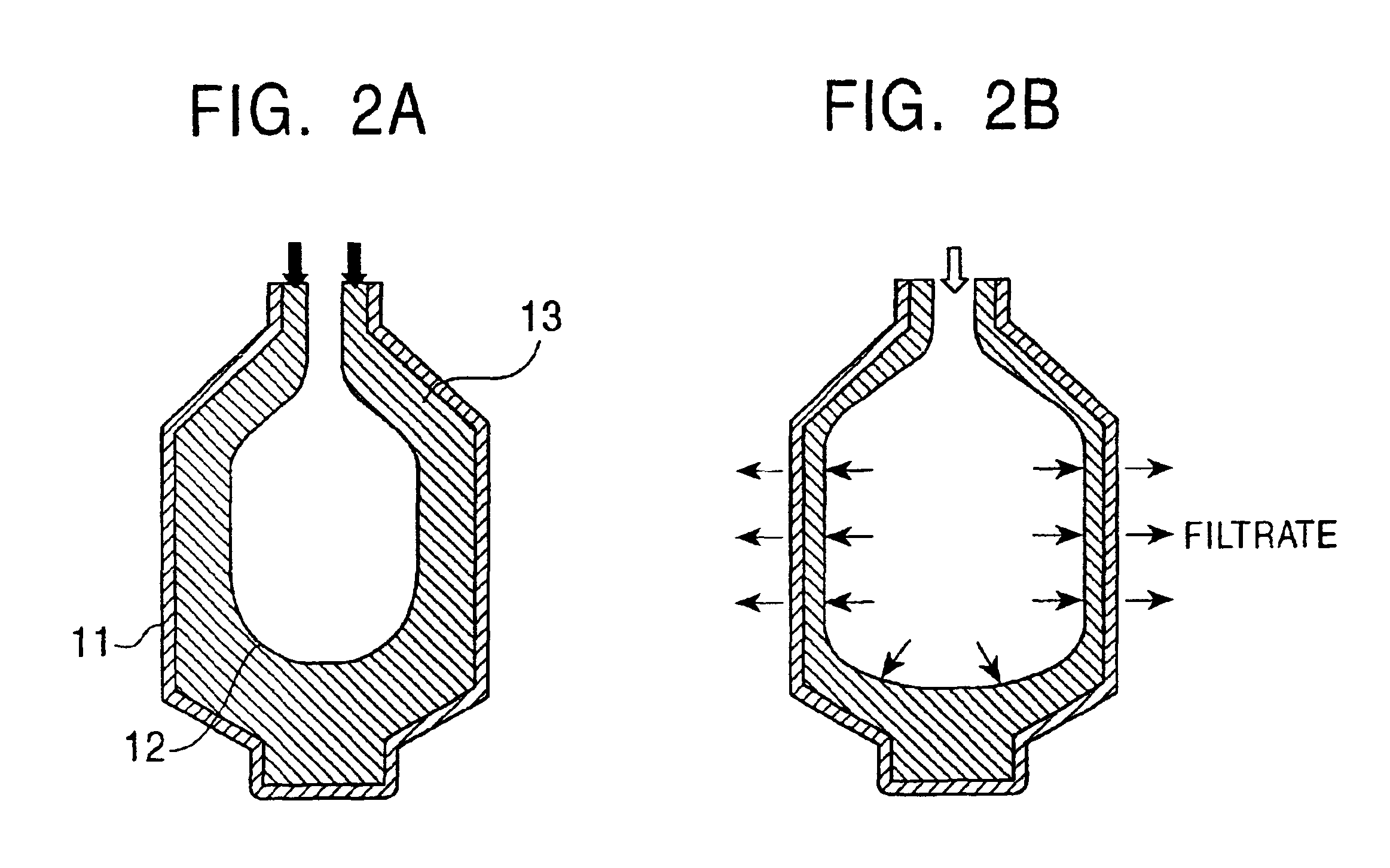
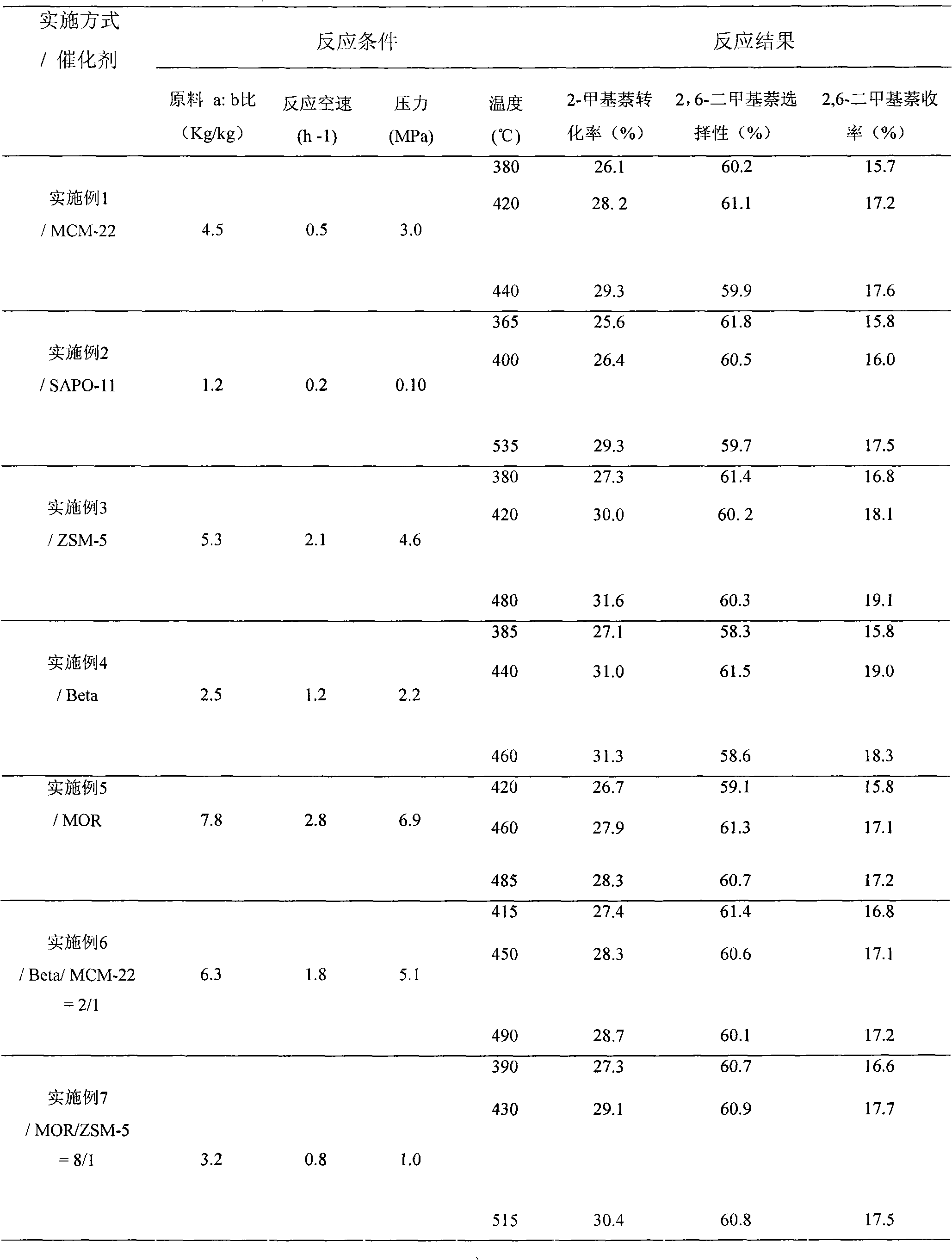
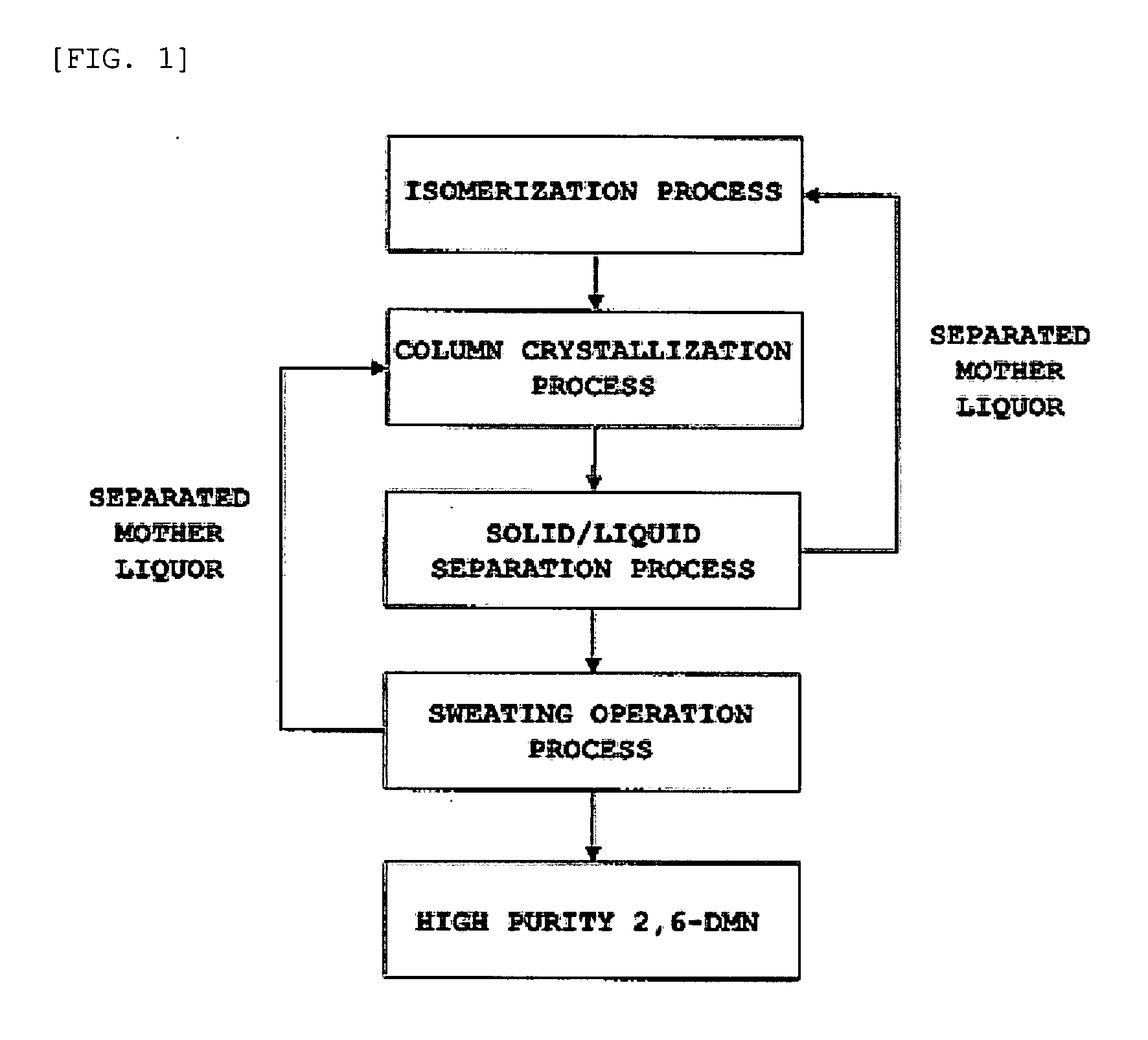
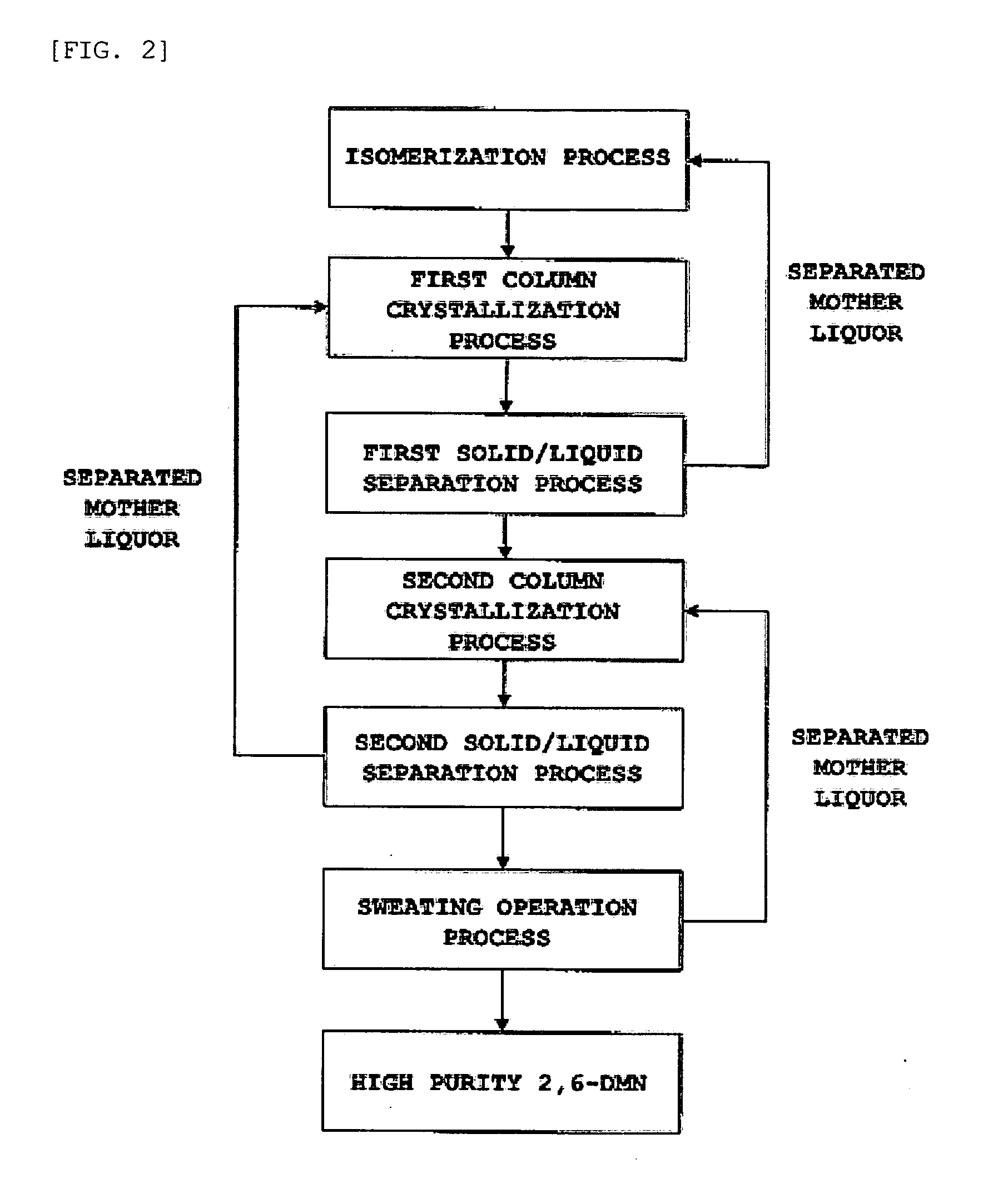
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "2,6-Dimethylnaphthalene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2,6-Dimethylnaphthalene (2,6-DMN) is a polycyclic aromatic hydrocarbon. It is one of the ten dimethylnaphthalene isomers, which are derived from naphthalene by the addition of two methyl groups. 2,6-DMN is of commercial importance as a starting material for high-performance polyester fibers and films. Polyethylene naphthalate (PEN) is made from the product of oxidation 2,6-DMN.
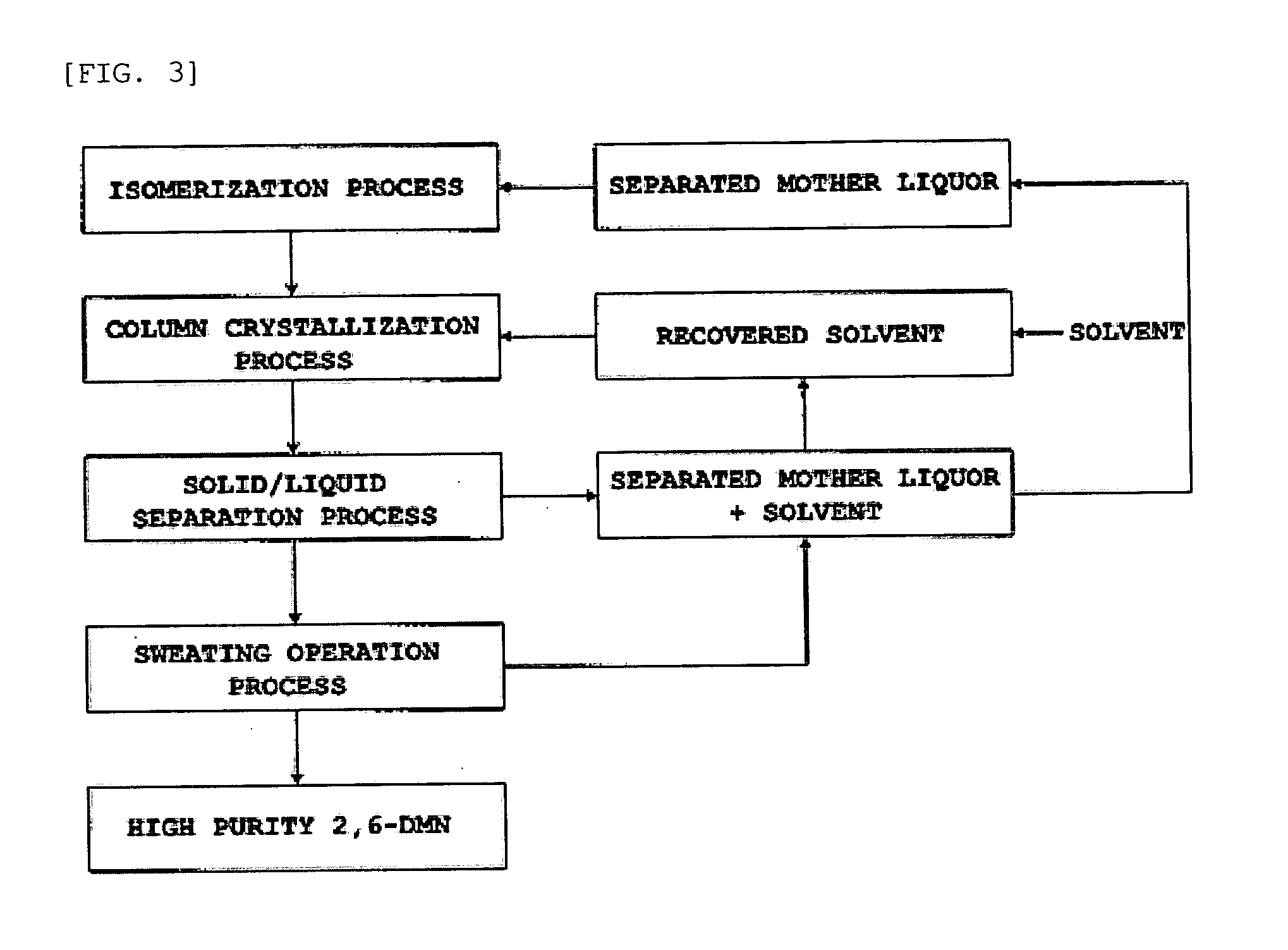
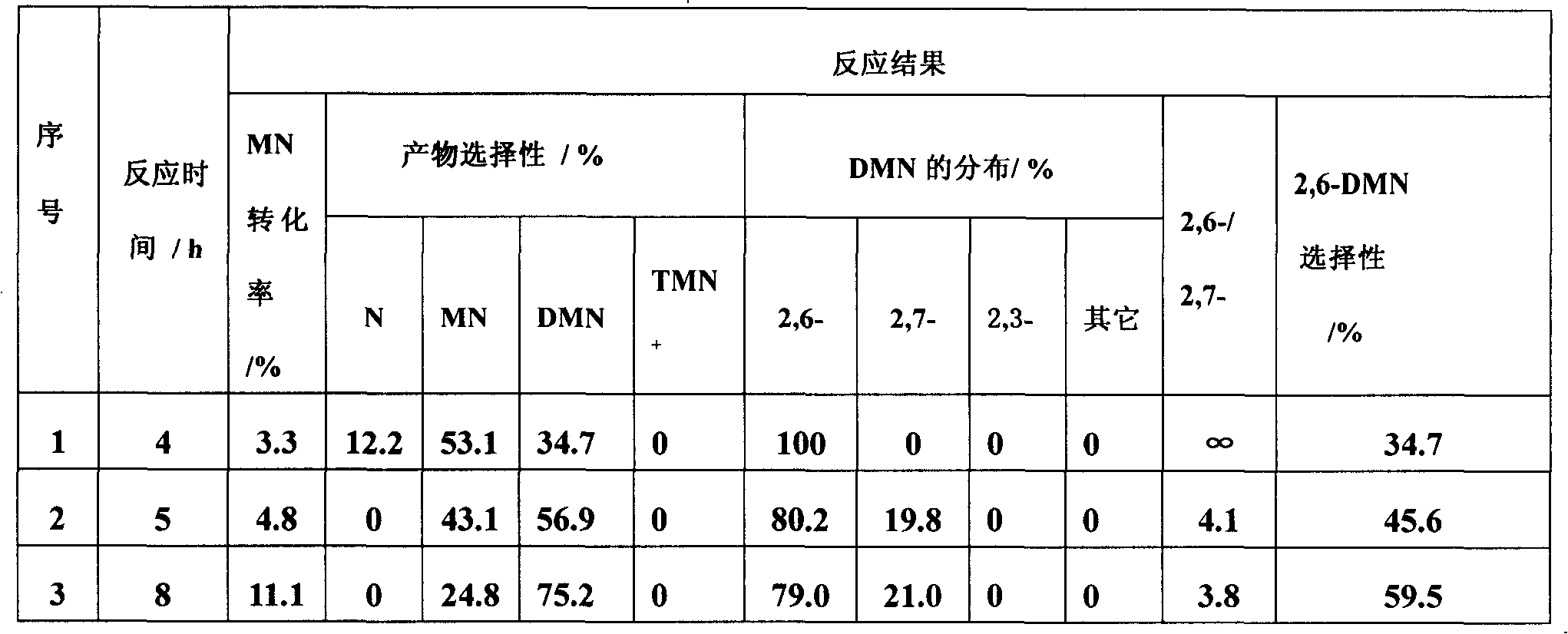
Isomerization of dimethylnaphthalene to produce 2,6-dimethylnaphthalene
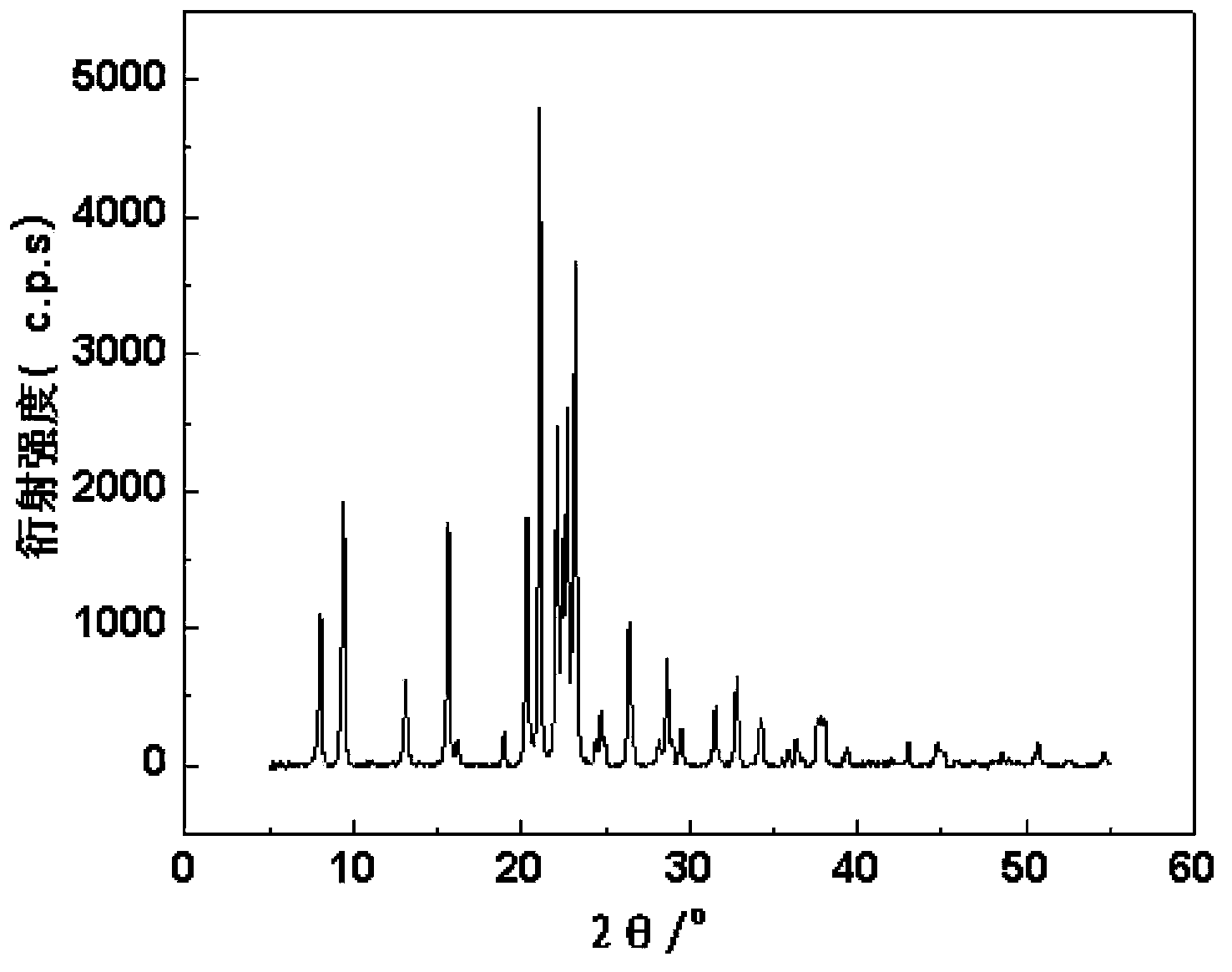
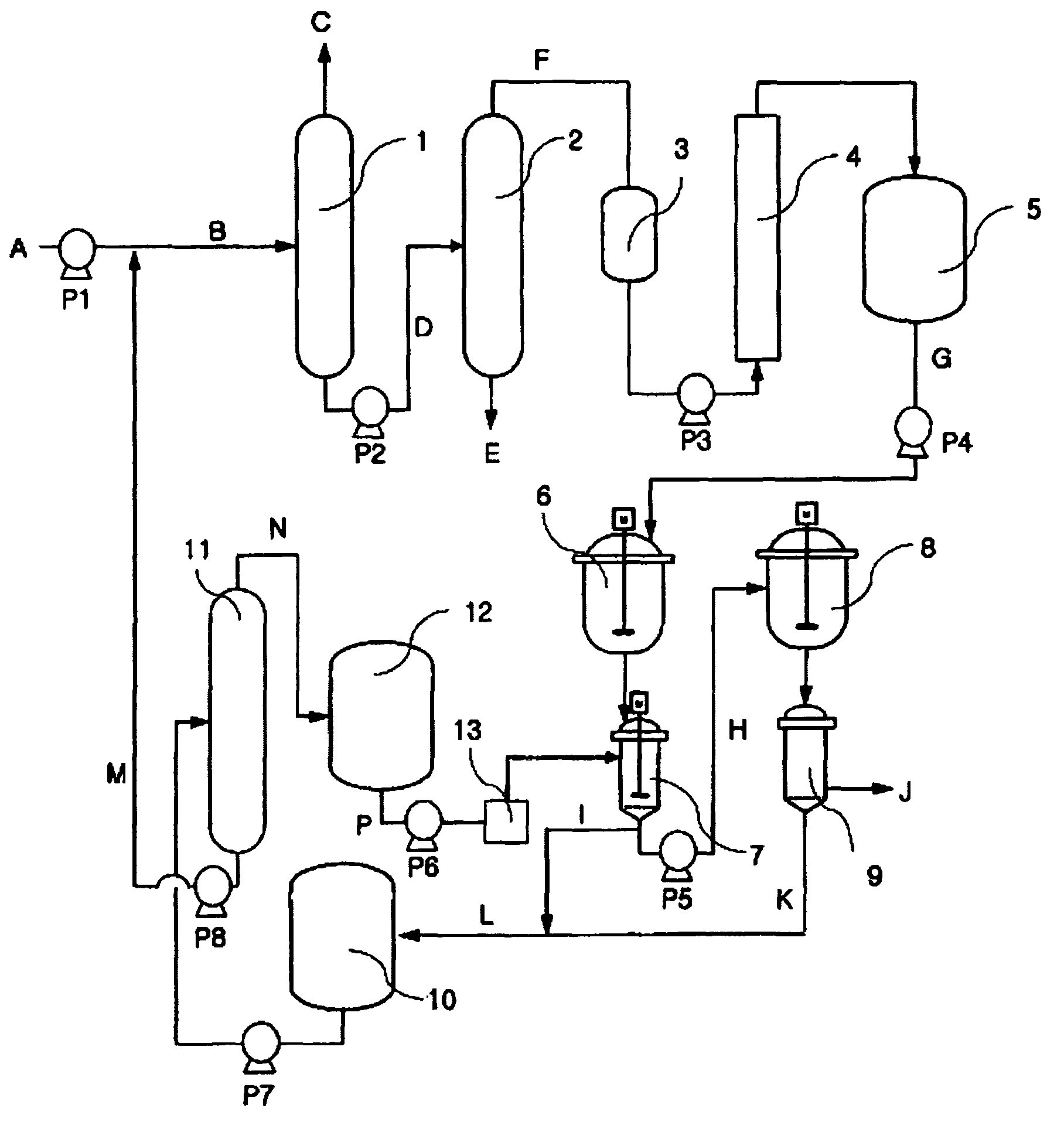
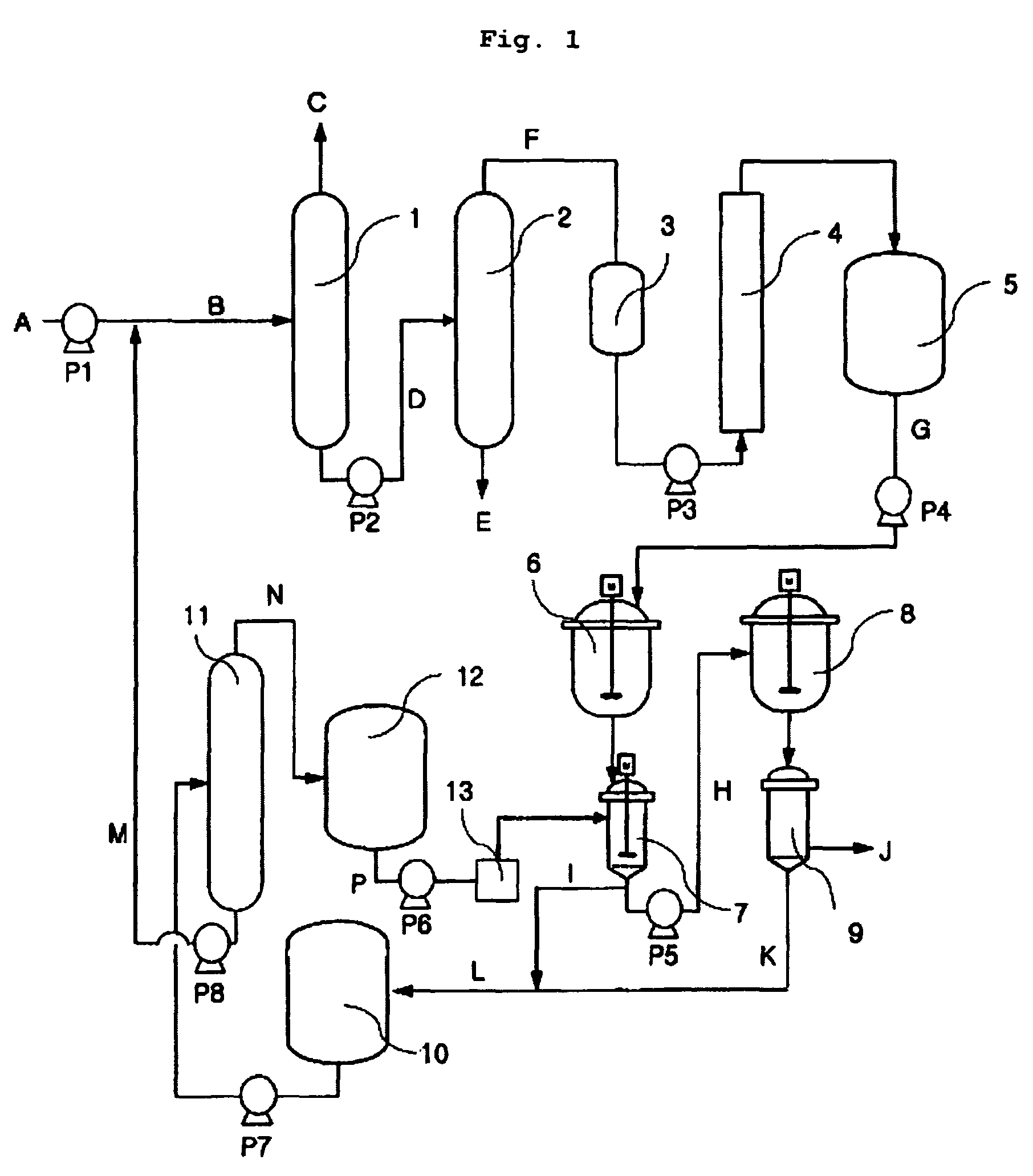
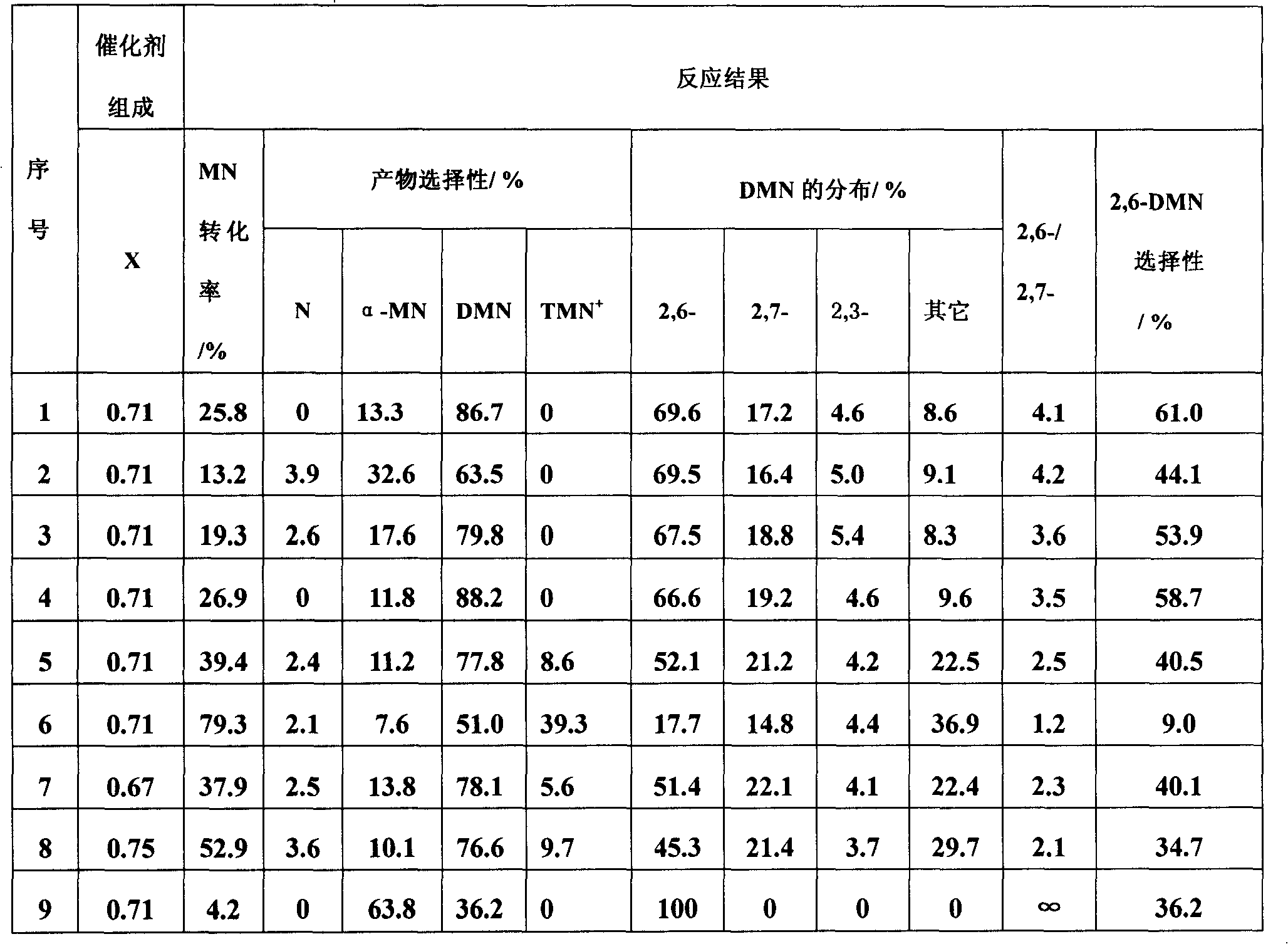
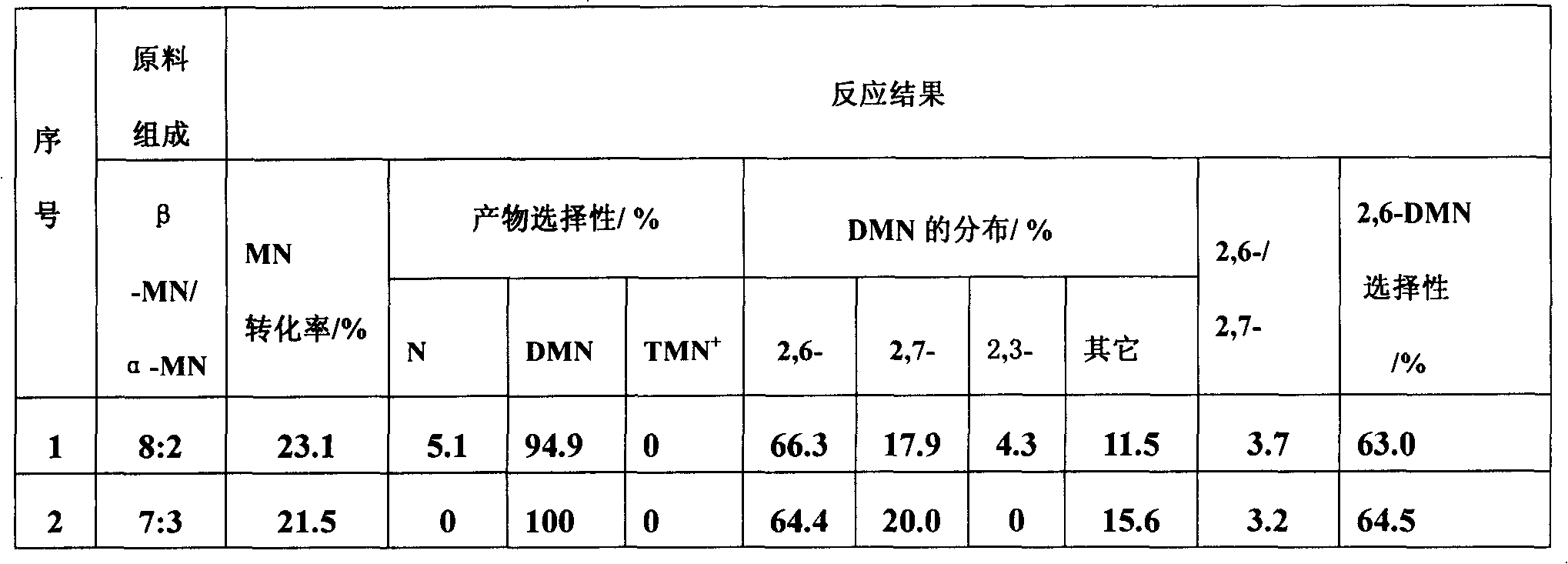
The present invention relates to a method of preparing 2,6-dimethylnaphthalene from a feed stream that contains hydrocarbons which contain dimethylnaphthalene isomers. The method includes the following steps: I. distillation and concentration of the dimethylnaphthalene isomers from the feed stream, to form a dimethylnaphthalene fraction, II. isomerization of the dimethylnaphthalene fraction to enrich the dimethylnaphthalene fraction in 2,6-dimethylnaphthalene, to form a 2,6-enriched dimethylnaphthalene fraction, III. purification of 2,6-dimethylnaphthalene from the 2,6-enriched dimethylnaphthalene fraction, wherein step II is conducted in the presence of a catalyst composition containing a synthetic zeolite characterized by an X-ray diffraction pattern including interplanar d-spacing ( ANGSTROM ) 12.36+ / -10.4 11.03+ / -0.2 8.83+ / -10.14 6.18+ / -0.12 6.00+ / -0.10 4.06+ / -0.07 3.91+ / -0.01 3.42+ / -0.06, wherein the purification includes crystallization under pressure.
Owner:KOBE STEEL LTD +1
Method for preparing 2,6-dimethylnaphthalene by catalyzing naphthalene alkylation reaction with CoAPO-11 molecular sieve
InactiveCN102746101AEasy to separateGood acid catalytic performanceMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sieveSolvent
The invention provides a method for preparing 2,6-dimethylnaphthalene by catalyzing naphthalene alkylation reaction with CoAPO-11 molecular sieve, relates to a method for preparing 2,6-dimethylnaphthalene, and is used for solving the problems that the traditional catalyst cannot simultaneously have high activity and high selectivity of 2,6-dimethylnaphthalene. The method comprises the steps of: activating the CoAPO-11 molecular sieve synthesized by an electrical heating method or microwave heating method for 2h, then mixing with naphthalene, alkylation reagents and solvent to prepare raw material liquid, and carrying out alkylation reaction to obtain 2,6-dimethylnaphthalene. According to the invention, the synthesized CoAPO-11 molecular sieve has higher catalytic reaction activity on naphthalene alkylation reaction, and higher selectivity of 2,6-dimethylnaphthalene; and the method is suitable for preparing 2,6-dimethylnaphthalene.
Owner:HEILONGJIANG UNIV
Method for preparing 2,6-dimethyl naphthalene by alkylation reaction of MgAPO-11 molecular sieve catalytic naphthalene
InactiveCN103265396AEasy to separateEasy to operateMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationAlkyl transferMolecular sieve
The invention discloses a method for preparing 2,6-dimethyl naphthalene by alkylation reaction of MgAPO-11 molecular sieve catalytic naphthalene. The method comprises the following steps of: I, activating an MgAPO-11 molecular sieve; and II, mixing naphthalene with an alkylation reagent and a solvent to prepare a feed solution, and carrying out alkylation reaction on the feed solution and the activated MgAPO-11 molecular sieve catalytic naphthalene to obtain the 2,6-dimethyl naphthalene. According to the method for preparing 2,6-dimethyl naphthalene by alkylation reaction of MgAPO-11 molecular sieve catalytic naphthalene, the used MgAPO-11 molecular sieve is synthesized by adopting a conventional electric heating method or a microwave heating method; the alkylation reaction of the MgAPO-11 molecular sieve catalytic naphthalene can be used for overcoming the defects that the homogeneous catalysts including anhydrous AlCl3 and the like are difficult to separate from the product, severe in device corrosion and severe in environment pollution, so that the reaction activity and 2,6-DMN (Dimethylnitrosamine) selectivity are higher, the 2,6- / 2,7-DMN ratio and anti-carbon deposition capacity are higher. Moreover, the reaction products and the catalysts are easy to separate. Besides, the method is simple to operate and convenient for large-scale production.
Owner:HEILONGJIANG UNIV
Preparation method of 2,6-DiMethylnaphthalene (DMN) by using SAPO-11 molecular sieve
InactiveCN102746102AEasy to separateEasy to operateMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationAlkyl transferMolecular sieve
The invention relates to a preparation method of the 2,6-DMN, in particular to a preparation method of the 2,6-DMN by using a SAPO-11 molecular sieve. The preparation method mainly solves the problems that catalysts cannot be provided with high activity and 2,6-DMN selectivity simultaneously, and the catalysts are easy to inactivate. The method includes activating the SAPO-11 molecular sieve synthesized by microwave radiation and heating, mixing naphthalene and alkylation reagents with a solvent according to a molar ratio of 1:(2-4):(6-12), and performing alkylation reaction to obtain the 2,6-DMN at a temperature of 350 DEG C to 450 DEG C, at a pressure of 2 MPa to 5 MPa, at a quality airspeed of 0.5-2h-1 and at a carrier gas flow rate of 20-60 mL / min. According to the preparation method, the synthesized SAPO-11 molecular sieve can shorten the crystallization time greatly, and has high catalytic activity to the alkylation reaction of the naphthalene and high selectivity and good anti-carbon deposition competence to the 2,6-DMN.
Owner:HEILONGJIANG UNIV
Selective methylation catalyst, method of catalyst manufacture and methylation process
InactiveUS7022637B2High selectivityIncrease conversionsMolecular sieve catalystsMolecular sieve catalystHalogenFluoride
Novel catalysts and processes in accordance with the invention can accomplish high selectivity and conversion of naphthalenic compounds such as the conversion of methylnaphthalene (2-MN) or naphthalene to 2,6-dimethylnaphthalene (2,6-DMN). The catalysts are prepared by treating, for example, a ZSM-5-type material with iron in the presence of a halogen such as a fluoride. The resulting catalyst includes iron, as well as a significant portion of aluminum present in the ZSM-5-type starting material. Processes for using the catalysts also are disclosed.
Owner:PENN STATE RES FOUND
Method for using CuSAPO-11 molecular sieve for preparation of 2,6-dimethylnaphthalene
InactiveCN105566052AEasy to separateEasy to operateMolecular sieve catalystsMolecular sieve catalystMolecular sieveAlkyl transfer
The present invention belongs to the technical field of preparation of 2,6-dimethylnaphthalene, and particularly relates to a method for using CuSAPO-11 molecular sieve for preparation of 2,6-dimethylnaphthalene. The method solves the technical problems that high activity and high 2,6-DMN selectivity of a molecular sieve catalyst used in 2,6-dimethylnaphthalene preparation methods in the prior art are difficult to achieved at the same time and stability of the catalyst is poor. The catalyst used in the method is the silicon phosphate copper and aluminum molecular sieve CuSAPO-11, a copper salt is added into a process for synthesis of silicon phosphate aluminum molecular sieve SAPO-11 by use a hydrothermal synthetic method for further synthesis of the new type silicon phosphate copper and aluminum molecular sieve CuSAPO-11. The molecular sieve catalyst has suitable acid property, high specific surface area and large mesoporous pore size, so that the molecular sieve catalyst shows higher catalytic activity in the alkylation reaction of naphthalene, the molecular sieve catalyst has high 2,6-dimethylnaphthalene selectivity, and the ratio of 2,6-dimethylnaphthalene to 2,7-dimethylnaphthalene is high.
Owner:TAIYUAN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for catalytically preparing 2,6-dimethylnaphthalene
ActiveCN108341734AHigh acid densityImprove reaction stabilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalystsMesitylene2,6-Dimethylnaphthalene
The invention discloses a method for catalytically preparing 2,6-dimethylnaphthalene through using magnetic ionic liquid. The method comprises the following steps of adding a mixed solution of methylnaphthalene and mesitylene in an ultrasonic reaction kettle provided with a stirrer, stirring to react for 35-45min in inert atmosphere, then adding a magnetic ionic liquid catalyst, reacting for 15-80min under the condition that temperature is 25-40 DEG C, the inert atmosphere exists, the stirring speed is 400-600r / min and the power of ultrasonic wave is 150-300W, and standing for 30min after completing reaction; a lower-layer catalyst can be directly recycled after subjected to magnetic filed separation; the mixed solution with an upper layer containing the 2,6-dimethylnaphthalene is furtherseparated and purified to obtain a 2,6-dimethylnaphthalene product and incompletely reacted raw materials. The method has the advantages of high catalytic activity, precise process, high reaction transformation rate, high 2,6-dimethylnaphthalene yield and the like.
Owner:广东和汇新材料有限公司
Method for synthetizing 2,6-dimethylnaphthalene with methanol, C10 arene and 2-methylnaphthalene through alkylation
InactiveCN102001907AReduce the introductionRich sourcesMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sieveAlkyl transfer
The invention relates to a method for synthetizing 2,6-dimethylnaphthalene with methanol, C10 arene and 2-methylnaphthalene through alkylation. The method uses the mixture of 2-methylnaphthalene, methanol and C10 arene which is C10 aromatic mixture mainly containing tetramethylbenzene and is a byproduct obtained from the aromatic device, as raw material to prepare 2,6-dimethylnaphthalene through alkylation in the existence of a molecular sieve catalyst. In particular, methanol is used as the raw material a, C10 arene is used as the raw material b and 2-methylnaphthalene is used as the raw material c; the raw materials are used to prepare the raw material mixture, wherein the ratio of a to c is 1-3:1(mol / mol) and the ratio of b to c is 1-6:1(mol / mol); and 2,6-dimethylnaphthalene is synthetized through alkylation in the existence of the molecular sieve catalyst under the conditions that the reaction temperature is 380-500 DEG C, the reaction pressure is 0-6.0MPa and the reaction air speed is 0.1-2h<-1>. By adopting the method, the C10 arene resource which is the byproduct with low value can be utilized, methanol with low price and rich source is introduced in the reactant and the reaction activity, selectivity and reaction stability can be effectively increased.
Owner:TONGJI UNIV
Process for the production of 2,6-dimethylnaphthalene from petrochemical streams
InactiveUS6388158B1Long catalyst lifeProlong lifeHydrocarbon by isomerisationMolecular sieve catalystsFractionationSolid acid
Process for the preparation of 2,6-dimethylnaphthalene comprising reacting with at least one aromatic hydrocarbon, in the presence of a zeolitic catalyst, a mixture of naphthalenes comprising a cut obtained by the fractionation of suitable petrochemical streams and subsequent treatment of the product thus obtained with a solid acid.
Owner:ENICHEM S P A 62 +1
Catalyst for compounding 2, 6-dimethylnaphthalene and preparing method thereof
ActiveCN102513146AMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sieveAmmonium bromide
The invention discloses a catalyst for compounding 2, 6-dimethylnaphthalene and a preparing method thereof. The weight ratio of main components of the catalyst is: Na 2O 0.25-0.5 parts, SiO 2 1.0 parts, tetrapropyl ammonium bromide (TPABr) 0.05-0.2 parts, Al2O3 0.01-0.03 parts, Fe2O3 0.01-0.03 parts, H2PO4- 0.03-0.06 parts and carbon aerogel 22-45 parts. The catalyst for compounding the 2, 6-dimethylnaphthalene and the preparing method of the catalyst are used in an alkylation reaction of 2-methylnaphthalene and methanol, the selectivity and yield of the 2, 6-dimethylnaphthalene respectively reach 50% and 17% and are obviously better than those of a common molecular sieve catalyst.
Owner:KAILUAN ENERGY CHEM
Method for preparing 2,6-dimethyl naphthalene by alkylation reaction of MgAPO-11 molecular sieve catalytic naphthalene
InactiveCN103265396BEasy to separateEasy to operateMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationAlkyl transferMolecular sieve
Owner:HEILONGJIANG UNIV
Process for producing 2,6-dimethylnaphthalene by dehydrocyclizing 1-(p-tolyl)-2-methylbutane and/or 1-(p-tolyl)-2-methylbutene using a reduced vanadium catalyst
PCT No. PCT / FI97 / 00095 Sec. 371 Date Sep. 15, 1998 Sec. 102(e) Date Sep. 15, 1998 PCT Filed Feb. 14, 1997 PCT Pub. No. WO97 / 30012 PCT Pub. Date Aug. 21, 1997The invention concerns a preparation process for 2,6-dimethylnaphthalene in which 1-(p-tolyl)-2-methylbutane and / or 1-(p-tolyl)-2-methylbutene are dehydrocyclisized using a reduced vanadium catalyst. 2,6-dimethylnaphthalene can be used as starting material when manufacturing polyethylenenaphthalate. The conversion of this method is good and the selectivity of 2,6-dimethylnaphthalene can be improved by reducing the catalyst.
Owner:OPTATECH CORP
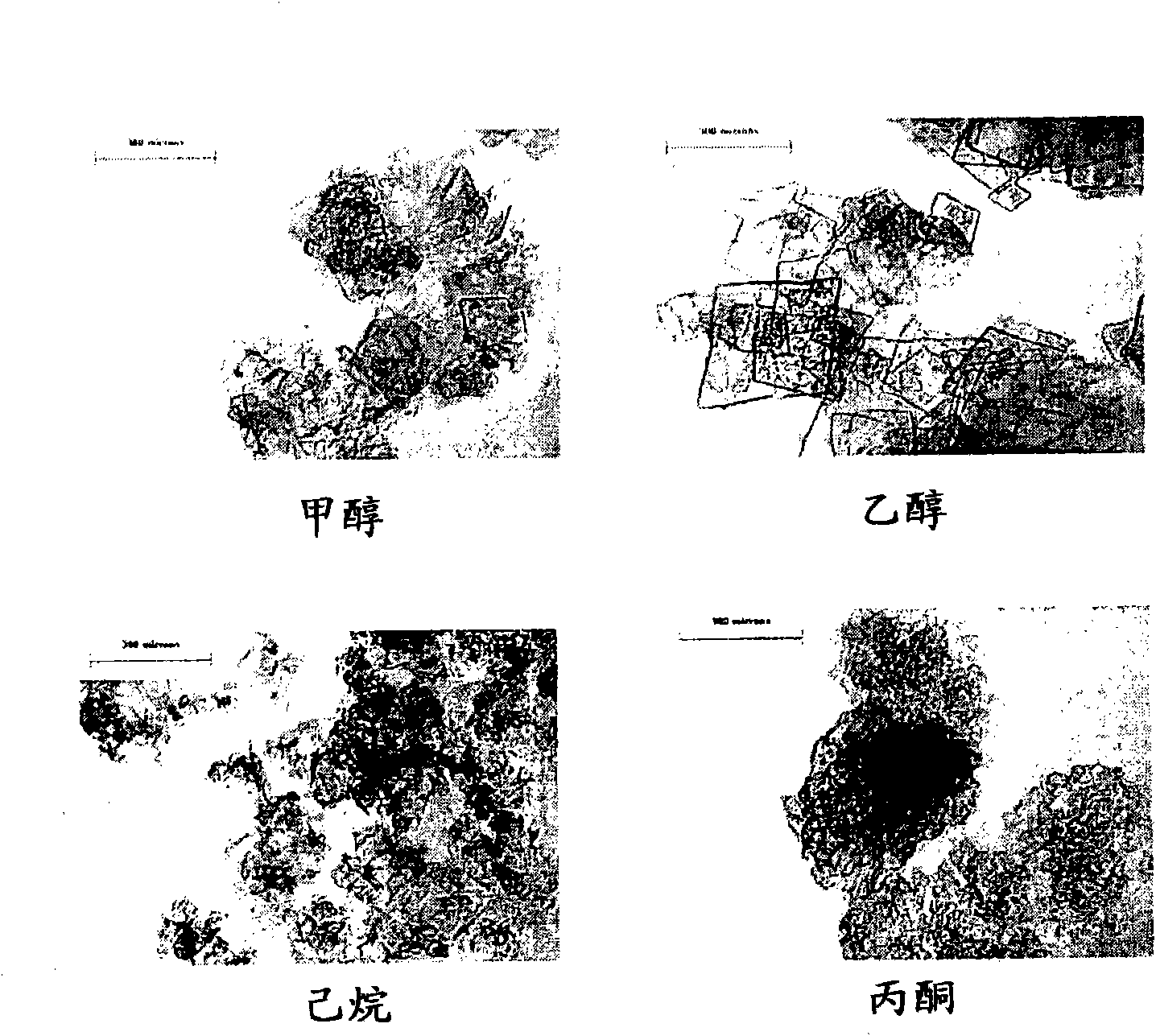
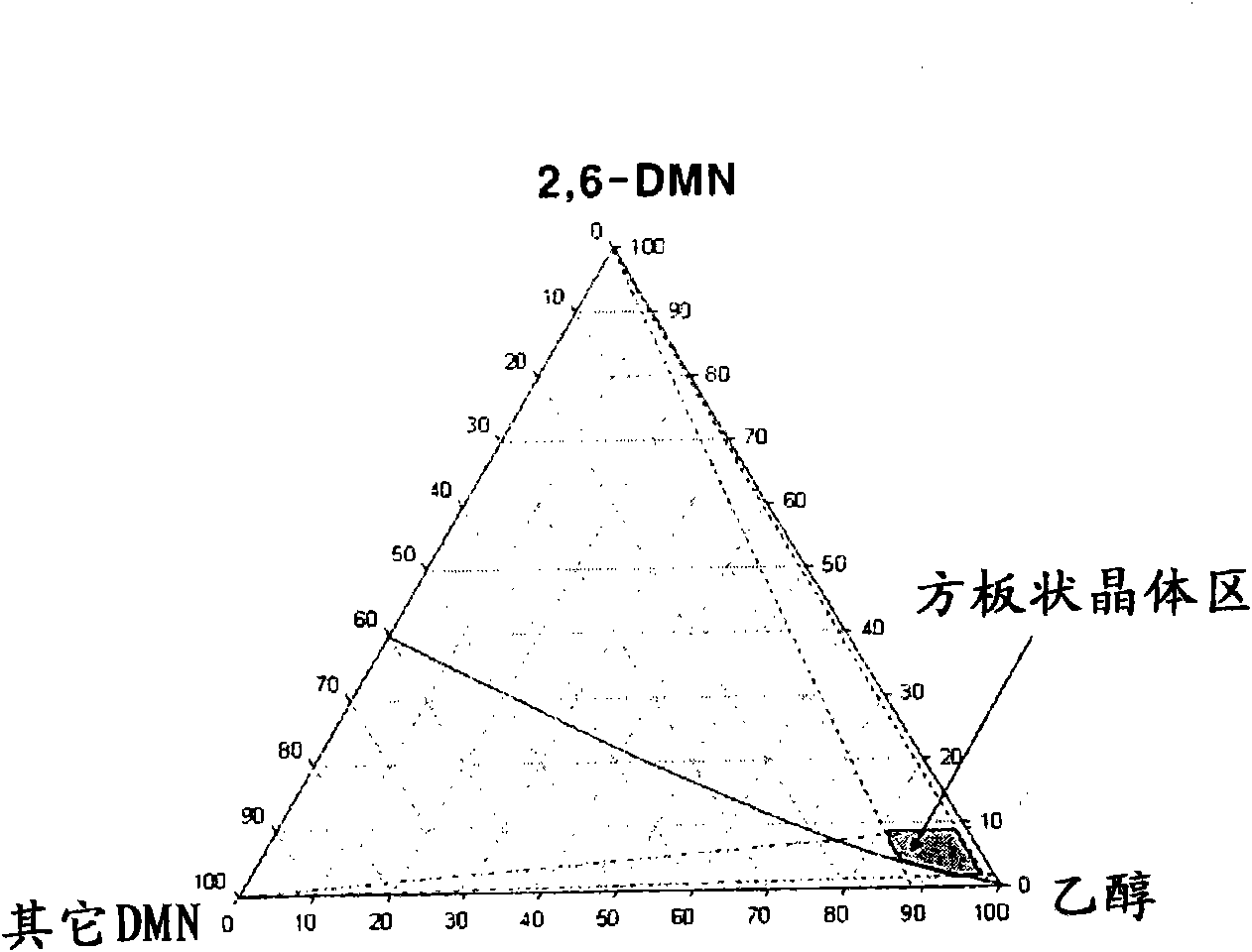
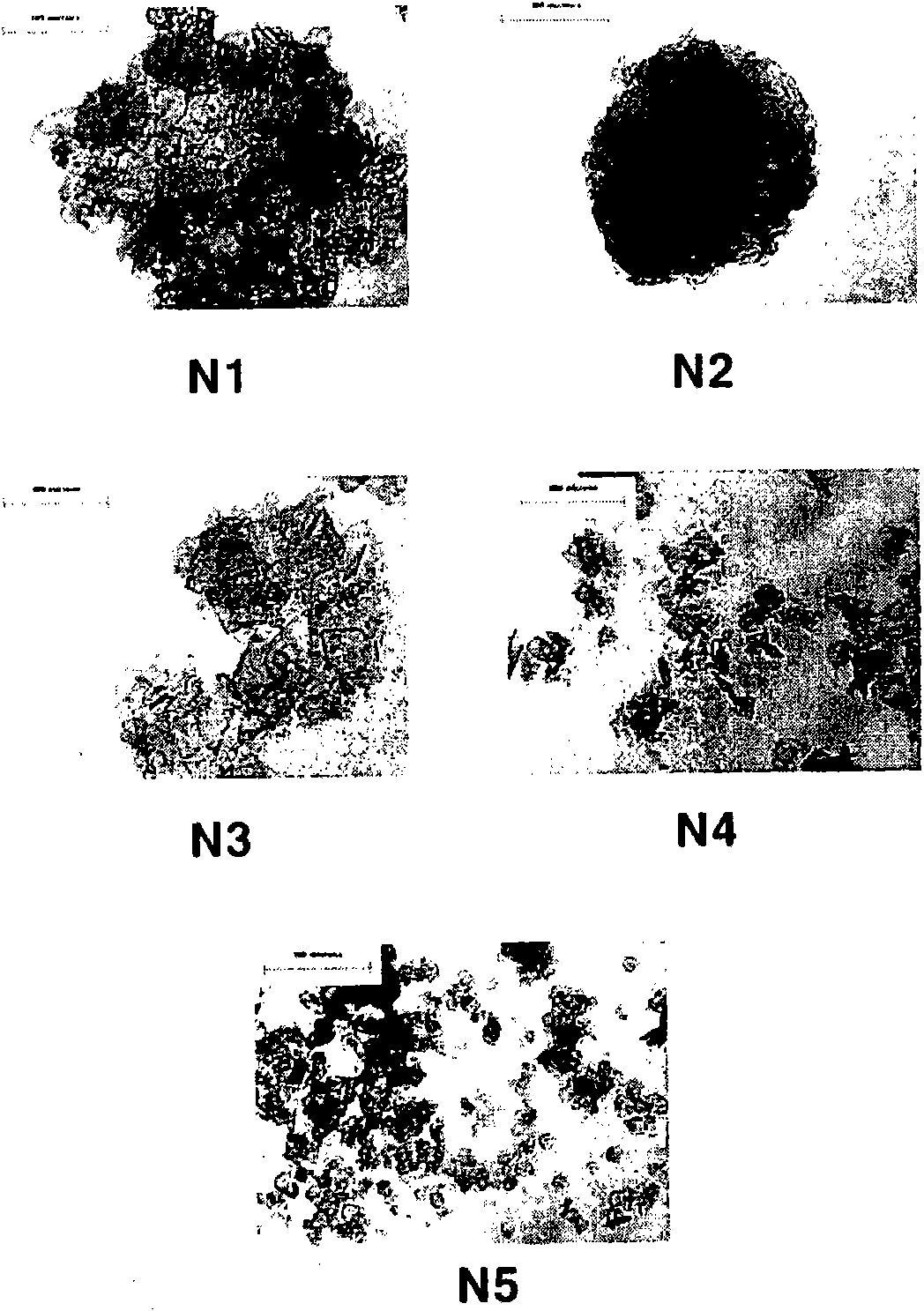
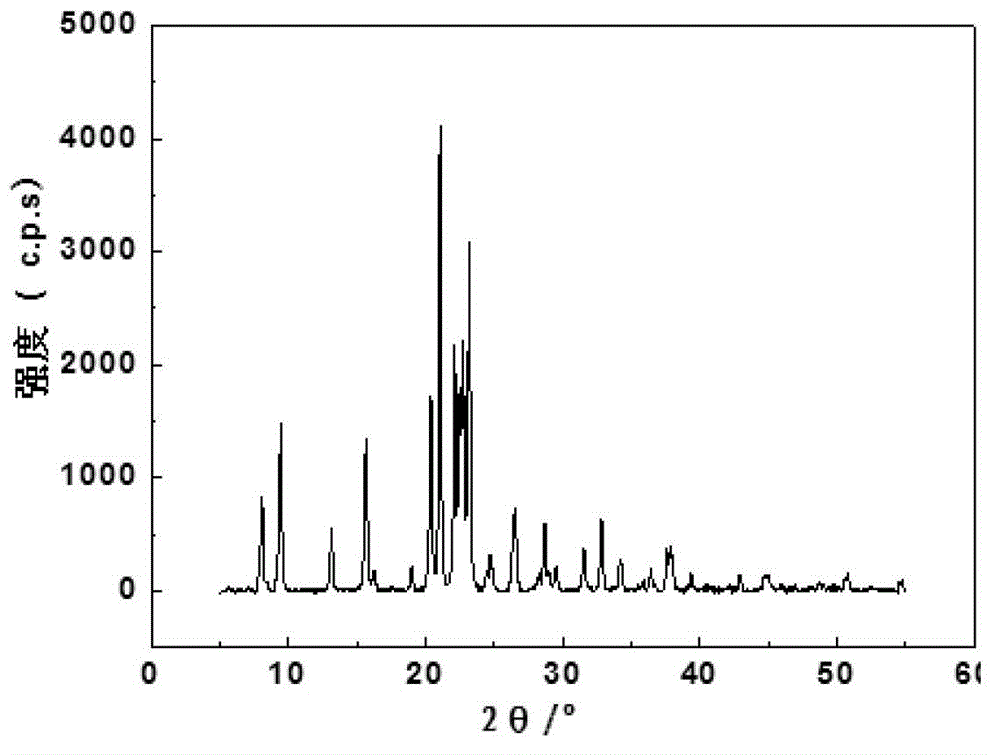
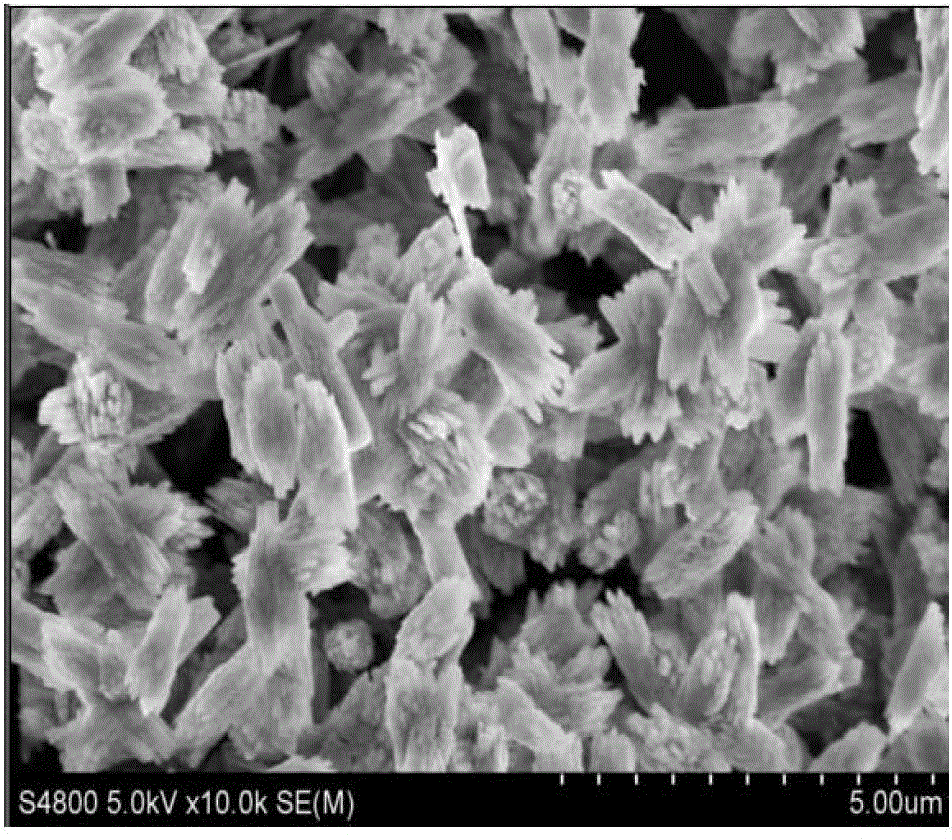
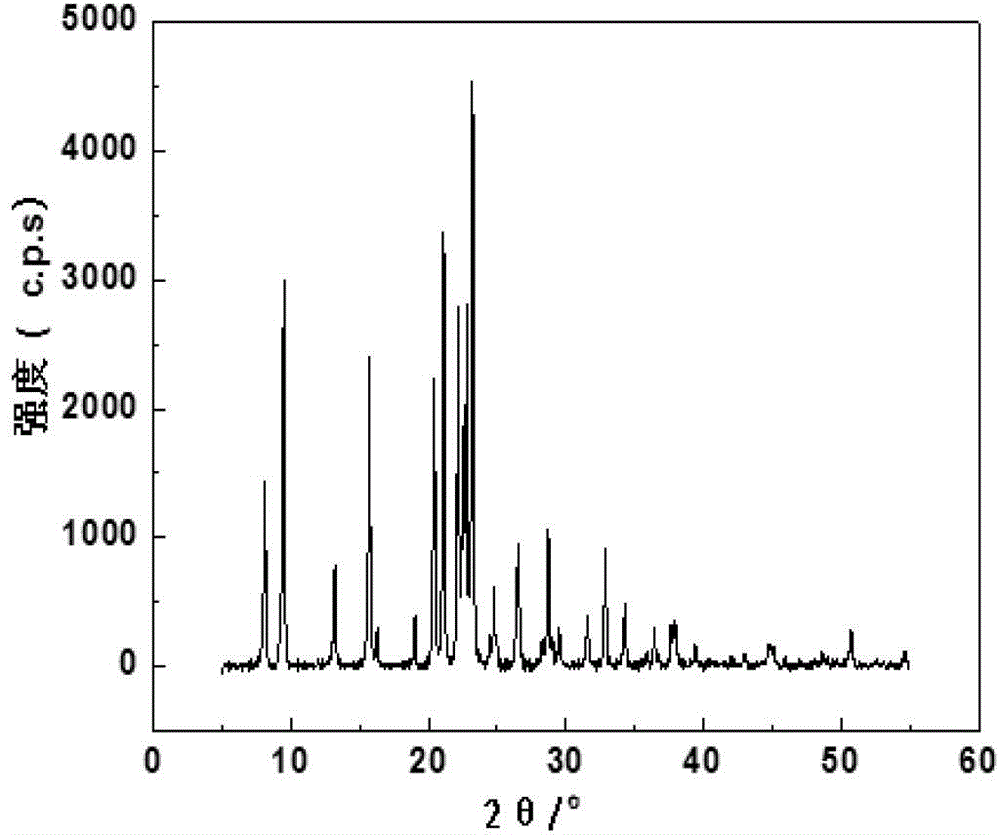
Method for achieving high purity separation and refinement by controlling morphology and particle size of 2, 6-dimethylnaphthalene crystals
InactiveCN102123976ASave energyEasy to operateOrganic compound preparationCrystallisation purification/separationSolvent2,6-Dimethylnaphthalene
The present invention relates to a method for achieving high purity separation and refinement by controlling morphology and particle size of 2, 6-dimethylnaphthalene. And more particularly, the present invention relates to a method for obtaining high purity 2, 6-dimethylnaphthalene crystals, in which crystallization is carried out with a solvent that enables the crystals to form a square-plate shape. During the process, crystallization variables such as, agitation speed, cooling rate, solvent and composition ratio, are adjusted to control morphology and particle size of 2, 6-dimethylnaphthalene and to remove aggregation, thereby obtaining 2, 6-dimethylnaphthalene crystals of high purity.
Owner:HYOSUNG CORP
Preparation method of 2,6-DiMethylnaphthalene (DMN) by using SAPO-11 molecular sieve
InactiveCN102746102BEasy to separateEasy to operateMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationAlkyl transferMolecular sieve
The invention relates to a preparation method of the 2,6-DMN, in particular to a preparation method of the 2,6-DMN by using a SAPO-11 molecular sieve. The preparation method mainly solves the problems that catalysts cannot be provided with high activity and 2,6-DMN selectivity simultaneously, and the catalysts are easy to inactivate. The method includes activating the SAPO-11 molecular sieve synthesized by microwave radiation and heating, mixing naphthalene and alkylation reagents with a solvent according to a molar ratio of 1:(2-4):(6-12), and performing alkylation reaction to obtain the 2,6-DMN at a temperature of 350 DEG C to 450 DEG C, at a pressure of 2 MPa to 5 MPa, at a quality airspeed of 0.5-2h-1 and at a carrier gas flow rate of 20-60 mL / min. According to the preparation method, the synthesized SAPO-11 molecular sieve can shorten the crystallization time greatly, and has high catalytic activity to the alkylation reaction of the naphthalene and high selectivity and good anti-carbon deposition competence to the 2,6-DMN.
Owner:HEILONGJIANG UNIV
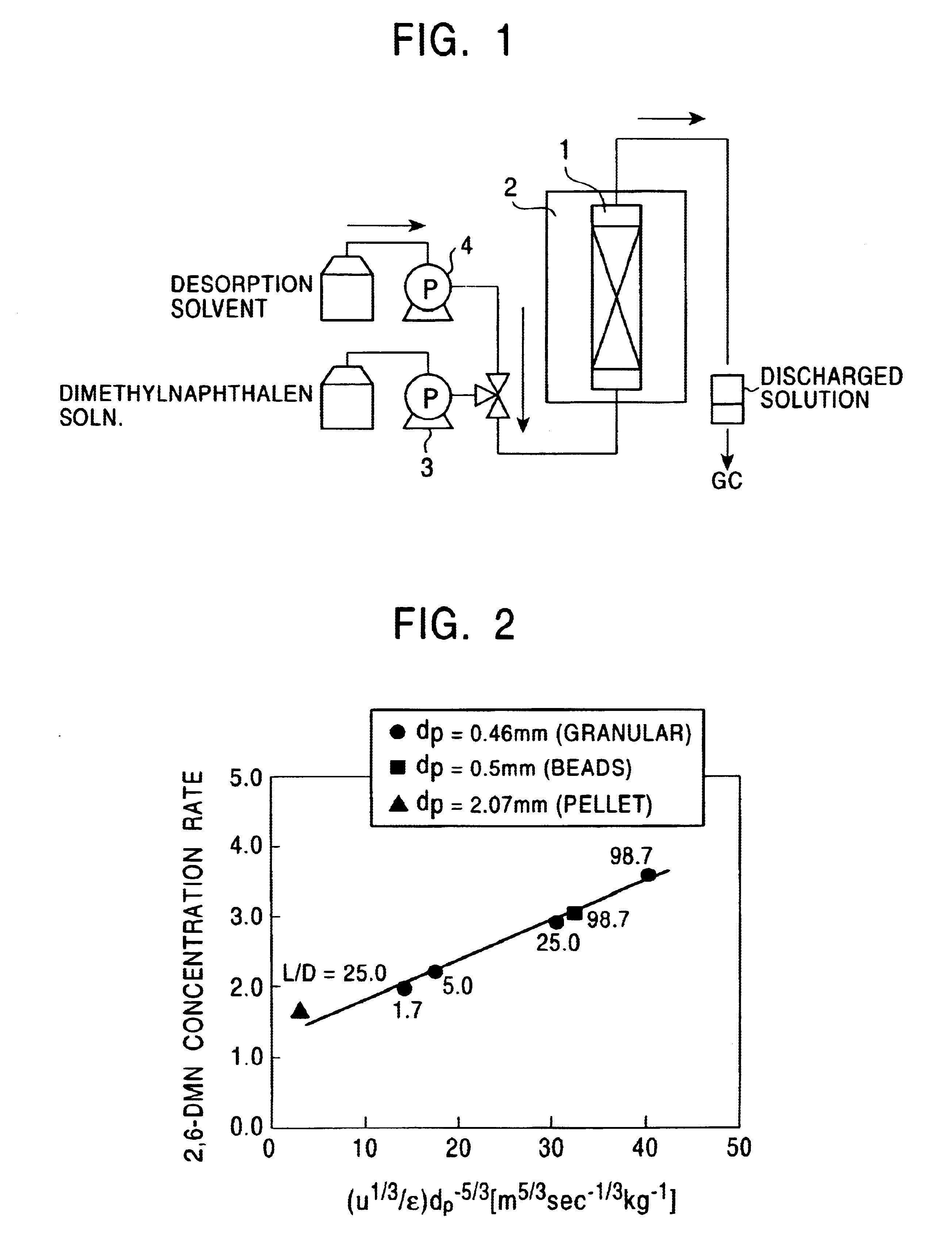
Method for concentrating 2,6-dimethylnaphthalene
InactiveUS6706939B2Solid sorbent liquid separationHydrocarbonsConcentration ratioVolumetric Mass Density
A method for concentrating 2,6-dimethylnaphthalene in a dimethylnaphthalene isomer mixture includes supplying the dimethylnaphthalene isomer mixture to an adsorption column packed with Y-type zeolite. In this instance, by setting the value derived from the expression (u<1 / 3> / epsilon)d<-5 / 3 >at 14 (m<5 / 3 >s<-1 / 3 >kg<-1>) or more, the concentration ratio of 2,6-dimethylnaphthalene to 2,7-dimethylnaphthalene can be 2.0 or more. u here represents the linear velocity (m / s) of the dimethylnaphthalene isomer mixture supplied to an adsorption column, epsilon represents the packing density (kg / m<3>) of Y-type zeolite, and d represents the grain size (m) of the Y-type zeolite.
Owner:KOBE STEEL LTD
Method for preparing 2,6-dimethylnaphthalene by catalyzing naphthalene alkylation reaction with CoAPO-11 molecular sieve
InactiveCN102746101BEasy to separateImprove redox performanceMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sieveAlkyl transfer
Owner:HEILONGJIANG UNIV
Method for catalyzing and preparing 2, 6-dimethylnaphalene by utilizing SAPO-31 molecular sieve
InactiveCN102491868BEasy to separateNot easy to inactivateHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sieveAlkyl transfer
A method for catalyzing and preparing 2, 6-dimethylnaphalene by utilizing an SAPO-31 molecular sieve relates to a method for preparing the 2, 6-dimethylnaphalene and aims at solving the problems of low selectivity of target product 2, 6-dimethylnitrosamine (DMN) and small ratio of 2, 6-DMN to 2, 7-DMN by means of an existing method for preparing the 2, 6-dimethylnaphalene continuously. The method comprises the steps of mixing raw materials to produce raw material liquid, performing alkylation reaction by adopting the SAPO-31 molecular sieve to produce a reaction product, and finally performing separation through eutectic melting crystallization to produce the 2, 6-dimethylnaphalene. The method has the advantages that 1 the selectivity of target product 2, 6-DMN is 30%-60%, the ratio of 2, 6-DMN to 2, 7-DMN is 2-9, and purity of produced 2, 6-DMN is 55%-90%, and 2 the reaction product and catalyst are easily separated and the production cost is reduced. The method is mainly used for preparing the 2, 6-dimethylnaphalen.
Owner:HEILONGJIANG UNIV
Method for producing naphthalenedicarboxylic acid
InactiveUS20070270609A1High purityOrganic compound preparationCarboxylic acid esters preparationAcetic acidDissolution
A method for producing naphthalenedicarboxylic acid comprising the steps of: dissolving 2,6-dimethylnaphthalene in acetic acid solvent; oxidizing the product from the dissolution process using oxygen and a diluent gas; crystallizing naphthalenedicarboxylic acid that has been produced from the oxidation process; and separating the crystallized naphthalenedicarboxylic acid, wherein the amount of the diluent gas being discharged from and recycled to the oxidation process is controlled during the oxidation process, and the amount of mother liquor being recycled to the dissolution process after crystallization is controlled during the separation process, is provided.
Owner:HYOSUNG CORP
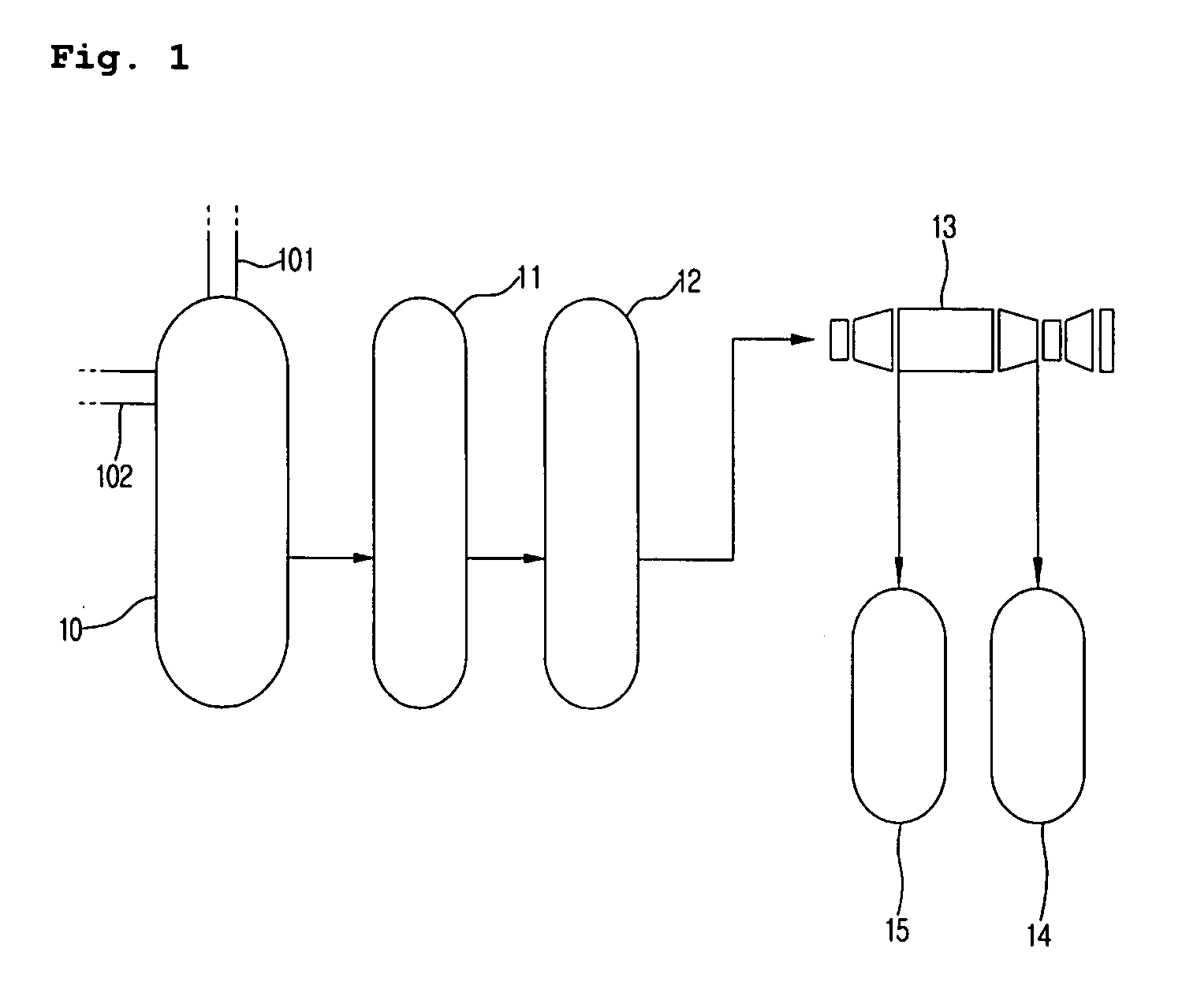
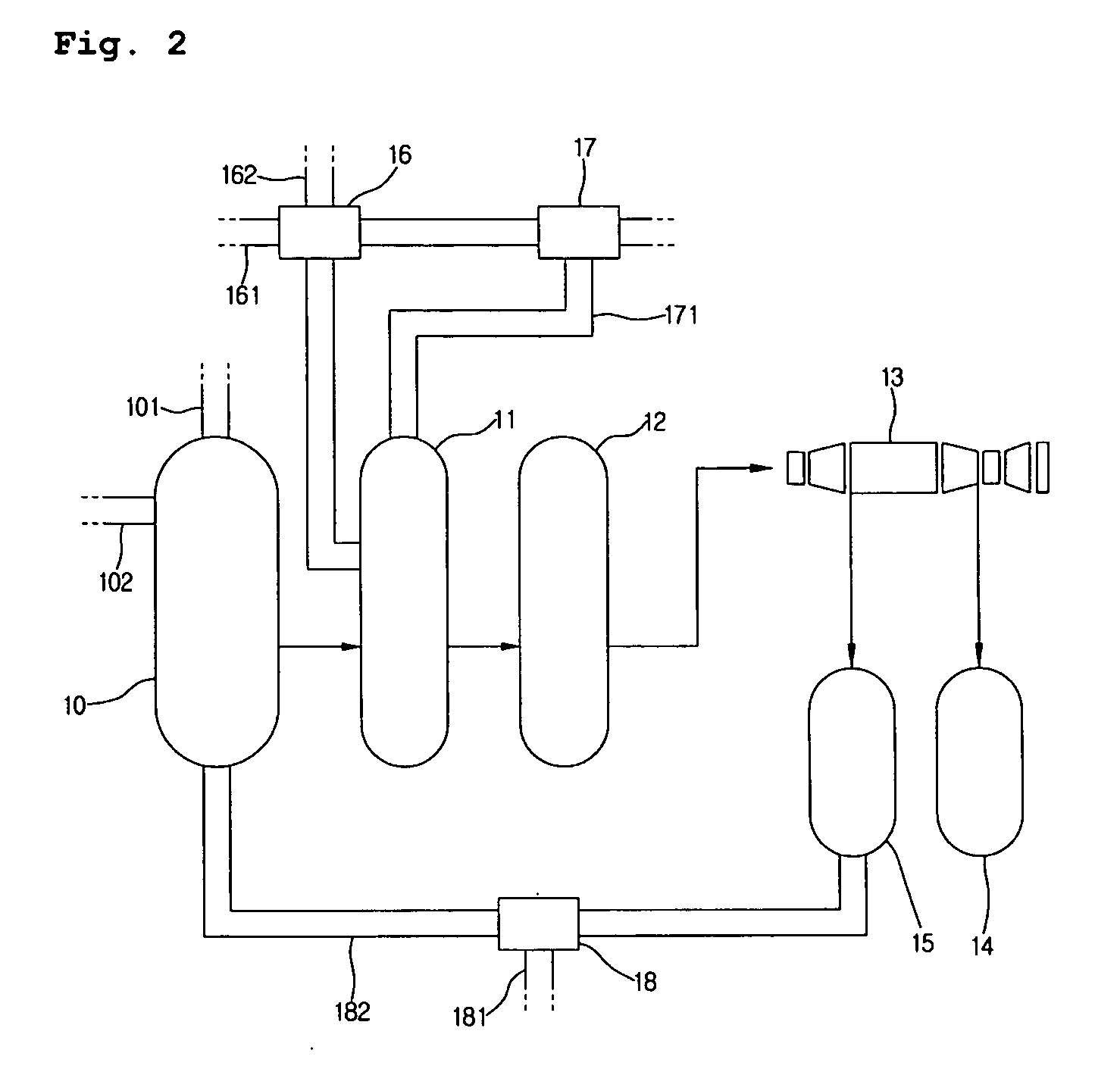
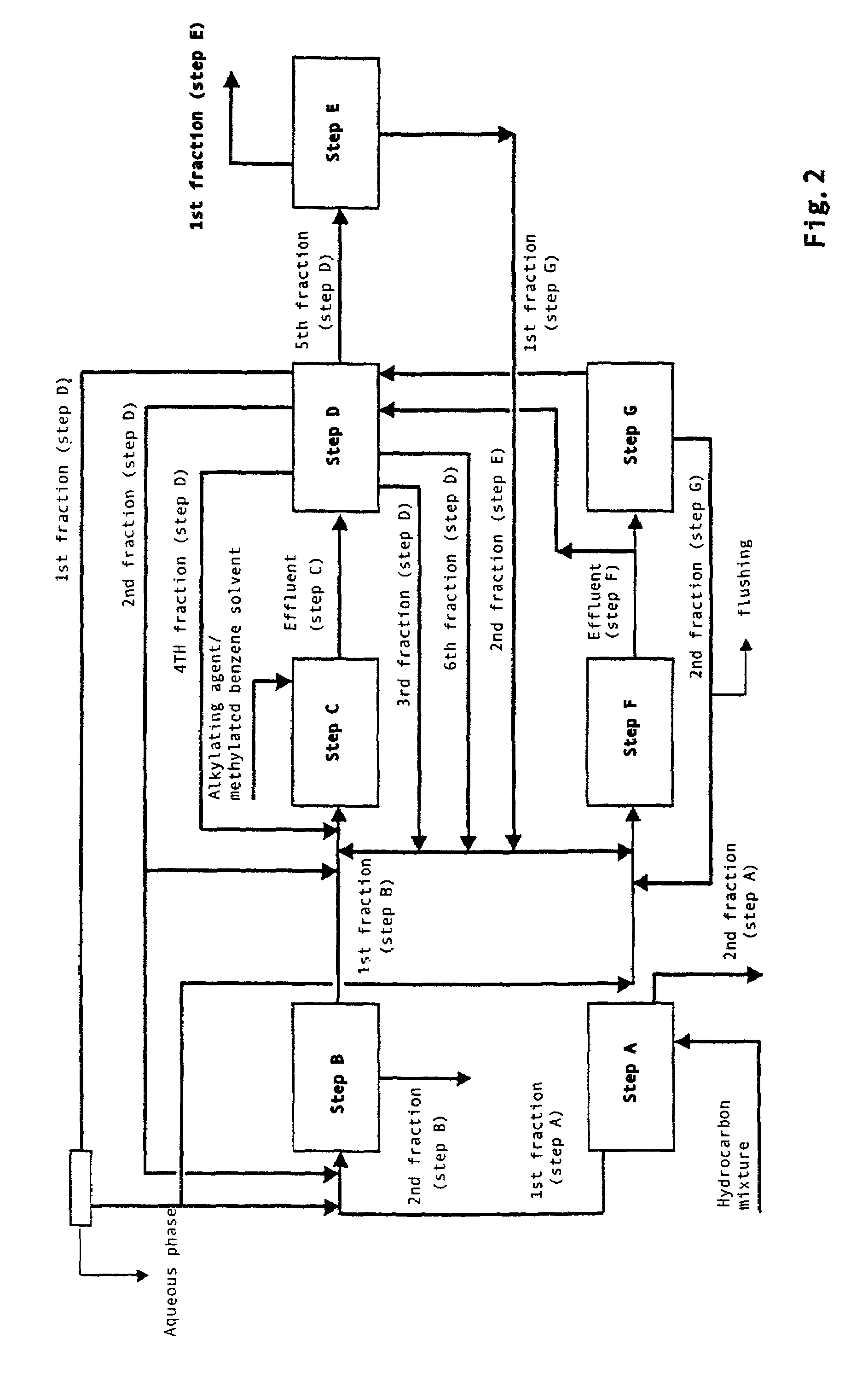
Integrated process for the production of 2,6-dimethylnaphthalene
InactiveUS7718836B2High selectivityHigh duration and stabilityHydrocarbon by isomerisationOrganic chemistry methodsHydrocarbon mixturesSolvent
The present invention relates to an integrated process for the production of high purity 2,6-dimethylnaphthalene starting from hydrocarbon mixtures containing naphthalene and / or isomers of methylnaphthalene and / or isomers of dimethylnaphthalene and / or isomers of polymethylnaphthalene, and from an alkylating agent, preferably methanol, reacted in the presence of a methylated benzene solvent or mixture of various methylated benzene solvents, preferably selected from toluene, xylene and trimethylbenzene, and a catalyst consisting of ZSM-12 zeolite and an inorganic ligand.
Owner:ENI SPA +1
Method for producing 2,6-dimethylnaphthalene by using amylene in fischer-tropsch reaction product
The invention relates to a method for producing 2,6-dimethylnaphthalene by using amylene in a fischer-tropsch reaction product, and the method is mainly used for solving the technical problem of how to produce a chemical product 2,6-dimethylnaphthalene which is in shortage in the market at present by using amylene in a fischer-tropsch product. In the method, a fischer-tropsch amylene separation unit, an alkylation unit, a dehydrocyclization unit, an isomerization unit and a separation unit are adopted, and the method comprises the following steps: through a five-step method, firstly, separating out amylene from the fischer-tropsch reaction product, carrying out alkylation reaction on olefin and benzene-series arene, carrying out dehydrocyclization reaction on the product subjected to the alkylation reaction, so as to generate naphthalene-series arene; separating out 2,6-dimethylnaphthalene by virtue of the separation unit; and converting dimethylnaphthalene of a non-target product into 2,6-dimethylnaphthalene of a target product through isomerization reaction, and then recycling in the separation unit again. According to the system, the technical problem of how to produce the 2,6-dimethylnaphthalene by using amylene in the fischer-tropsch reaction product is relatively well solved; and the method can be applied to industrial production of 2,6-dimethylnaphthalene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for producing 2,6-dimethylnaphthalene
InactiveUS6894202B1High purityHydrocarbonsCrystallisation purification/separationSolid componentFiltration
An object of the present invention is to provide a method for manufacturing 2,6-DMN, in which even when a mixture containing DMN isomers which includes 5 wt % or more of 2,7-DMN is used, a highly pure 2,6-DMN can be obtained. The method for manufacturing the highly pure 2,6-dimethylnaphthalene of the present invention comprises performing cooling crystallization of a mixture containing dimethylnaphthalenes which includes 2,6-dimethylnaphthalene, performing solid-liquid separation to obtain a solid component, and washing the solid component using a solvent, wherein the solid-liquid separation performed after the cooling crystallization includes press filtration. In the present invention, the pressure of the press filtration is preferably 10 kg / cm2 or more, and according to the method of the present invention, even when a DMN mixture containing 5 wt % or more of 2,7-DMN is used as a feedstock, a highly pure 2,6-DMN can be manufactured, and in addition, even when a DMN mixture containing less than 25 wt % of 2,6-DMN is processed by cooling crystallization, a highly pure 2,6-DMN can be manufactured.
Owner:KOBE STEEL LTD
Method for synthesizing 2,6-dimethylnaphthalene with 2-methylnaphthalene and C10 aromatics by transferring alkyl group
InactiveCN101973841AHigh yieldDelay inactivationHydrocarbonsHydrocarbon preparationMolecular sievePetrifaction
The invention relates to a method for synthesizing 2,6-dimethylnaphthalene with 2-methylnaphthalene and C10 aromatics by transferring an alkyl group, in particular to a method for synthesizing the 2,6-dimethylnaphthalene by transferring the alkyl group through a molecular sieve based catalyst by using the 2-methylnaphthalene and the C10 aromatics (which is an aromatics mixture using tetramethylbenzene as a main component, wherein the carbon atom number of the mixture is 10, and the mixture is a by-product of a petrifaction aromatics device) as raw materials. The method comprises the following steps of: blending the C10 aromatics a and the 2-methylnaphthalene b which are used as the raw materials into a mixture raw material by a weight ratio of a:b=1-5:1; keeping the reaction airspeed to be 0.1-3 h<-1> at a reaction temperature of 360-540 DEG C and a reaction pressure of 0-7.0 MPa; and realizing an alkyl group transfer reaction through the molecular sieve based catalyst to synthesize the 2,6-dimethylnaphthalene. In the invention, the catalyst has low inactivation speed and longer service life, and meanwhile, the reaction selectivity and the yield of the 2,6-dimethylnaphthalene can be effectively improved; in addition, the mixture of the C10 aromatics is used as the raw material, and thus the production process of the 2,6-dimethylnaphthalene is effectively simplified; and the utilization of the C10 aromatics resource which is a byproduct and has low carbon value is also beneficial to the reducing of the production cost.
Owner:TONGJI UNIV
Method for catalyzed synthesis of 2,6-dimethylnaphthalene by using ion liquid
InactiveCN101391937BMild reaction conditionsEasy to operateOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsAlkyl transferRoom temperature
The invention discloses a method for preparing 2, 6-dimethylnaphthalene by ionic liquid catalysis, relating to a method for preparing dimethylnaphthalene by ionic liquid catalysis. The invention solves the problems in the existing method for preparing 2, 6-dimethylnaphthalene that: the process is complex, byproducts are numerous, the selectivity of the 2, 6-dimethylnaphthalene is poor and separating the 2, 6-dimethylnaphthalene from products is difficult. The method comprises the following steps: methylnaphthalene, an alkyl transfer agent and a solvent are mixed; then, the ionic liquid catalyst occupying 10 to 75 percent of the total weight of the mixed solution is added in the mixed solution; reaction is carried out under the protection of inert gas at the temperature of 10 to 50 DEG C for 0.5 to 8h; after that, the mixed solution is cooled to room temperature; and the 2, 6-dimethylnaphthalene is obtained through separation after the decantation of an upper layer of the reaction solution in layered reaction solution. The selectivity of the 2, 6-dimethylnaphthalene obtained by adopting the method is 66.4 to 100 percent; no multi-methylnaphthalene byproducts such as trimethyl-naphthalene and the like are generated; and the method for preparing 2, 6-dimethylnaphthalene has simple process and low production cost.
Owner:HEILONGJIANG UNIV
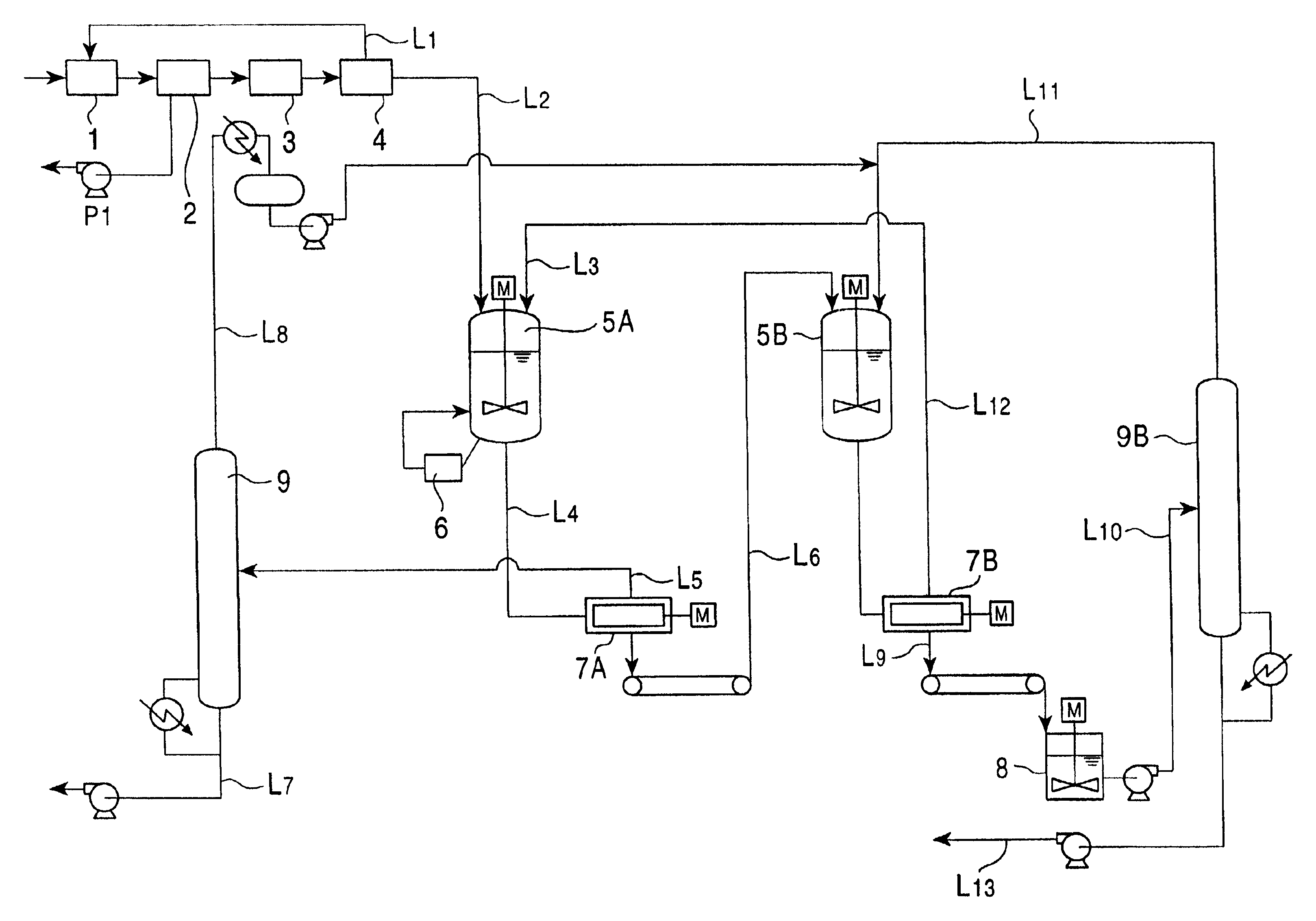
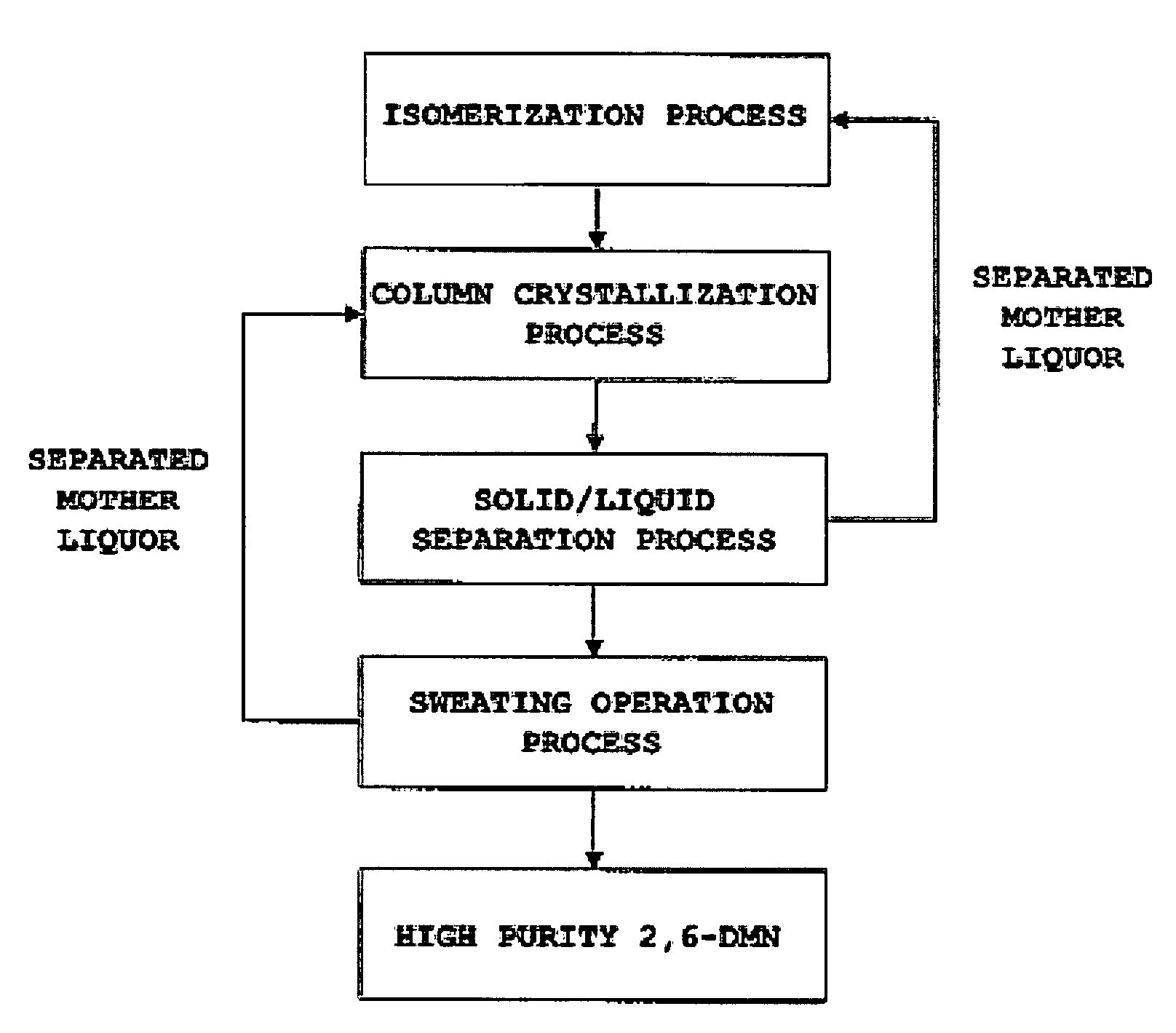
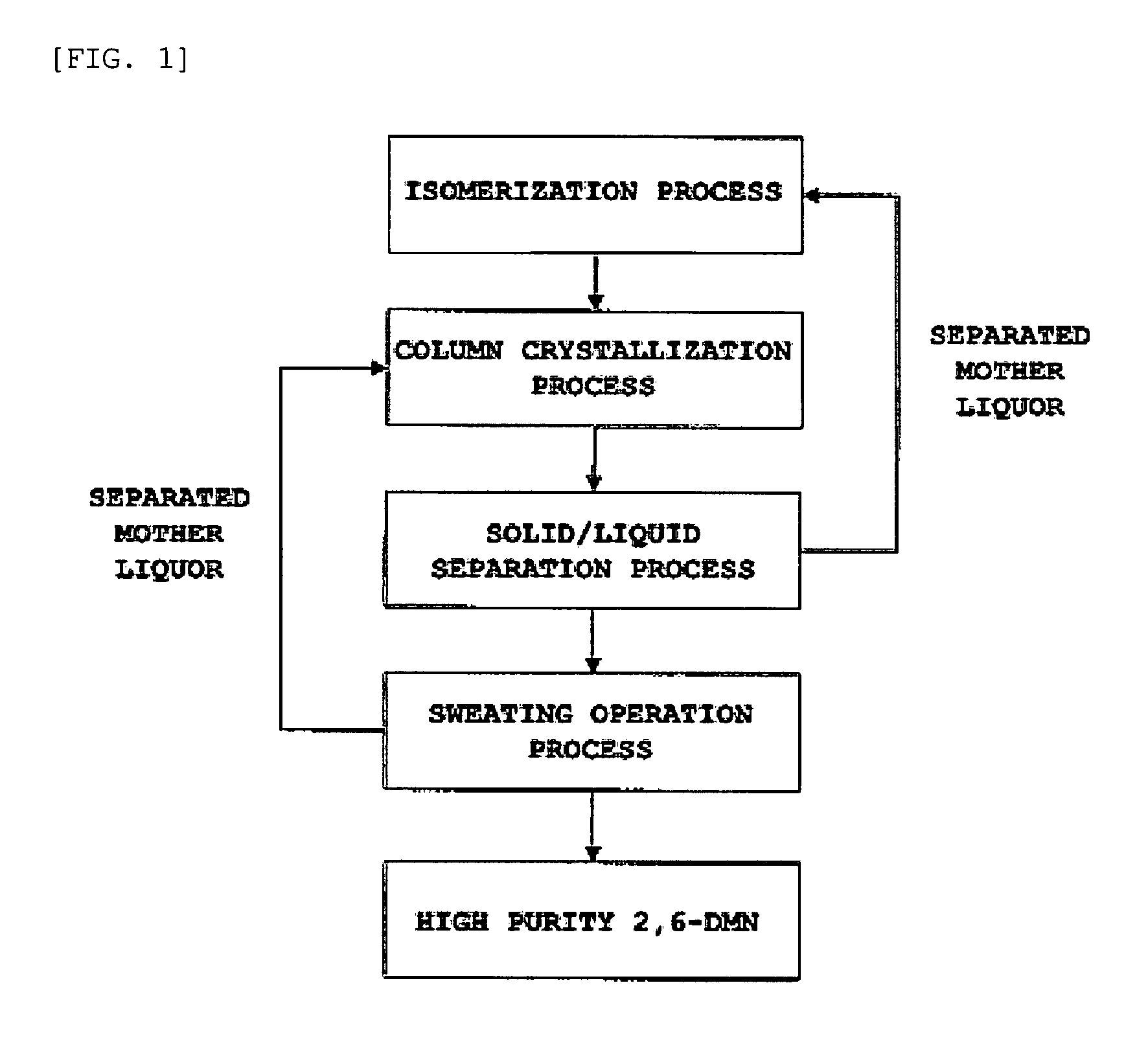
Method for separating and purifying 2,6-dimethylnaphthalene
ActiveUS20070255083A1HydrocarbonsCrystallisation purification/separationCombined method2,6-Dimethylnaphthalene
A method for separating and purifying 2,6-dimethylnaphthalene, is provided in which 2,6-dimethylnaphthalene of high purity is obtained from a mixture of dimethylnaphthalene isomers with a high yield, by means of a combined process of column melt crystallization and sweating operation.
Owner:HYOSUNG CHEM CORP
Method for extracting catalyst for 2,6-dimethylnaphthalene from coal tar
InactiveCN102728390BIncrease contentEasy to operateMetal/metal-oxides/metal-hydroxide catalystsChemical modification purification/separationChemical industryIsomerization
The invention relates to a method for extracting a catalyst for 2,6-dimethylnaphthalene from coal tar, belonging to the field of catalysis in chemical industry. The method comprises the following steps: washing kieselguhr which is used as a supporter with acid, carrying out activating treatment by vapor and roasting, dissolving Pt-containing reagent in a hydrochloric acid solution, and slowly and dropwisely adding the kieselguhr subjected to activating treatment; and standing the impregnant at room temperature over night, drying, cooling to room temperature, dropwisely adding a nickel-containing solution onto the kieselguhr, aging, drying, and reducing with hydrogen to obtain the composite catalyst. The kieselguhr subjected to acid washing is used as a supporter to support Pt and Ni so as to prepare the composite catalyst; and thus, the invention solves the problems of high catalyst carbon deposition amount, short service life and low catalytic activity in the isomerization process of extracting 2,6-dimethylnaphthalene from coal tar, increases the isomerization conversion rate and enhances the recovery rate of 2,6-dimethylnaphthalene extracted from coal tar.
Owner:韩钊武
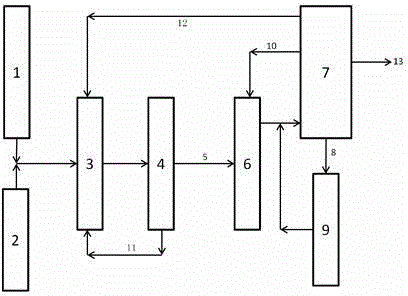
Method and System for Separation and Purification of High-Purity 2,6-Dimethylnaphthalene by Continuous Crystallization
InactiveUS20100010281A1Save energy consumptionReduced fixed investmentChemical industryHydrocarbonsContinuous flowIndustrial scale
Provided is a method for the separation and purification of high-purity 2,6-dimethylnaphthalene from a reaction mixture of dimethylnaphthalenes by continuous crystallization. According to the method, shell-tubetype crystallization apparatuses are used to perform crystallization operations under a continuous flow of a reaction mixture of dimethylnaphthalenes, which is obtained from the synthesis of dimethylnaphthalenes using o-xylene and butadiene as starting materials. As a result, high-purity 2,6-dimethylnaphthalene is separated and purified in a high yield from the reaction mixture. In addition, the method is advantageous in terms of energy saving when compared to conventional separation methods and enables continuous separation and purification of 2,6-dimethylnaphthalene on an industrial scale. A system for implementing the method is further provided.
Owner:HYOSUNG CORP
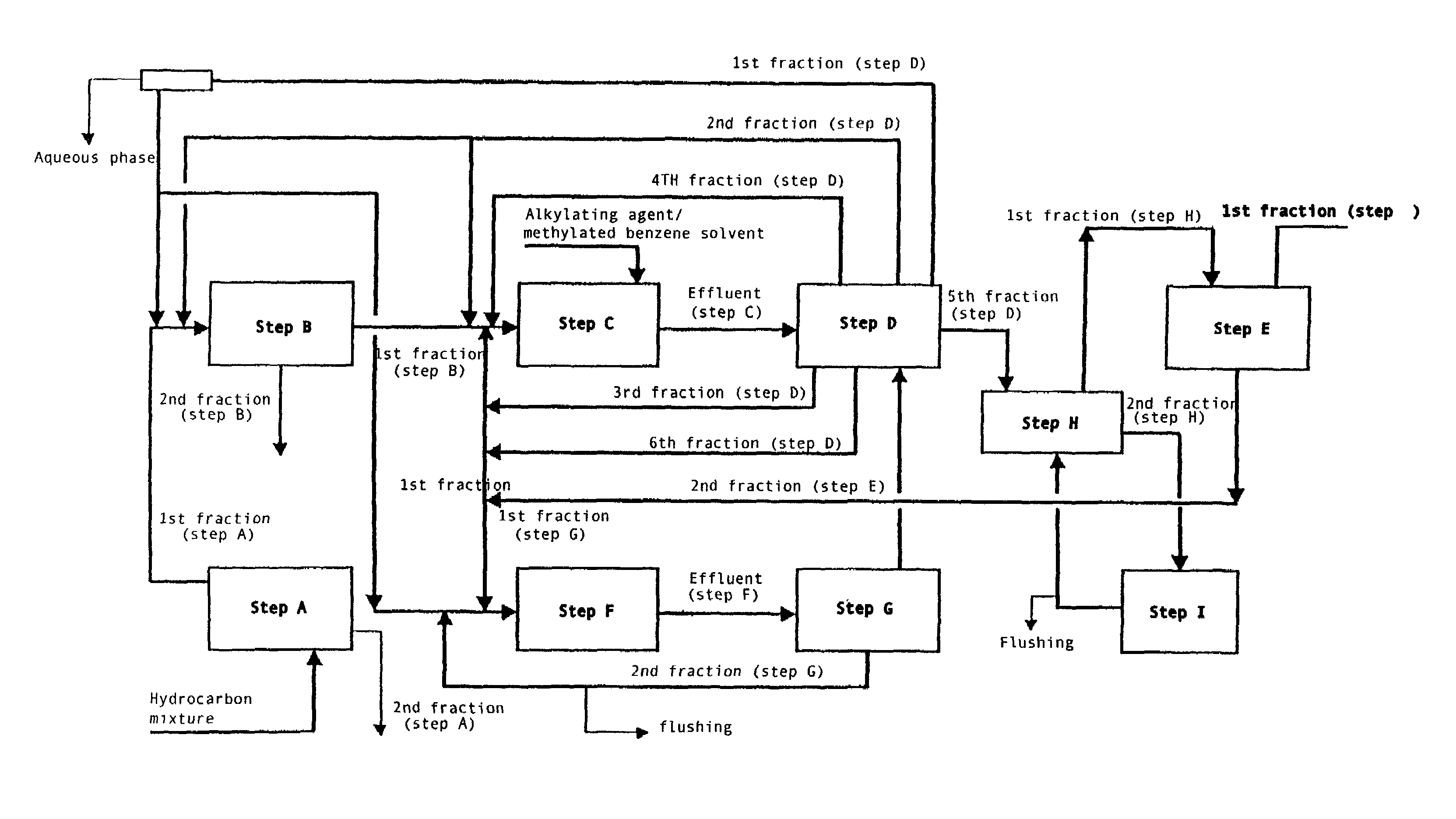
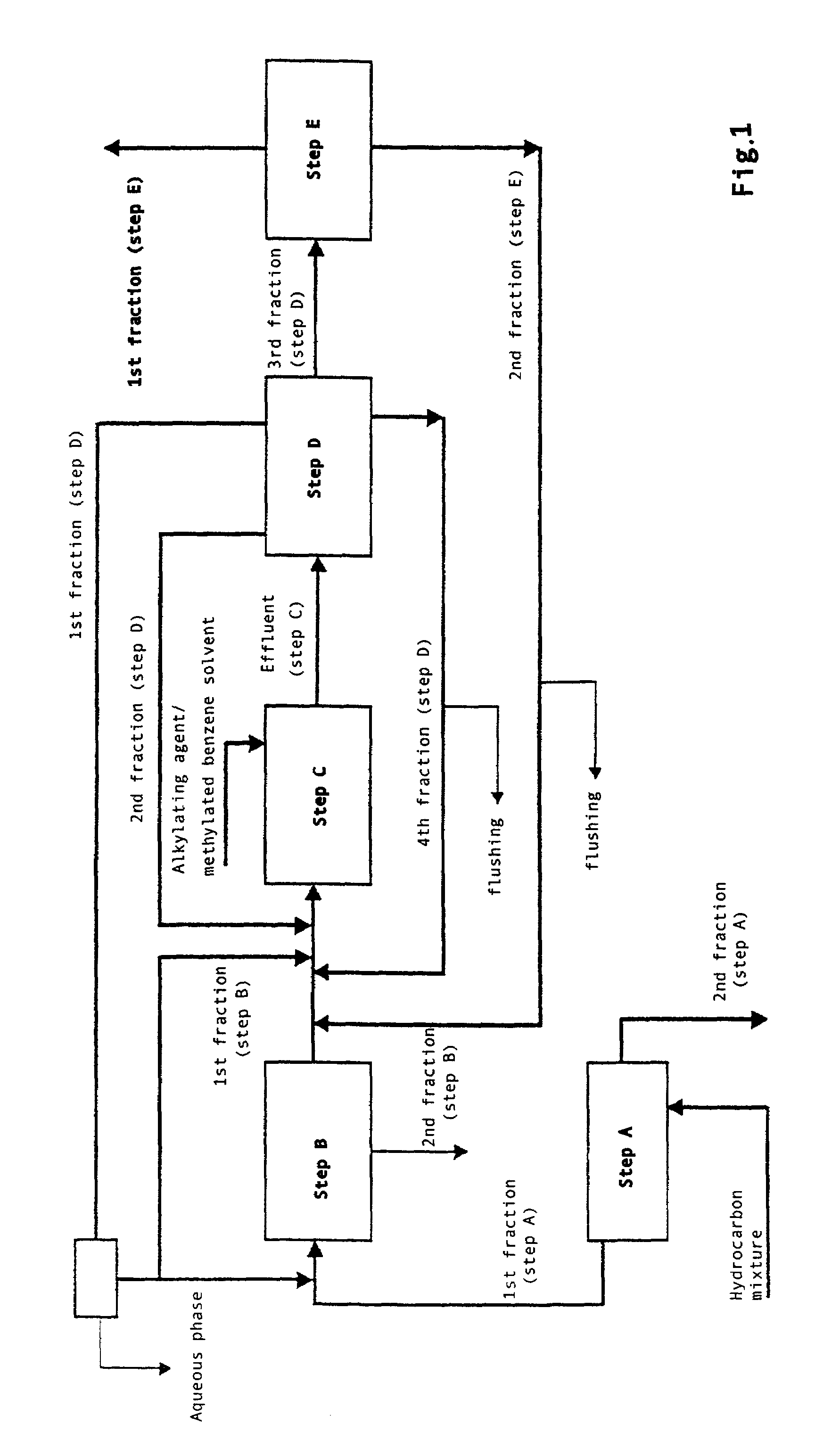
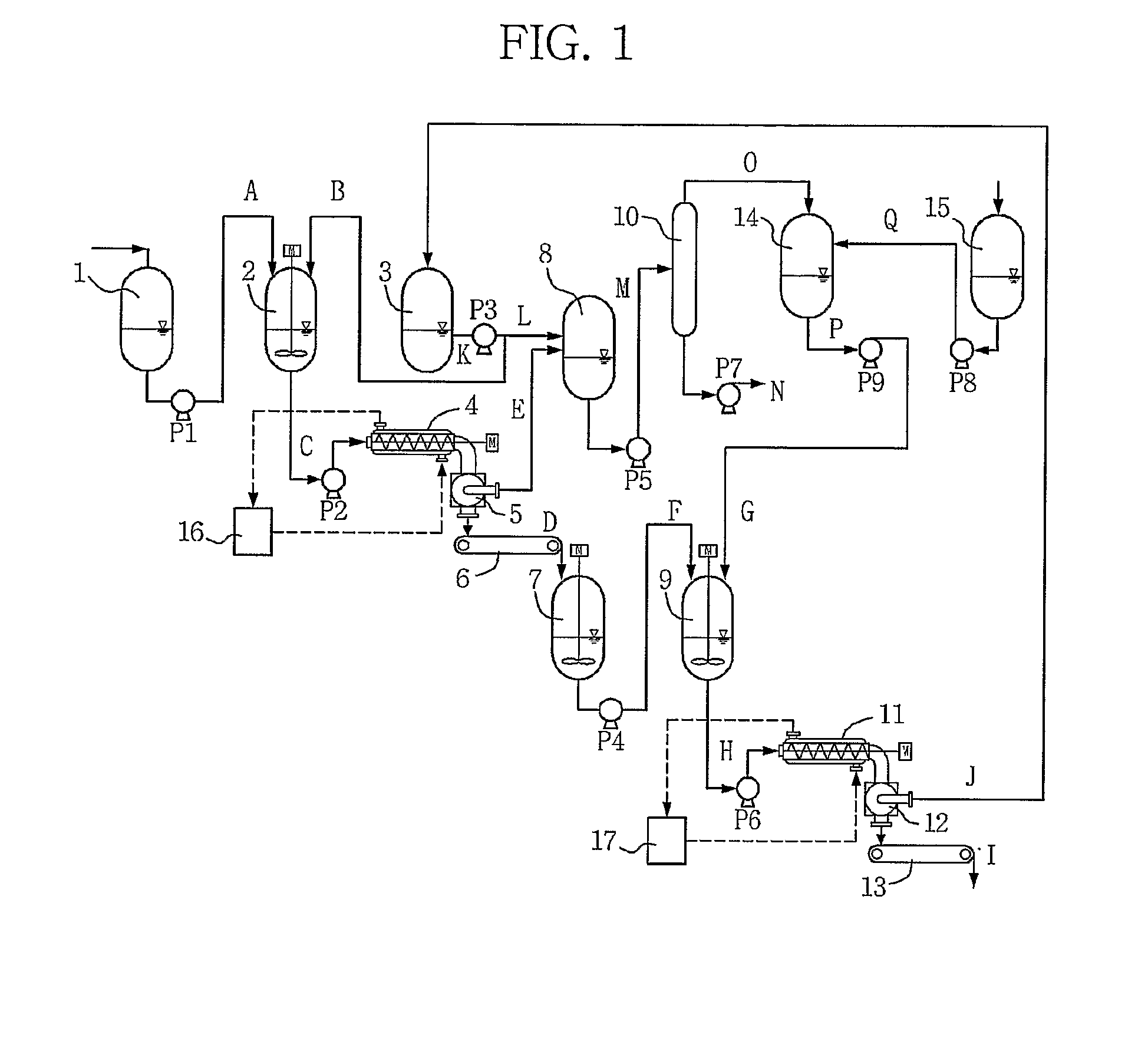
Method for obtaining 2,6-dimethylnaphthalene using isomerization and crystallization processes
High-purity 2,6-dimethylnaphthalene is prepared by (1) subjecting a dimethylnaphthalene isomer mixture rich in 1,5-dimethylnaphthalene, high boiling point materials, unreacted 1,5-dimethyltetralin, and low boiling point materials, which are produced from a dehydrogenation reaction of 1,5-dimethyltetralin, to separation, using a distillation column; subjecting the dimethylnaphthalene mixture separated by the distillation column to liquid state isomerization in the presence of an isomerization catalyst; (3) a first crystallization (melt crystallization process) by cooling the product of liquid state isomerization with a refrigerant without a solvent to form crystals; and (4) a second crystallization (solution crystallization process) of mixing the crystals of the first crystallization step with a solvent to form crystals.
Owner:HYOSUNG CHEM CORP
Ionic liquid catalyzed transalkylation process for preparing 2,6-dimethyl naphthalene
InactiveCN101020619BWide adaptabilityGood choiceHydrocarbon by saturated bond conversionChemical recyclingPolyesterSolvent
The ionic liquid catalyzed transalkylation process for preparing 2, 6-dimethyl naphthalene includes: mixing 2-methyl naphthalene or mixed methyl naphthalene, transalkylating agent and solvent in the molar ratio of 1 to (0.5-3) to 12; adding ionic liquid catalyst in 20-75 % of the total weight of the mixture liquid, and transalkylating reaction at the temperature of 20 deg.c to reflux temperature under the protection of inert gas for 2-11 hr to obtain 2, 6-dimethyl naphthalene. The present invention has simple technological path, reuse of the catalyst, no environmental pollution, greatly raised 2, 6-dimethyl naphthalene selectivity and lowered production cost.
Owner:HEILONGJIANG UNIV
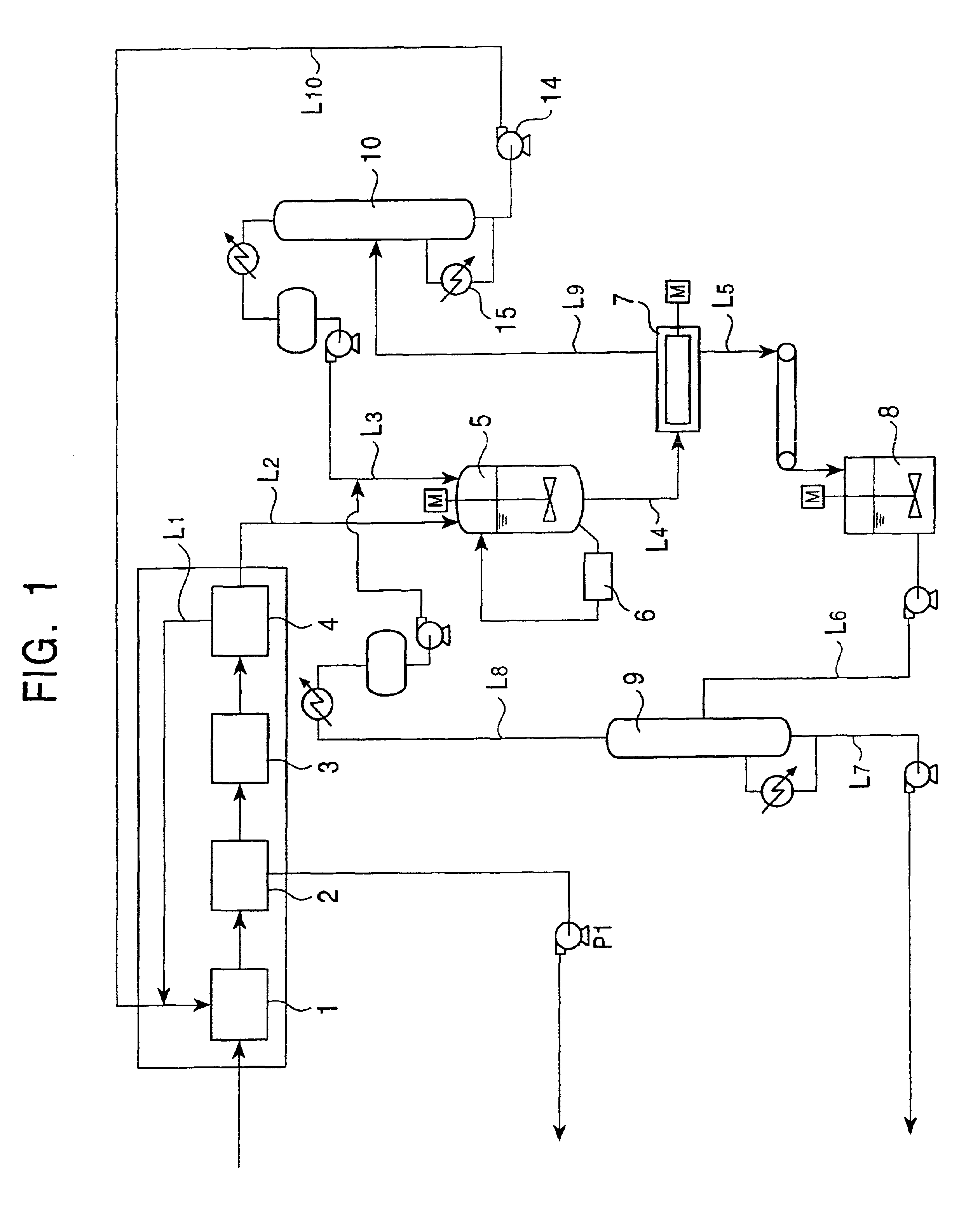
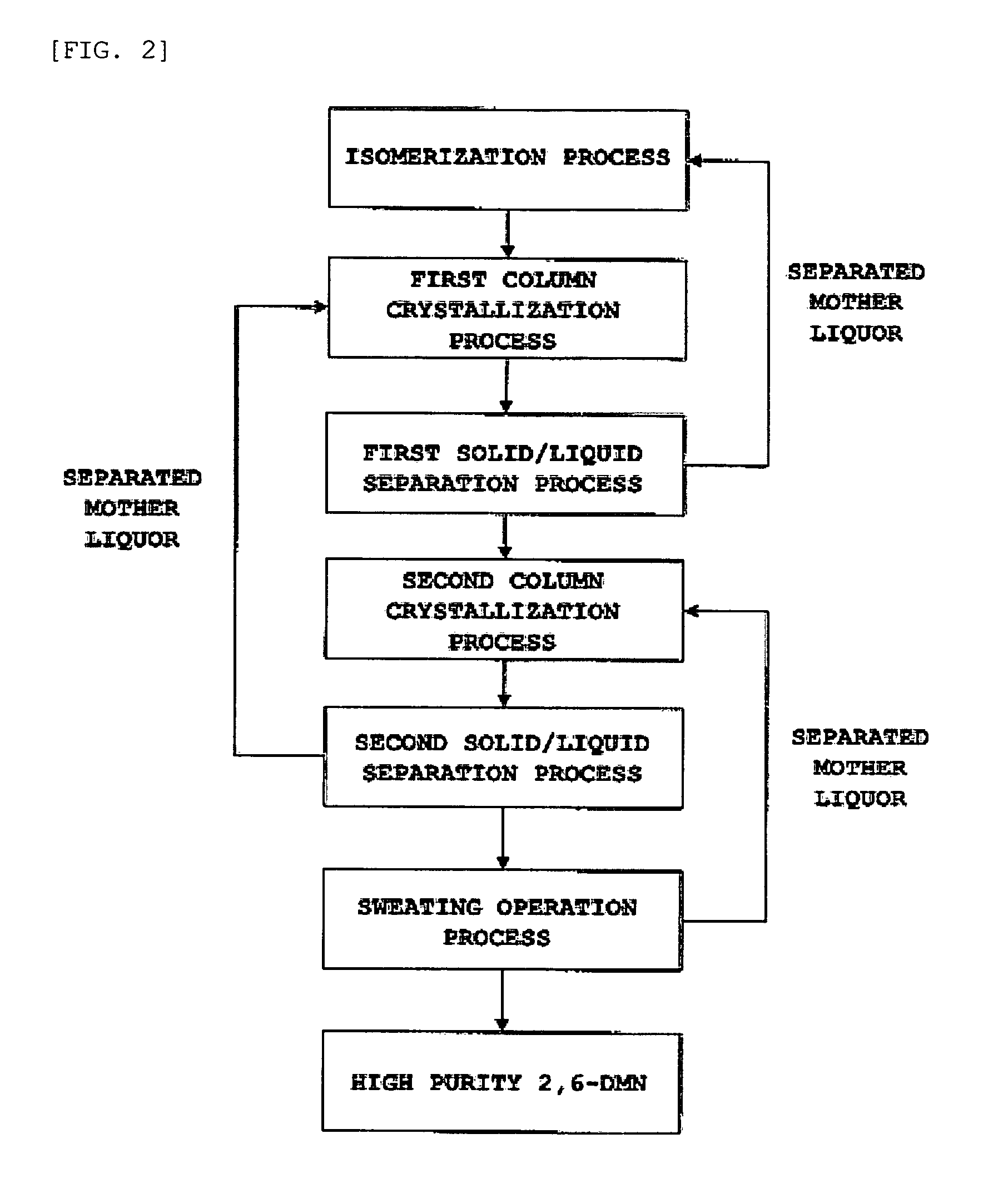
Method for separating and purifying 2,6-dimethylnaphthalene
ActiveUS7605296B2HydrocarbonsCrystallisation purification/separationCombined method2,6-Dimethylnaphthalene
A method for separating and purifying 2,6-dimethylnaphthalene, is provided in which 2,6-dimethylnaphthalene of high purity is obtained from a mixture of dimethylnaphthalene isomers with a high yield, by means of a combined process of column melt crystallization and sweating operation.
Owner:HYOSUNG CHEM CORP
Pseudomonas SP. HN-72 and purification method of 2,6-naphthalene dicarboxylic acid using the same
Provided are a novel microorganism and a method for purifying 2,6-naphthalene dicarboxylic acid with high purity using the microorganism. The microorganism is Pseudomonas sp. Strain HN-72 isolated from soil and has the ability to convert 2-formyl-6-naphthoic acid contained as an impurity in a crude naphthalene dicarboxylic acid, which is an oxidation product of 2,6-dimethylnaphthalene, to 2,6-naphthalene dicarboxylic acid. The Pseudomonas sp. strain HN-72 has excellent effects in producing high-purity 2,6-naphthalene dicarboxylic acid in high yield.
Owner:HYOSUNG CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com