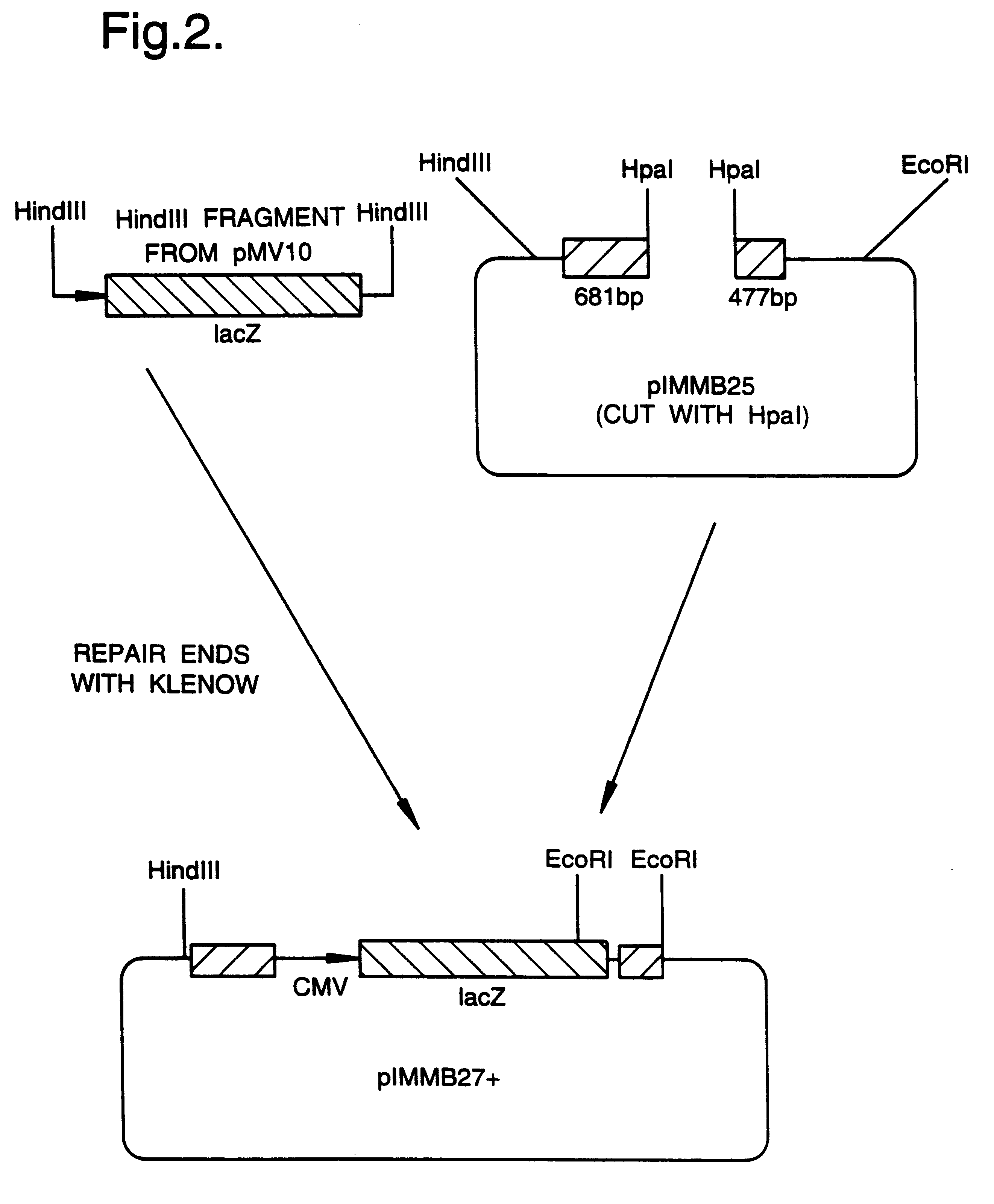
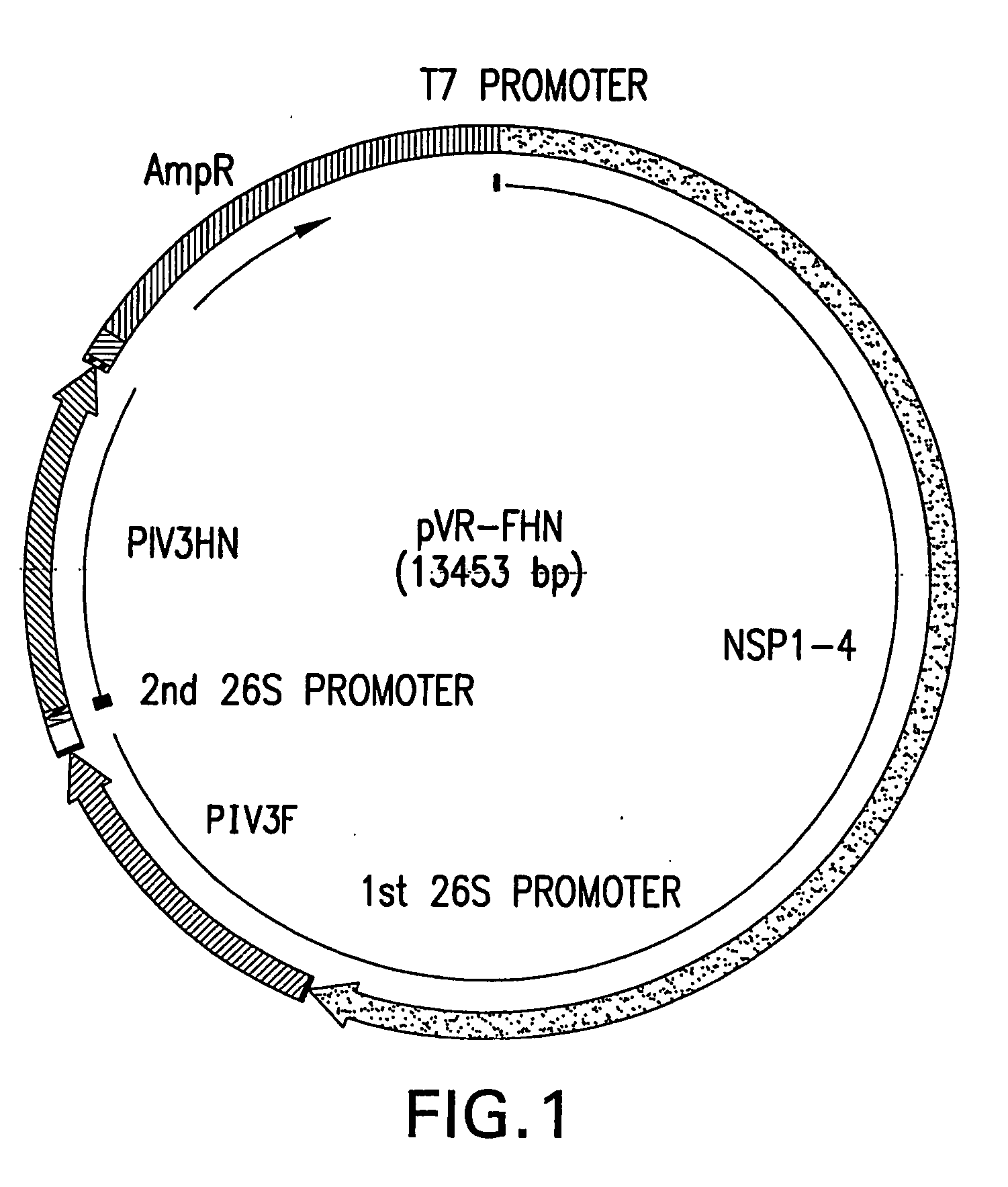
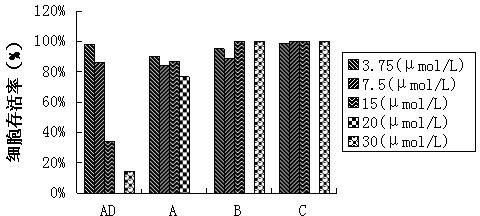
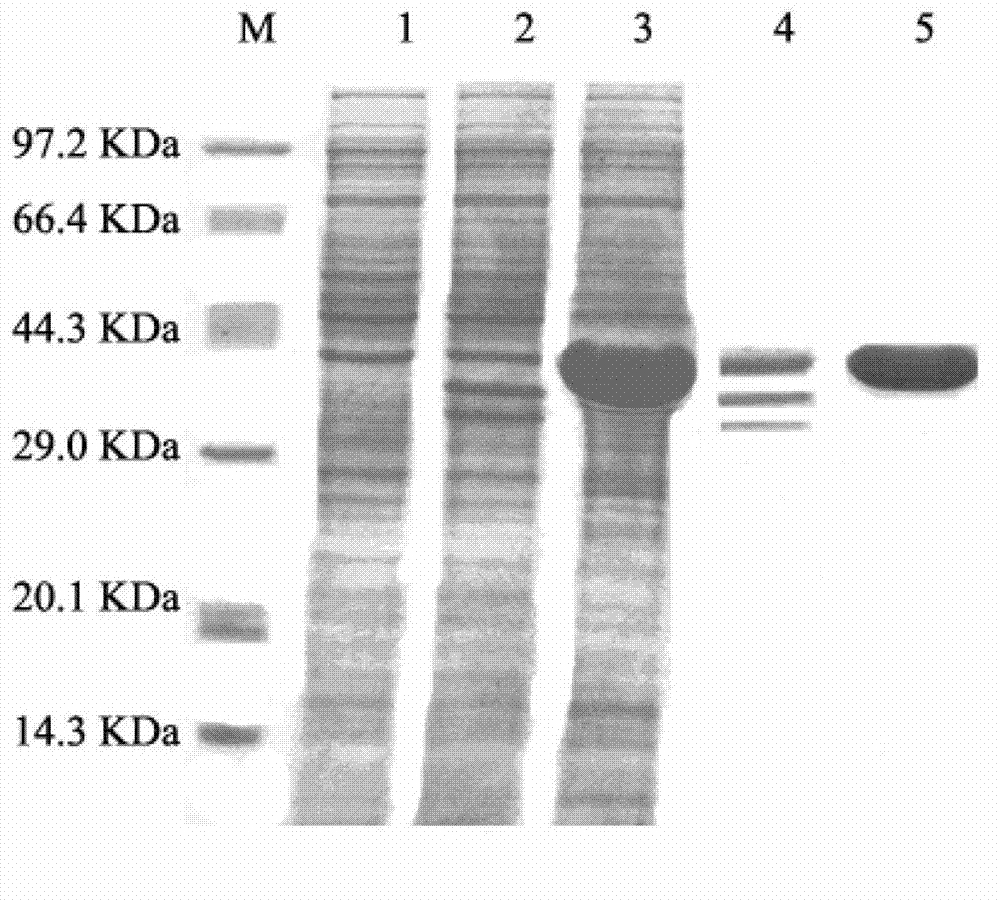
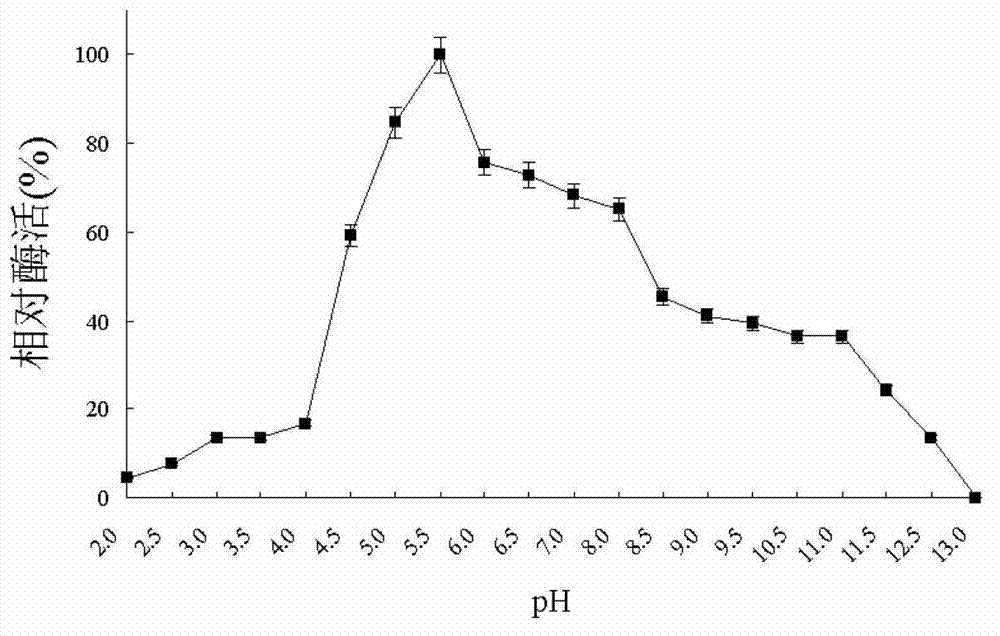
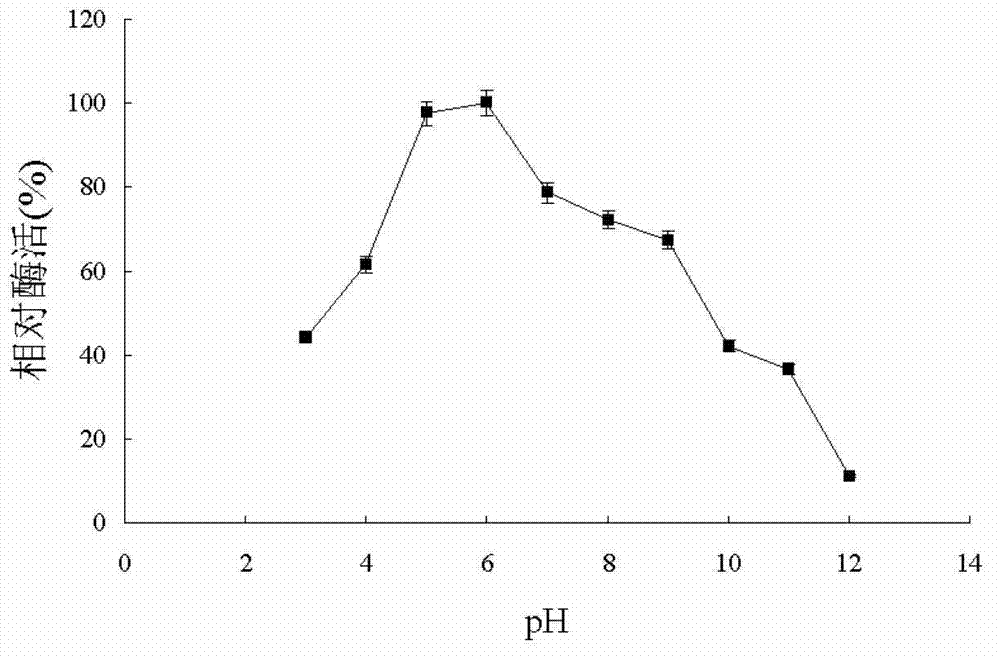
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
233 results about "Cytopathic change" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytopathic effect or cytopathogenic effect (abbreviated CPE) refers to structural changes in host cells that are caused by viral invasion. The infecting virus causes lysis of the host cell or when the cell dies without lysis due to an inability to reproduce. Both of these effects occur due to CPEs.
Anti-viral treatment and assay to screenfor Anti-viral agent
InactiveUS20130085133A1Improve palatabilityImprove stabilityBiocideSugar derivativesCytopathic effectAntiviral drug
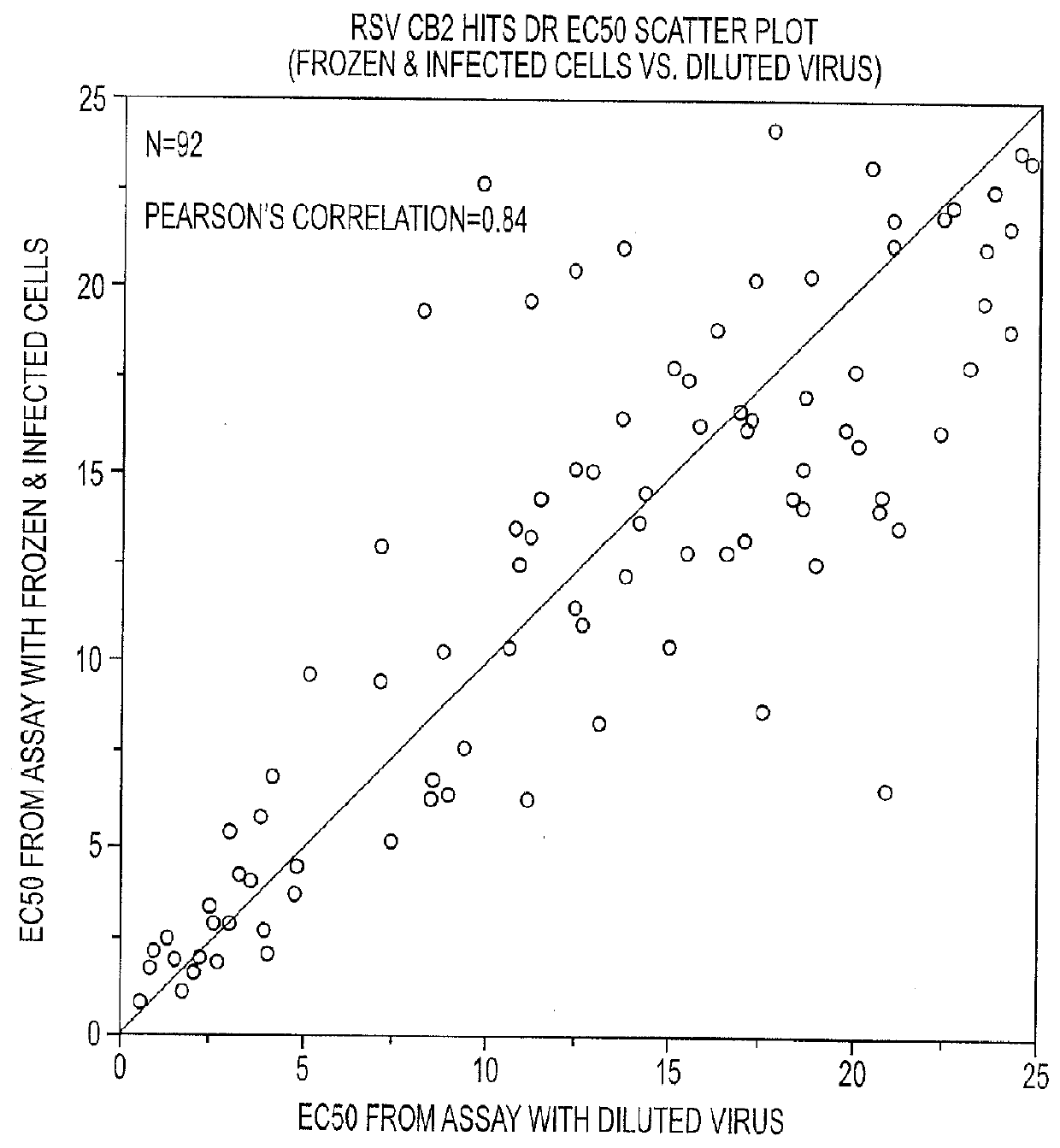
The present disclosure relates to novel compounds of formulas (1) through (19) and to a method for treating humans infected with a virus including various respiratory viruses such as members of the Paramyxoviridae family (respiratory syncytial virus (RSV), human metapneumovirus (HMPV), human parainfluenza virus (HPIV), measles virus, and mumps virus) with a compound of formulas (1) through (19). The present disclosure also relates to a cytopathic effect (CPE)-based assay that will assess virus-induced CPE for screening of compounds for treating viral diseases or inhibiting a virus.
Owner:SOUTHERN RES INST & IP
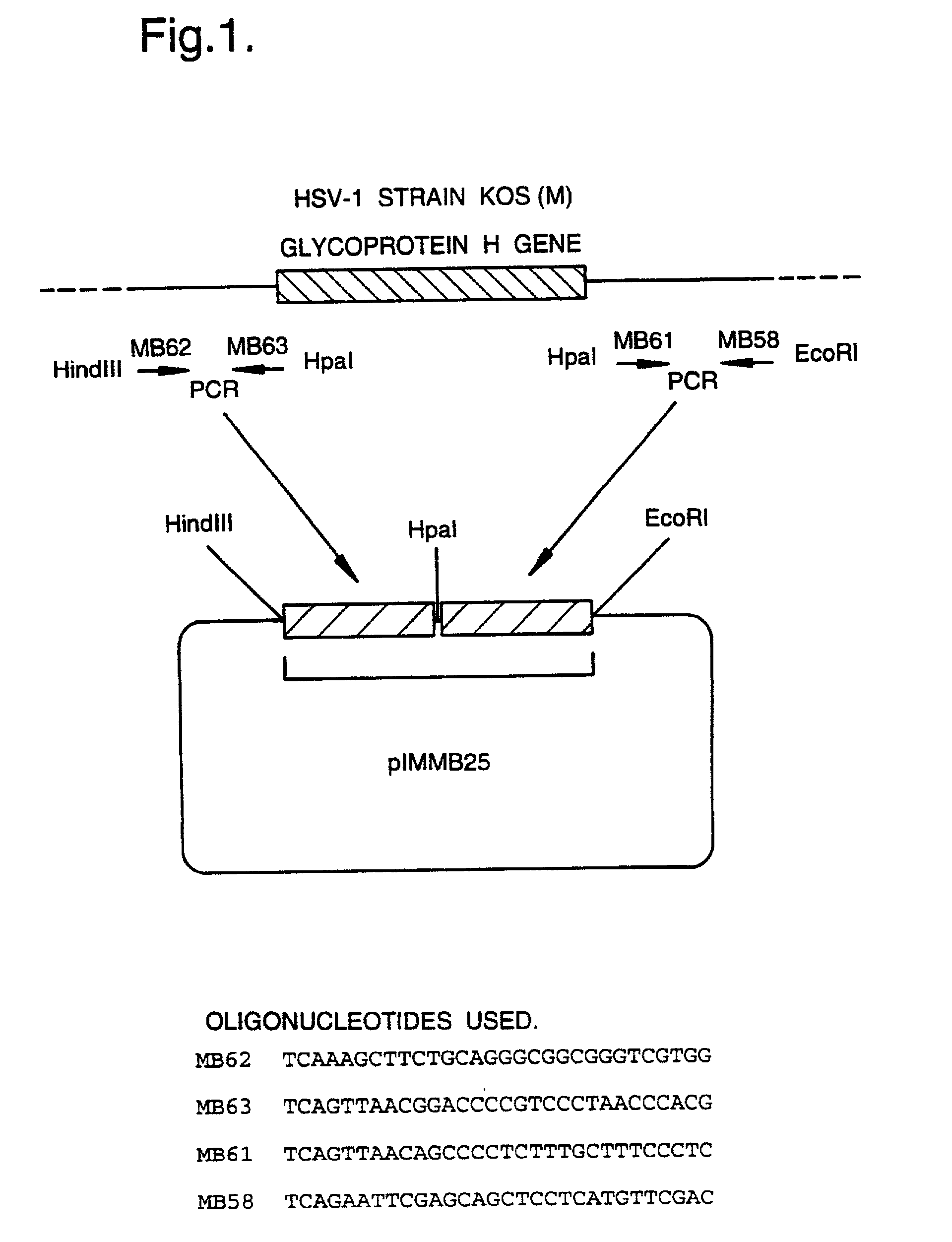
Recombinant virus vectors
InactiveUS6319703B1Risk minimizationReduced zero riskBiocidePeptide/protein ingredientsInfected cellCytopathic effect
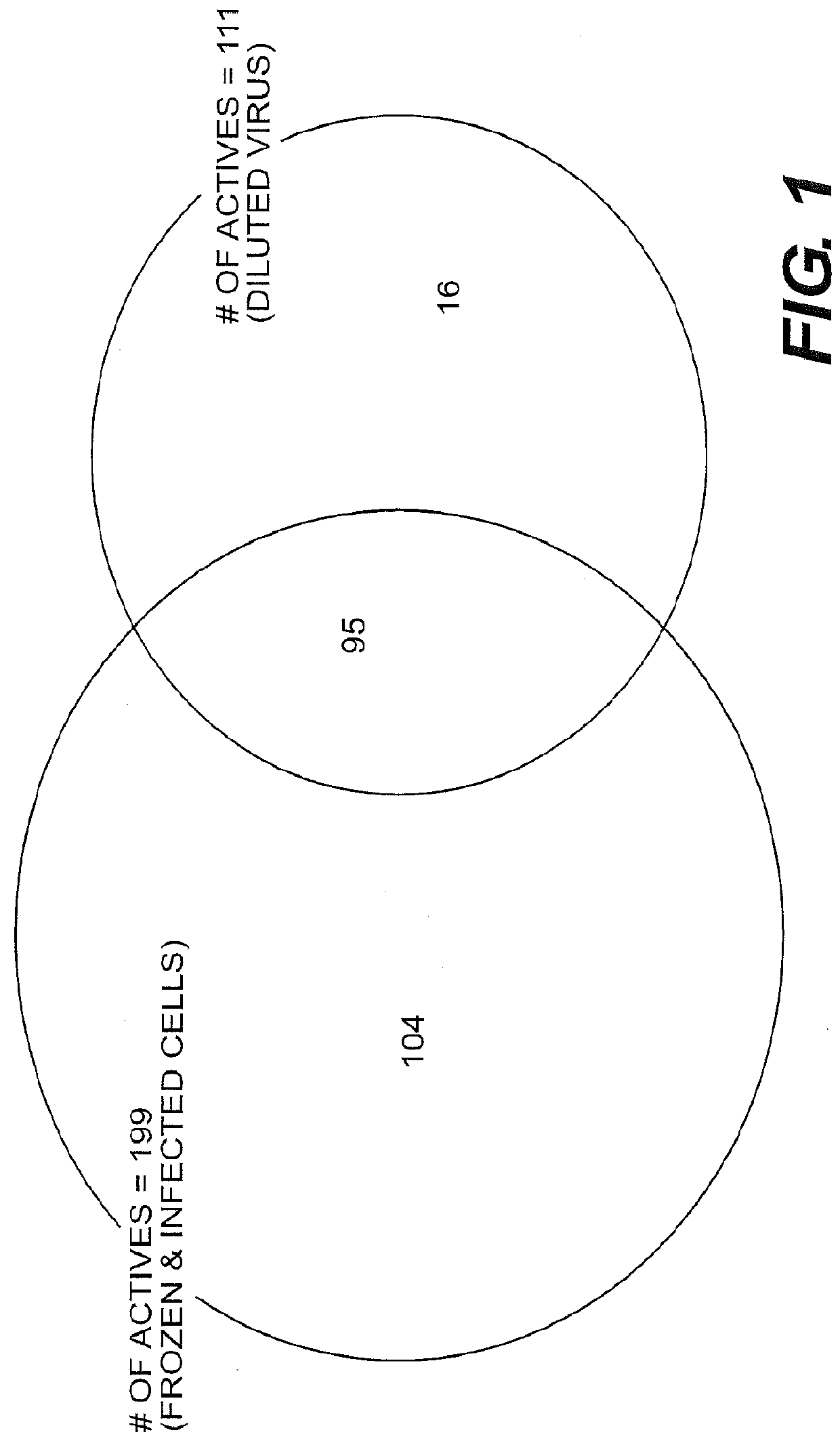
A mutant herpesvirus that can be used a recombinant virus vector includes (a) a mutation such that the mutant virus has a reduced ability in comparison with a parent type to cause lysis of an infected cell, and (b) an inactivating mutation in a gene essential for the production of infectious virus. An example is a HSV1 mutant lacking the essential glycoprotein gH gene and having a mutation impairing the function of the gene product VP16. A heterologous gene can be carried at the site of the inactivated essential gene, e.g. a gene suitable for administering gene therapy. The vector has an increased margin of safety over known herpesvirus vectors in respect of incidence of cytopathic effects and / or risk of infection.
Owner:SPECK PETER G
Triple vaccine of pig transmissible gastroenteritis, pig epidemic diarrhea and pig rotavirus
InactiveCN101491673AAvoid pollutionDoes not destroy nutrientsViral antigen ingredientsDigestive systemDiseaseCytopathic effect
The invention provides a method for preparing triple vaccine for preventing porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus. The method comprises the following steps: inoculating a host-cell line with a 90 percent grown monostratum against a porcine transmissible gastroenteritis virus, a porcine epidemic diarrhea virus and a porcine rotavirus respectively, and adding a cell maintenance media into the host-cell lines respectively to be cultured at 37 DEG C; after cytopathic effect reaches over 75 percent, collecting viruses to be stored at 20 DEG C below zero for standby; mixing the viruses according to 10 TCID50 in 1:1:1, and simultaneously adding Freund's complete adjuvant and immunopotentiator into the mixture to inactivate the mixture by formaldehyde at 37 DEG C for 24 hours; and adding an oil adjuvant into the mixture to prepare a vaccine of water-oil-water preparation. The method can be used for preparing the triple vaccine for preventing the porcine transmissible gastroenteritis, the porcine epidemic diarrhea and the porcine rotavirus so as to solve the problem that the diseases do not have an effective medicine to treat currently.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Fusogenic, self-propagating blebs as immunogenic compositions
InactiveUS20070128222A1Avoid infectionReduce severitySsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
Method for industrially producing pseudorabies vaccine by using bioreactor
InactiveCN102038946AHigh titerHigh degree of automation controlMicroorganism based processesAntiviralsSide effectCytopathic effect
The invention relates to a method for industrially producing a pseudorabies vaccine by using a bioreactor. The method comprises the following steps of: (1) sterilizing a microcarrier and the bioreactor, inoculating cells for vaccine production, culturing the cells, and after the cells on the microcarrier form a compact monolayer, inoculating pseudorabies virus, and continuously culturing to allow the virus to reproduce; (2) after more than 80 percent of cells are subjected to a cytopathic effect, stopping culturing, and obtaining virus liquid to prepare the pseudorabies vaccine. In the method, the pseudorabies vaccine is produced by culturing the cells in high density by the bioreactor microcarrier culture technology; and compared with the conventional roller bottle production process, the invention has the advantages that: the method has high automation control degree, saves manpower, reduces cost and is small in production land, the production can be monitored in real time, the scale is easy to expand, the produced virus has high titer and small difference among batches, and the product has stable quality and small side effect.
Owner:WUHAN CHOPPER BIOLOGY
Compositions and methods of use of phorbol esters
Methods and compositions containing a phorbol ester or a derivative of a phorbol ester are provided for the treatment of cytopathic diseases. Cytopathic diseases may be caused by a variety means suchas viral infections like HIV and AIDS, or the development of neoplasms in a mammalian subject. The methods and compositions of the invention are effective for inhibiting de novo HIV infection, upregulating viral expression from latent provirus, inhibiting HIV-induced cytopathic effects, down regulating the HIV receptor, increasing ThI cytokine expression, decreasing Th2 cytokine expression, increasing ERK phosphorylation, inducing apoptosis in malignant cells, inducing remission, maintaining remission, as chemotherapeutic agents, as well as for decreasing symptoms of cytopathic diseases and opportunistic infections that may accompany such diseasesl. Additional compositions and methods are provided which employ a phorbol ester or derivative compound in combination with at least one additional agent such as those used in HAART protocols, therapeutic agents used to treat opportunistic infections due to HIV, or chemotherapeutic agents to yield more effective treatment tools against cytopathic diseases in mammalian subjects.
Owner:BIOSUCCESS BIOTECH CO LTD
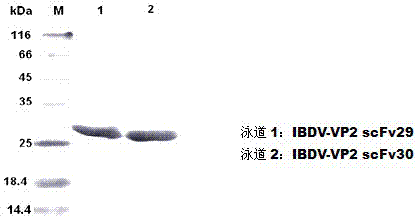
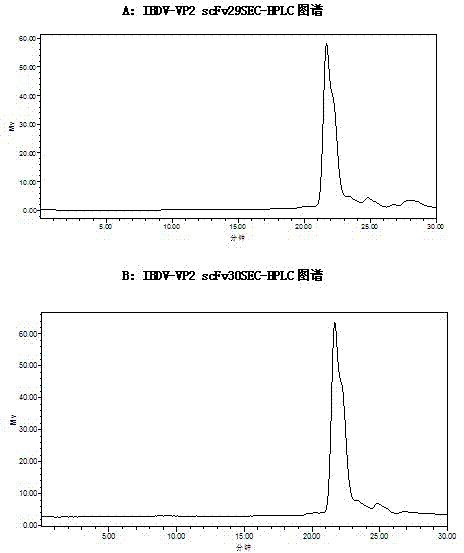
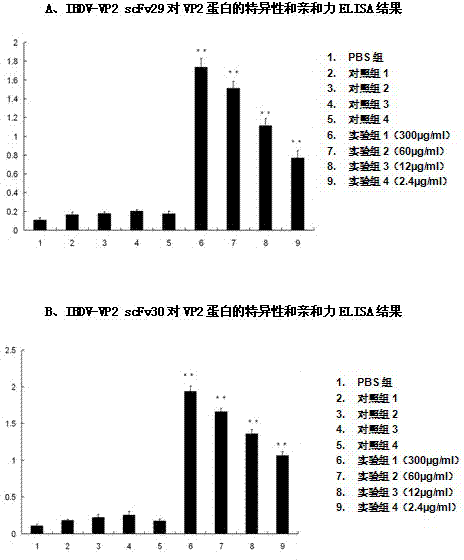
Two ScFv (Single Chain Variable Fragment ) antibodies, encoding genes and application thereof for preparing preparation for treating or preventing infectious bursal disease of chicken
InactiveCN103483449AStrong specificityGood treatment effectBacteriaImmunoglobulins against virusesAnimal virusDisease
The invention discloses two types of ScFv (Single Chain Variable Fragment) antibodies, encoding genes of the antibodies and applications of the antibodies for preparing the preparations for treating or preventing infectious bursal disease of chicken. Two single-chain antibodies (namely, the ScFv antibodies) are provided; and the ScFv antibodies can be specially bound with the protein 2 (VP2) (Virus Protein 2) in an IBDV (Infectious Bursal Disease Virus) structure and a plurality of IBDV strains to block the cytopathic effect (CPE) of the chicken embryo fibroblast by the IBDV so as to protect the young chicken infected with IBDV. The immune serum and egg yolk antibody are tedious in preparation, high in production cost, unstable in effect, difficult to control industrialized production quality, capable of causing horizontal disease spread and the like in a use process; the ScFv antibodies disclosed by the invention can overcome the above disadvantages and have the advantages of strong specificity, good treatment effect, controllable industrialized production quality, capability of preventing the horizontal disease spread caused by the egg yolk antibody and the like; and therefore, by virtue of the ScFv antibodies, a new situation is opened up in the IBDV prevention and treatment history, and even in the entire prevention and treatment history for animal virus diseases.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
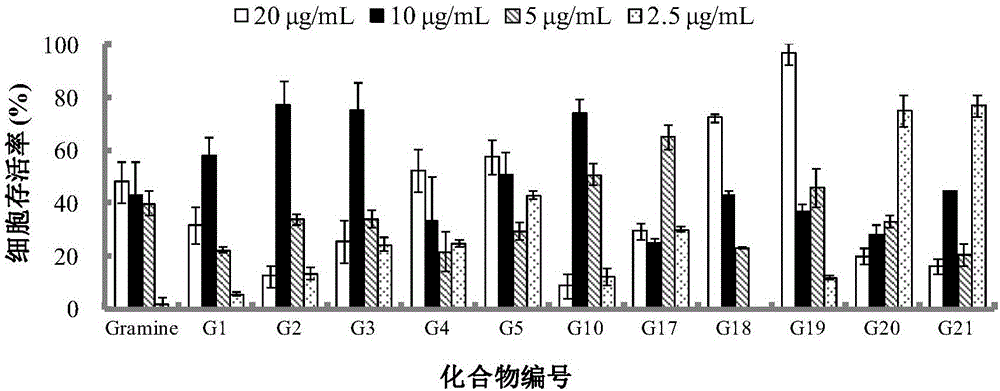
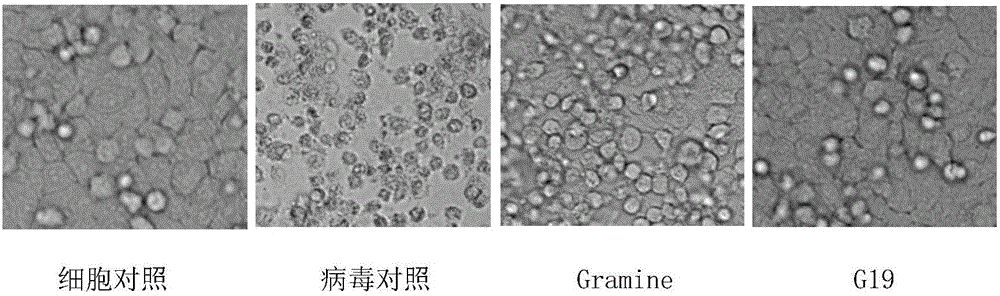
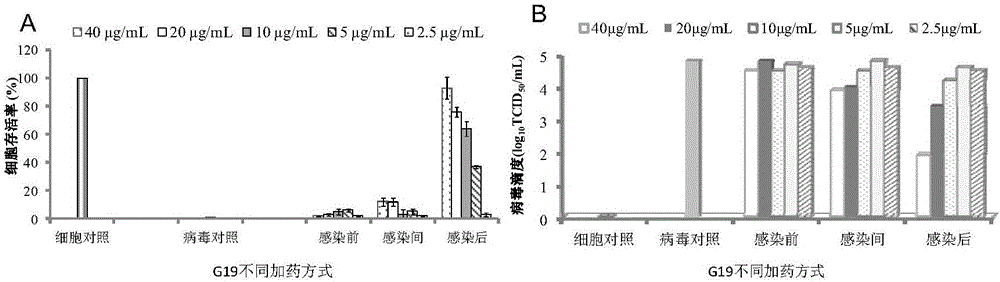
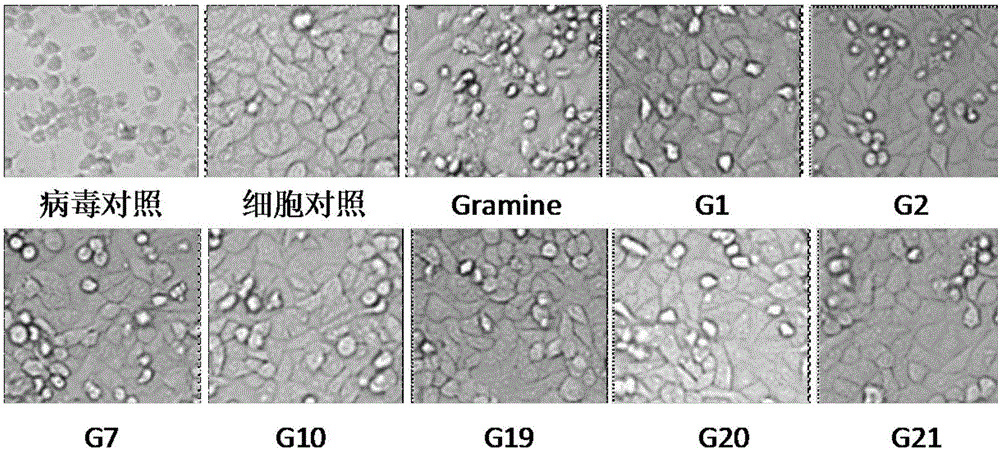
Applications of Gramine and derivatives thereof to preparation of medicaments for resisting adenovirus Type 7
InactiveCN106668002ALow priceEasy to buyOrganic active ingredientsAntiviralsAntiviral drugCytopathic effect
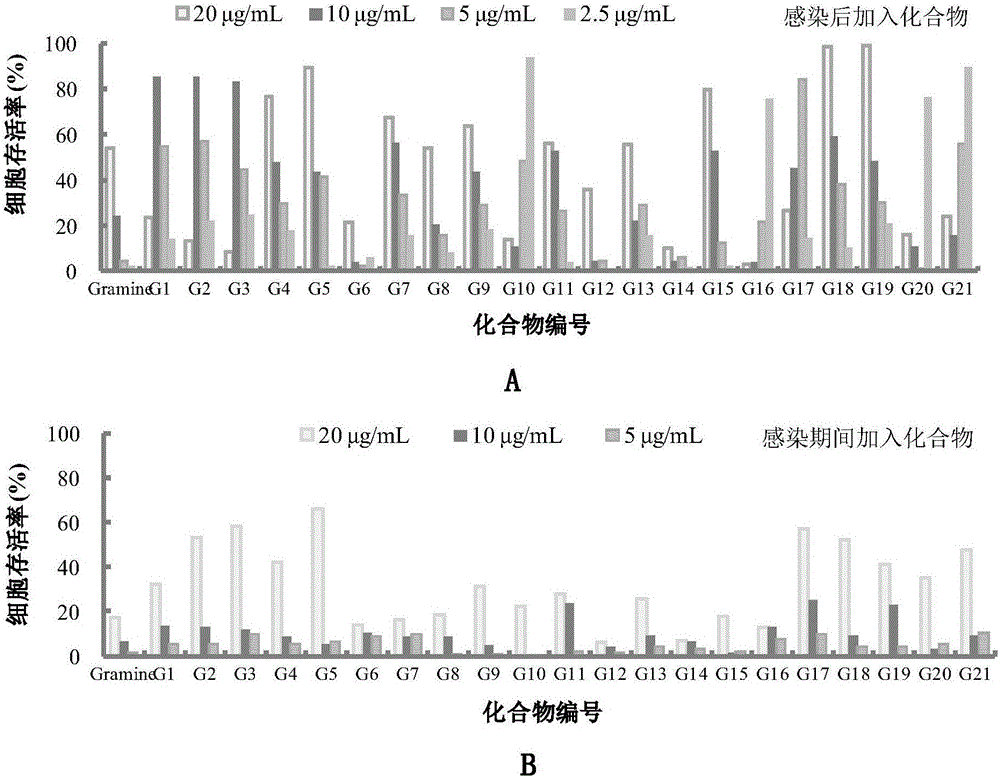
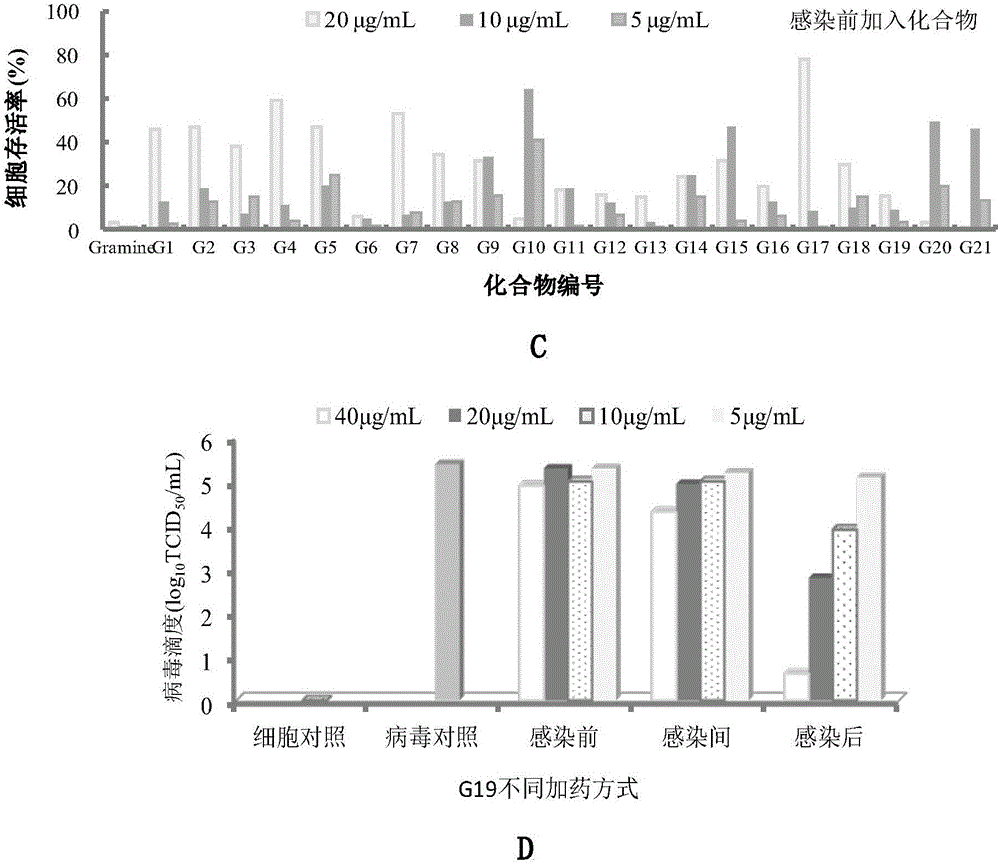
The invention belongs to the field of antiviral drugs. The invention provides applications of Gramine and derivatives thereof to preparation of medicaments for resisting adenovirus Type 7. Gramine derivatives are compounds shown in G1,G2,G3,G4,G5,G10,G17,G18,G19,G20, and G21. Experiments for researching activity of Gramine derivatives for resisting ADV7, pharmacology research of Gramine derivatives for resisting ADV7, and tests of inhibition effects of G19 for ADV7 duplication show that Gramine derivatives can inhibit cytopathic effects (CPE) generated by ADV7 on host cell Hela, enhance cell survival rate, inhibit yield of progeny virus, and mainly inhibit the duplication phase of ADV7 virus in host cells. The Gramine and derivatives thereof have potential for preparing anti-ADV1 virus medicaments, and the compounds have the advantages of simple synthesis process, and economy and fastness; the compounds are easy for large-scale production, and have clinic application prospects.
Owner:HUBEI UNIV OF TECH
Recombinant virus vectors
InactiveUS20020037575A1Free from riskUseful persistenceBiocidePeptide/protein ingredientsHeterologousInfected cell
A mutant herpesvirus that can be used as a recombinant virus vector comprises (a) a mutation such that the mutant virus has a reduced ability in comparison with a parent type to cause lysis of an infected cell, and (b) an inactivating mutation in a gene essential for the production of infectious virus. An example is a HSV1 mutant lacking the essential glycoprotein gH gene and having a mutation impairing the function of gene product VP16. A heterologous gene can be carried at the site of the inactivated essential gene, e.g. a gene suitable for administering gene therapy. The vector has an increased margin of safety over known herpesvirus vectors in respect of incidence of cytopathic effects and / or risk of reversion.
Owner:SPECK PETER G
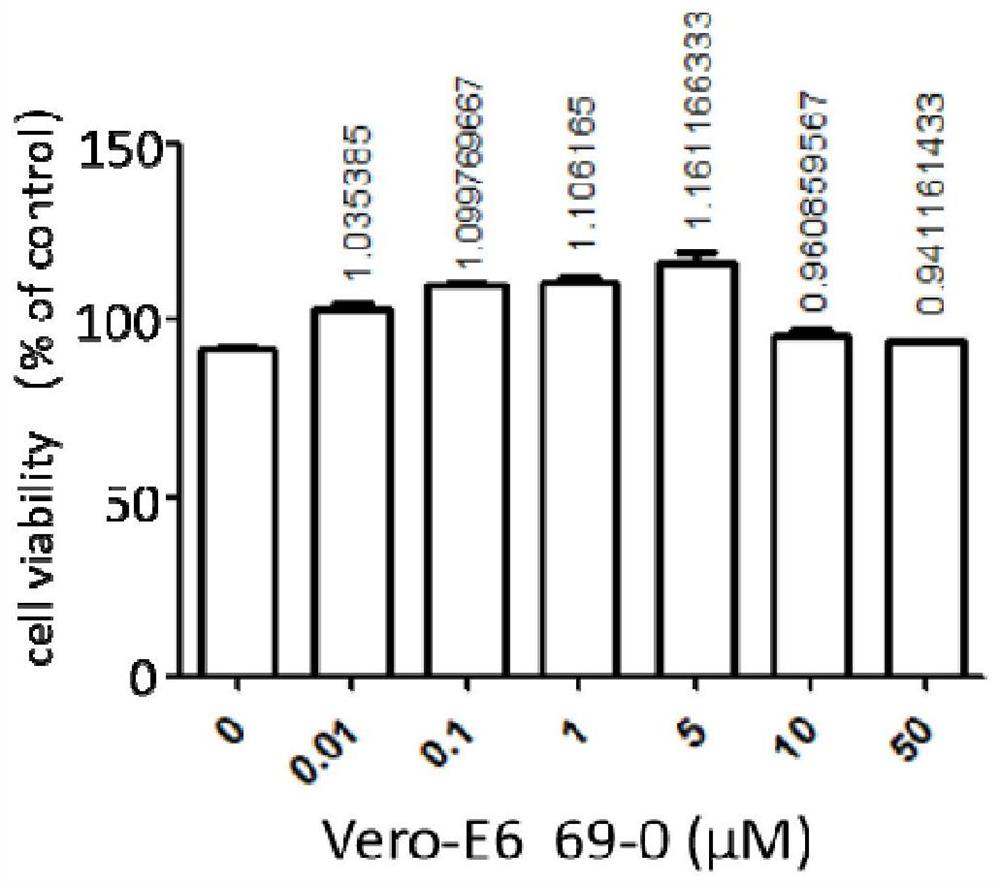
Application of compound to treatment of SARS-CoV-2 infection
InactiveCN111991401AInhibition of replicationHigh activityAntiviralsRespiratory disorderCytopathic effectSide effect
The invention provides an application of a compound 69-0 and / or tautomers and / or pharmaceutically acceptable salts thereof to prevention, alleviation and / or treatment of COVID19 / SARS-CoV-2. Products containing the compounds can effectively inhibit replication or reproduction of SARS-CoV-2 or homologous variant viruses of SARS-CoV-2 and the cytopathic effect produced by SARS-CoV-2. The compound 69-0 has good curative effect, good safety and low toxic and side effects. The invention also provides combined therapeutic pharmaceutical composition. The combined therapeutic pharmaceutical compositioncan effectively inhibit proliferation of SARS-CoV-2 in cells, and has good curative effect, low safety and low toxic and side effects, can reduce drug resistance of viruses and has obvious synergistic effect.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA +1
Binary inactivated vaccine against Japanese encephalitis virus and porcine parvovirus and preparation method thereof
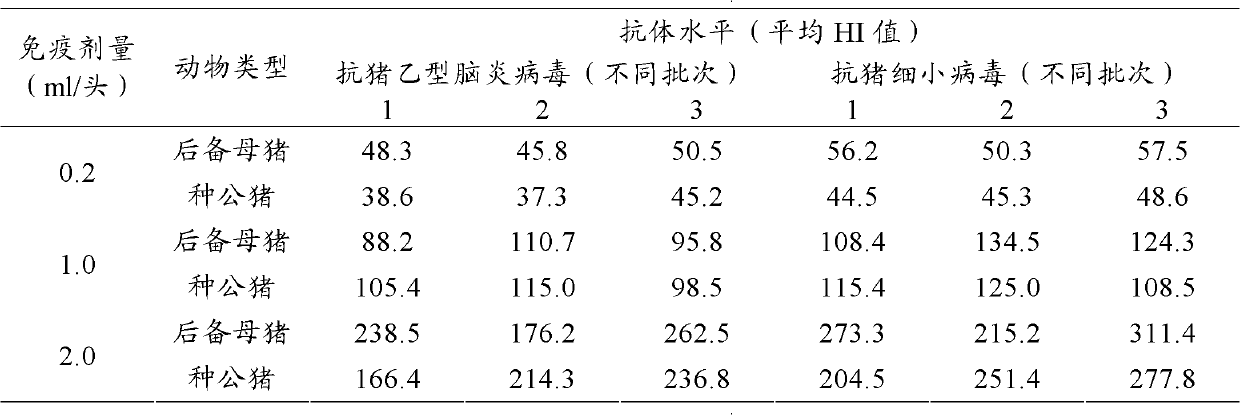
ActiveCN102886043AExcellent shelf lifeImproving immunogenicityViral antigen ingredientsAntiviralsCytopathic changeBiomedical engineering
The invention provides a preparation method of a binary inactivated vaccine against a Japanese encephalitis virus and a porcine parvovirus, which comprises the following steps of: (1) inoculating the animal cells to a microcarrier for performing the absorption culture and multiplication culture processes of the cells in a tidal biological reactor; (2) inoculating the Japanese encephalitis virus and the porcine parvovirus on the cells being subjected to multiplication culture; (3) when the inoculated animal cell CPE (Cytopathic Effect) reaches above 75%, harvesting a viral solution; and (4) freezing and thawing the harvested viral solution twice at minus 20 DEG C, inactivating after the concentration, and adding an emulsifier for emulsification to obtain the binary inactivated vaccine against Japanese encephalitis virus and porcine parvovirus. The binary inactivated vaccine provided by the invention has the advantages of good immunogenicity, stable growth and high titer of the preserved seed virus; and the tidal biological reactor is adopted to respectively culture and harvest two viruses with high titers, and the two viruses are directly emulsified and prepared after the concentration and the inactivation, so that the binary inactivated vaccine can be continuously produced in a large scale.
Owner:PU LIKE BIO ENG
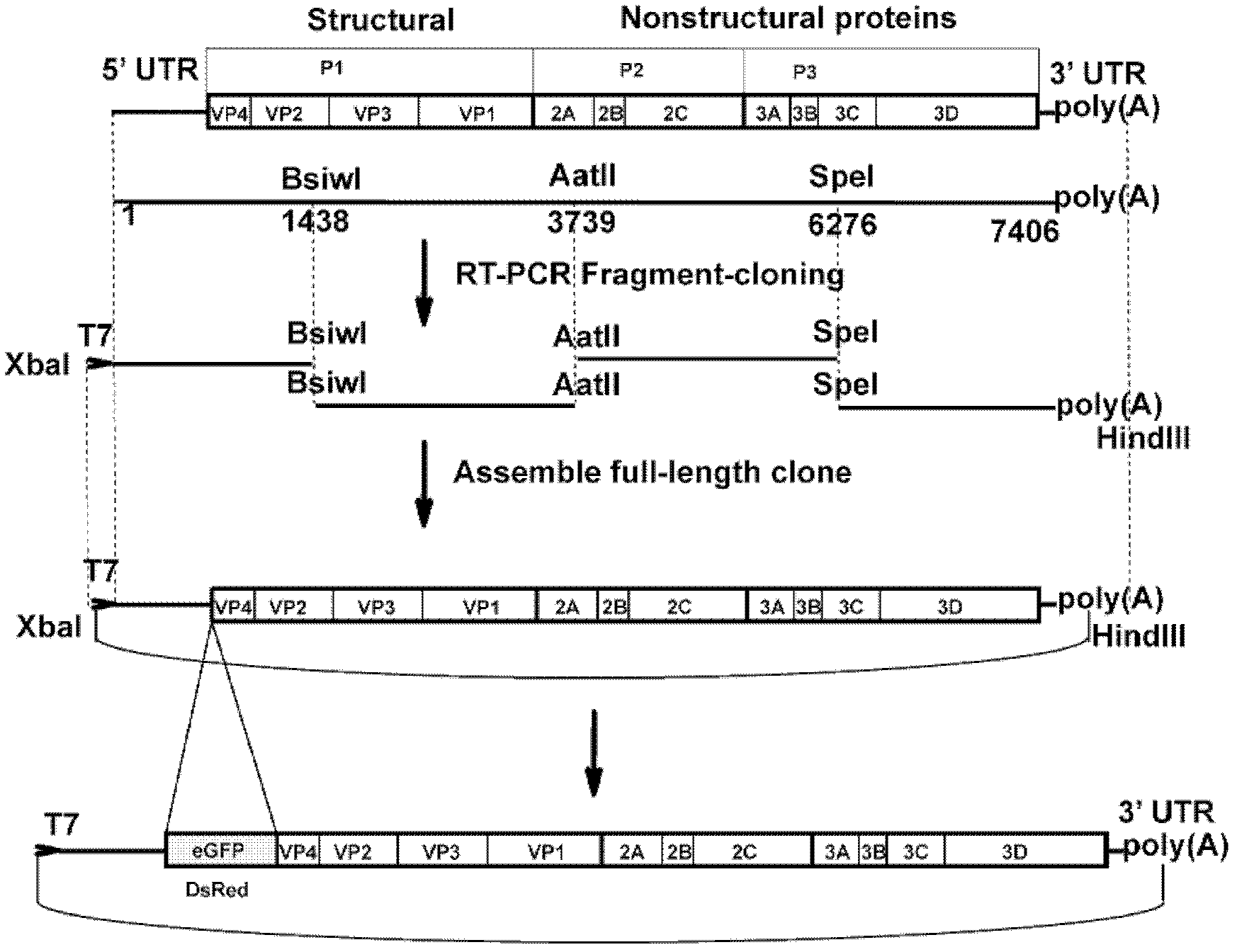
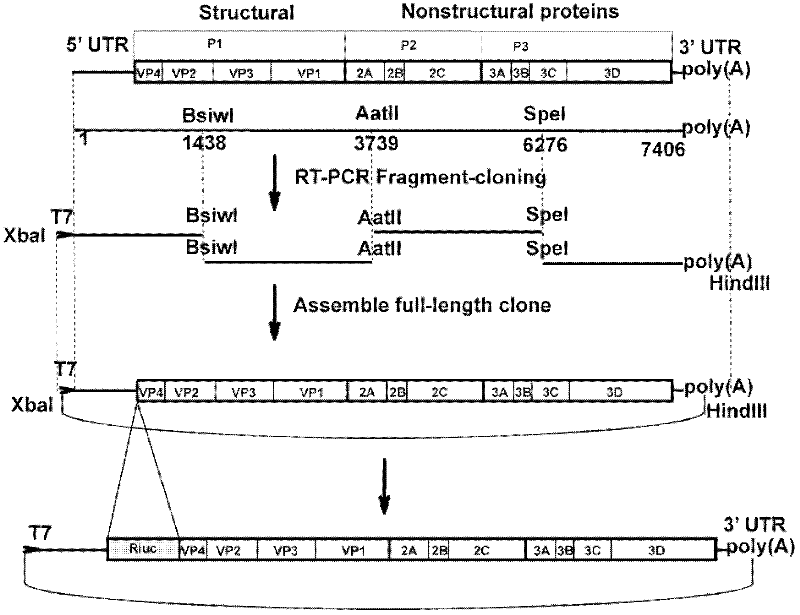
Preparation method and application of enterovirus 71 type full-length infectious clone with tags
InactiveCN102517317AEffective generationEfficiently obtainedMicrobiological testing/measurementViruses/bacteriophagesCytopathic effectTotal rna
The invention discloses a preparation method and application of enterovirus (EV) 71 type full-length infectious clone with tags. The preparation method comprises the following steps of: (1) extracting total RNA; (2) performing reverse transcription-polymerase chain reaction (RT-PCR) amplification on the EV71; (3) constructing EV71 subclone; (4) constructing recombinant clone containing EV71 genes; and (5) constructing the enterovirus 71 type full-length infectious clone with eGFP and DsRed. Tests, such as cytopathic effect, fluorescence detection, gene detection, virus plaque, medicine inhibition and the like, prove that the EV71 full-length infectious clone with the tags is obtained successfully. The invention has high application value in the aspects of research and development of animal models, virus replication and pathogenesis, medicine screening and medicine action mechanism, vaccine and diagnostic reagents and the like.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Cell culture method for duck flavivirus
InactiveCN102220290AStable proliferationObvious cytopathicViruses/bacteriophagesDiseaseCytopathic effect
The invention provides a cell culture method for duck flavivirus. The method comprises the following steps: inoculating to attach an anchorage-dependent kidney cell BHK21 of a baby hamster to the surface of a cell culture container to form a monolayer; inoculating the monolayer with virus; culturing the monolayer inoculated with the virus in an incubator with 5% CO2 at 37 DEG C; and continuously passing by taking a cell culture product as an inoculum for the next passage. According to the cell culture method provided by the invention, after the virus is continuously passed for 5-7 generations on the monolayer, stable multiplication can be obtained; and after the cell is inoculated for 3-4 days, an obvious cytopathic effect can be formed. The method provides a convenient and visual virus operating platform for studying the biological characteristics of the virus and the molecular basis of virus pathopoiesia and for the vaccine research developed for effectively preventing and controlling the disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Fusogenic, self-propagating blebs as immunogenic compositions
InactiveUS7541038B2SsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
TK, ge and gT gene deletion strain of porcine pseudorabies virus variant and application of TK, ge and gT gene deletion strain
InactiveCN106834236AFight infectionNo reverse mutationVirus peptidesMicroorganism based processesCytopathic effectVariant strain
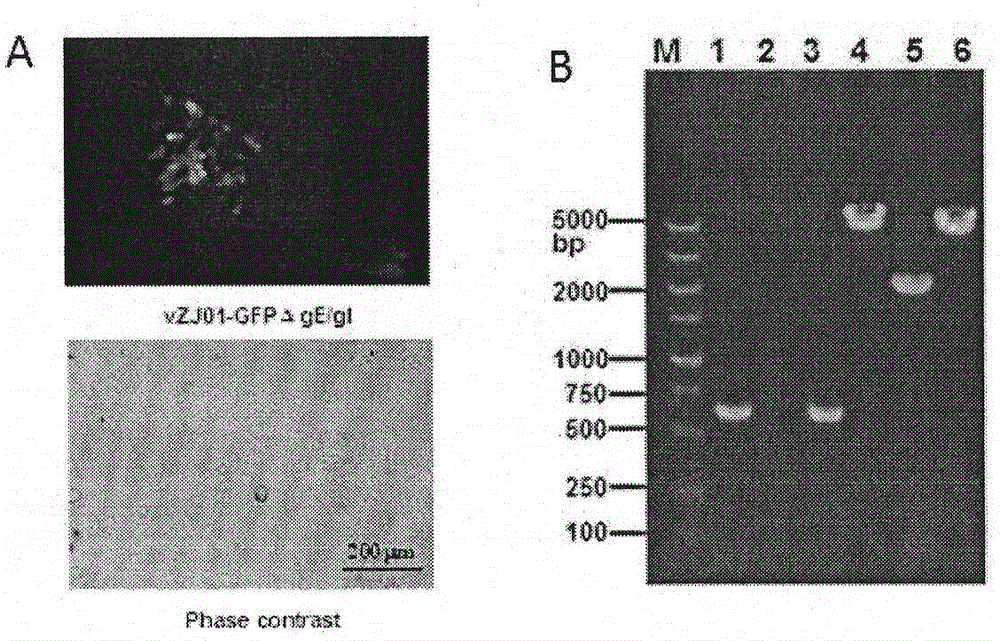
The invention discloses a TK, ge and gT gene deletion strain of a porcine pseudorabies virus (PRV) variant ZJ01 and application of the TK / ge / gT gene deletion strain. The strain is the TK / ge / gT gene deletion strain ZJ01delta TK / gE / gI (ZJ01R in short) of the PRV ZJ01 strain. According to the in-vitro passage test results, gE / gI gene deletion strains ZJ01delta gE / gI of a recombinant virus ZJ01R and the PRV ZJ01 strain are consistent with cytopathic effect generated by a parental virus ZJ01, and virus titer is similar with the ZJ01 strain; a target gene deletion region of the recombinant virus ZJ01R continuously and stably exists without resilience mutation; according to the pig immunity and attacking protection test, after the recombinant virus ZJ01R is immune, a pig is free of clinical symptoms and pathological changes; after being immunized by 21 days, the pig can generate a PRV neutralizing antibody with relatively high level, and can resist challenging infection of a fatal PRV variant strain ZJ01.
Owner:NANJING AGRICULTURAL UNIVERSITY
Construction method of SOD (superoxide dismutase) gene modified mesenchymal stem cell and application thereof
InactiveCN102660580AHigh activityConducive to survivalPeptide/protein ingredientsGenetic material ingredientsCytopathic effectSuperoxide dismutases
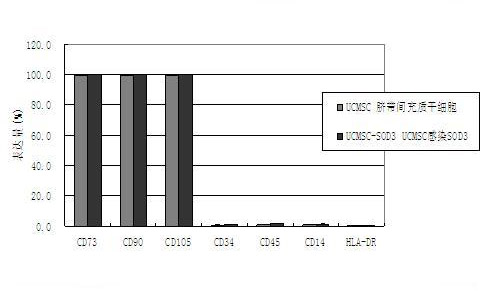
The invention provides a construction method of a SOD (superoxide dismutase) gene modified mesenchymal stem cell. The construction method comprises the following steps: (A) connecting an SOD3 gene to an adenovirus vector to obtain a recombinant adenovirus vector; B) co-transfecting the recombinant adenovirus vector and a packaging plasmid to a 293 cell line by utilizing a transfecting agent, culturing until the 293 cell line completely generates a cytopathic effect, collecting an adenovirus strain, storing the strain and confirming that the SOD3 gene is connected to the adenovirus vector; C) amplifying the adenovirus strain, infecting the 293 cell line, collecting the adenovirus supernatant, and determining the titer after ultrafiltration, purification and concentration; and D) infecting the mesenchymal stem cell in different conditions respectively by the adenovirus, and determining the best infection condition to obtain the SOD gene modified mesenchymal stem cell. The EC-SOD (extracellular superoxide dismutase) gene over-expressed SOD gene modified mesenchymal stem cell can be prepared by the method, and can be applied to treatment of mouse with radiation so as to prolong the survival period of the radiated mouse.
Owner:SHENZHEN BEIKE BIOTECH
Methods for treating viral conditions
InactiveUS20130171103A1Infection controlProlongation of the cell cycleBiocideOrganic chemistryCytopathic effectViral replication
Compounds that selectively inhibit viral replication or the production of viral RNA or DNA, viral protein or virus induced cytopathic effects and compositions comprising such Compounds are described. Also described are methods of inhibiting viral replication or the production of viral RNA or DNA, viral protein or virus induced cytopathic effects using such Compounds and methods for treating viral infections involving the administration of such Compounds. The Compounds may be administered as a single agent therapy or in combination with one or more additional therapies to a human in need of such treatments.
Owner:PTC THERAPEUTICS INC
Method for building 7-day-old mouse model infected with enterovirus 71
The invention discloses a method for building a 7-day-old mouse model infected with enterovirus (EV) 71, comprising the following steps: taking 7-day-old suckling mice and injecting liquid virus into the brains of the mice, thus obtaining the mouse model infected with EV 71. The injection volume of the liquid virus is 20mu L and the liquid virus is prepared by the following steps: inoculating the EV71 seed into MA104 cells in terms of inoculation amount of 200mu L / 100mL cell culture bottle, culturing the MA104 cells in 5% of CO2 at 37 DEG C and observing the cytopathic effect every day; on the second day after inoculating EV71, observing that about 70% of the MA104 cells are agglutinated and crimpled, freeze-thawing the cells in the whole bottle and then centrifuging the cells and taking the supernatant, thus obtaining the liquid EV71. The method successfully builds the stable 7-day-old mouse model infected with EV71, provides foundations for the scientific research personnel to research the EV71 etiology and pathogenic mechanism and for screening efficient antivirus drugs and developing vaccines and has broad application prospect.
Owner:INST OF BASIC MEDICINE OF SAMS
Preparation and application of efficient cell inactivation vaccine against new duck reovirus disease
PendingCN105854011ARaise antibody levelsFight infectionViral antigen ingredientsAntiviralsDiseaseAntigen
The invention relates to preparation and application of an efficient cell inactivation vaccine against new duck reovirus disease. The preparation method comprises the following steps: an antigen preparation step: inoculating a virus NDRV-TH11 strain with immortalized duck embryo fibroblast to obtain cytotoxicity with 80% of cytopathic effect, and performing cryopreservation at -20 DEG C; and a vaccine preparation step: inactivating the obtained cytotoxicity and mixing with a homemade nano adjuvant and stirring uniformly to obtain the efficient cell inactivation vaccine against new duck reovirus disease. After the duck is immunized by the efficient cell inactivation vaccine against new duck reovirus disease prepared in the invention, the duck can effectively resist the attack of a virulent strain of the new duck reovirus.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Application of andrographolide C15 substitution derivative in manufacturing anti-hepatitis drug
ActiveCN102600129ATo clarify the anti-BVDV activity in vitroOrganic active ingredientsDigestive systemBovine Viral Diarrhea VirusesCytopathic effect
The invention discloses the application of an andrographolide C15 substitution derivative in manufacturing an anti-hepatitis drug, which belongs to the technical field of pharmaceutical chemistry. According to the invention, MDBK (mardin-darby bovine kidney cells) and BVDV (bovine viral diarrhea virus) are utilized to study the inhibitory action of the compound on cytopathic effect caused by the virus through an external screening model. Through the screening of a large number of andrographolide derivatives, the compound with a structure of general formula 1 is found to significantly inhibit MDBK cytopathic effect caused by the BVDV; the andrographolide C15 substitution derivative has high efficiency and low toxicity, thereby being used for manufacturing medicaments for treating and preventing hepatitis and having good prospect in development and application. The general formula 1 is shown in the description.
Owner:ZHENGZHOU UNIV
Application of two nitrogen-containing heterocyclic ester compounds to preparation of drugs with resistance to enterovirus 71
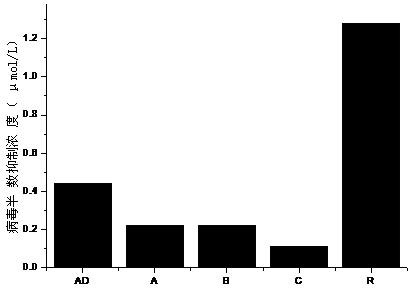
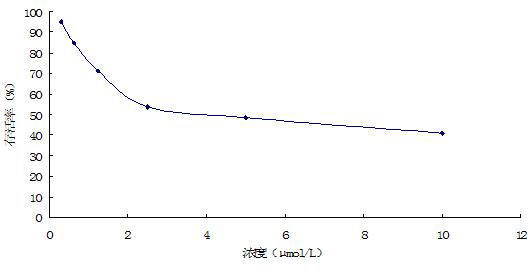
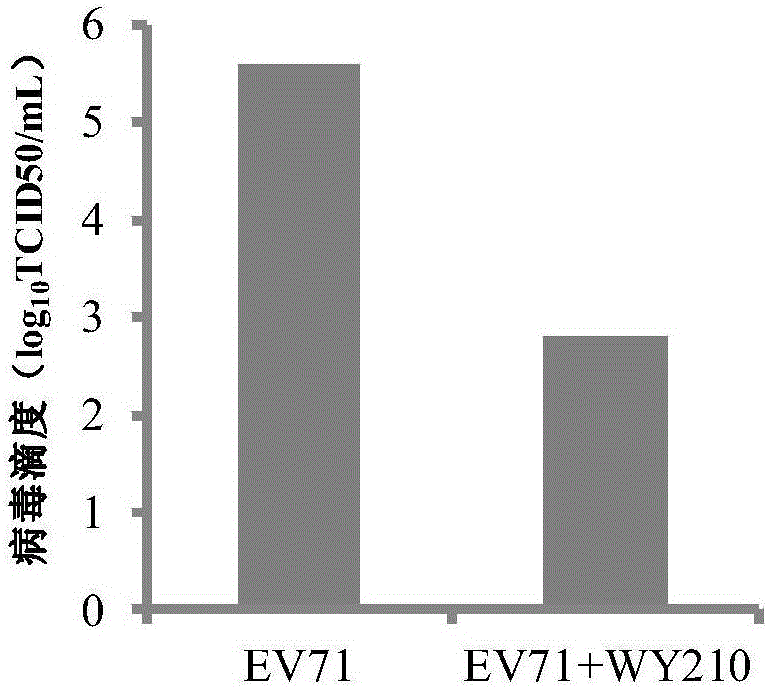
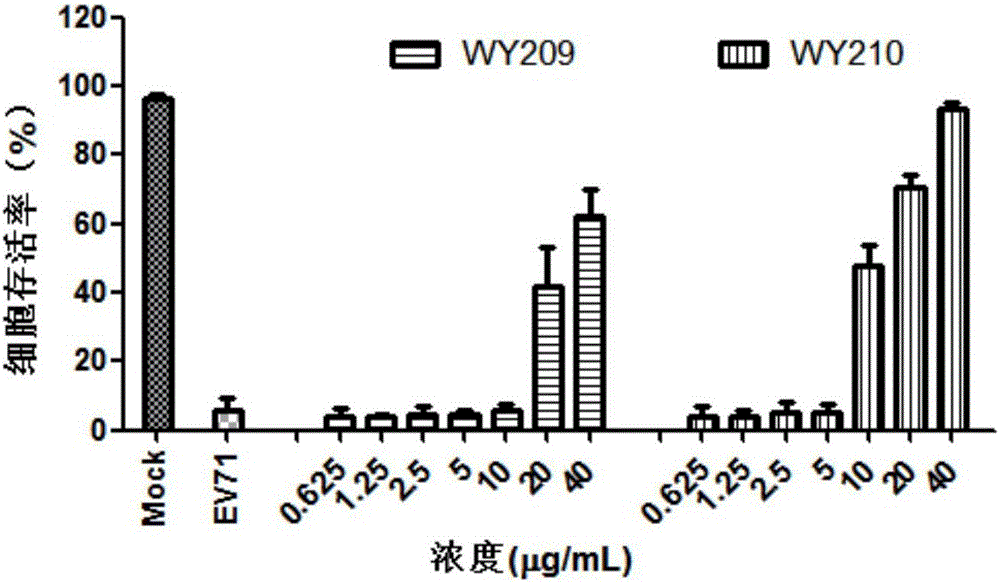
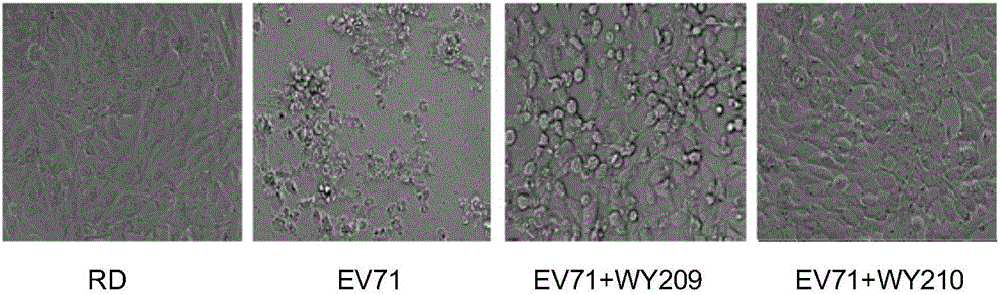
ActiveCN106822120ALow priceEasy to buyOrganic active ingredientsAntiviralsAntiviral drugCytopathic effect
The invention belongs to the field of antiviral drugs and provides application of two nitrogen-containing heterocyclic ester compounds to preparation of drugs with resistance to enterovirus 71 (EV71). The nitrogen-containing heterocyclic ester compounds are compounds expressed as formulas WY209 and WY210. Through research experiment of inhibition of novel nitrogen-containing heterocyclic ester compounds on activity of EV71, WY209 and WY210 can inhibit cytopathic effect (CPE) generated by EV71 in host cells RD; the cell survival rate is increased; the progeny virus yield is reduced; the apoptosis of the host cells caused by infection of EV71 can be inhibited; the application shows that the nitrogen-containing heterocyclic ester compounds have potentials for preparing the drugs with resistance to the EV71 virus; the synthesis process of the compounds is simple, economical and rapid; the large-scale production of the compounds is easy to implement; the compounds have clinical application prospects.
Owner:HUBEI UNIV OF TECH
Attenuation of encephalitogenic alphavirus and uses thereof
InactiveUS20100015179A1SsRNA viruses positive-senseViral antigen ingredientsUltrasound attenuationCytopathic effect
The present invention is drawn to generating attenuated and less cytopathic forms of New World alphaviruses that can be used in immunogenic compositions as vaccines against both Old and New World alphaviruses. In this regard, the present invention discloses that the N-terminal, ˜35-aa-long peptide of VEEV, EEEV and, most likely, of WEEV capsid proteins plays the most critical role in the downregulation of cellular transcription and development of cytopathic effect. The identified, VEEV-specific peptide, CVEE30-68, includes two domains with distinguished functions. The integrity of both domains determines not only the intracellular distribution of CVEE, but is also essential for direct capsid function in the inhibition of transcription. The replacement of the N-terminal fragment of CVEE by its SINV-specific counterpart in VEEV TC-83 genome does not affect virus replication in vitro, but makes it less cytopathic and more attenuated in vivo.
Owner:FROLOV ILYA V +2
Gramine analogue with heterocyclic structure and application of gramine analogue with heterocyclic structure to preparation of anti-CVB3 virus medicines
InactiveCN105748474ALow priceEasy to buyOrganic active ingredientsAntiviralsCytopathic effectViral nucleic acid
Owner:HUBEI UNIV OF TECH
Pharmaceutical composition for treatment of cancers
ActiveUS20050031594A1Shrink tumorEffective treatmentBiocideSsRNA viruses positive-senseAbnormal tissue growthCytopathic effect
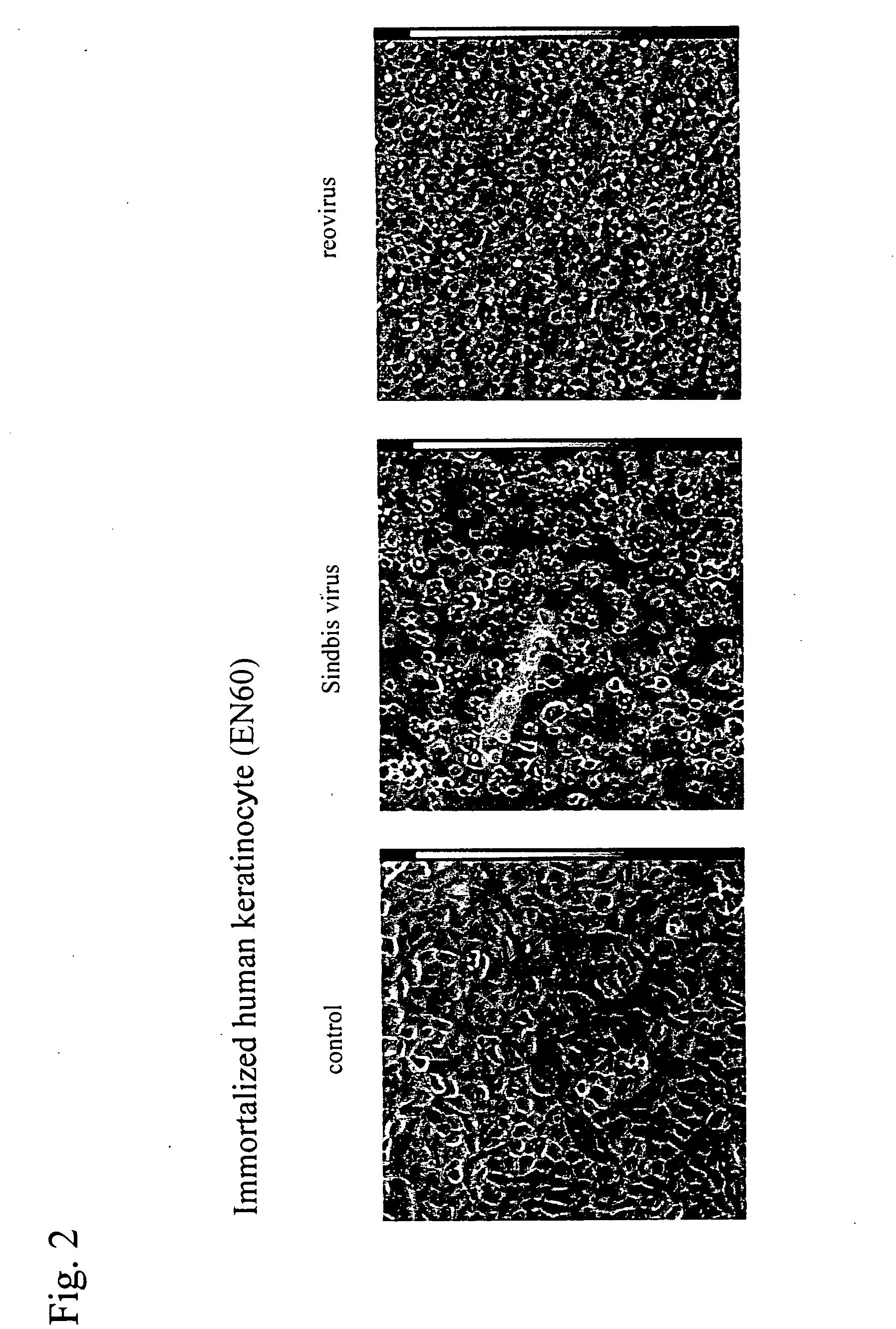
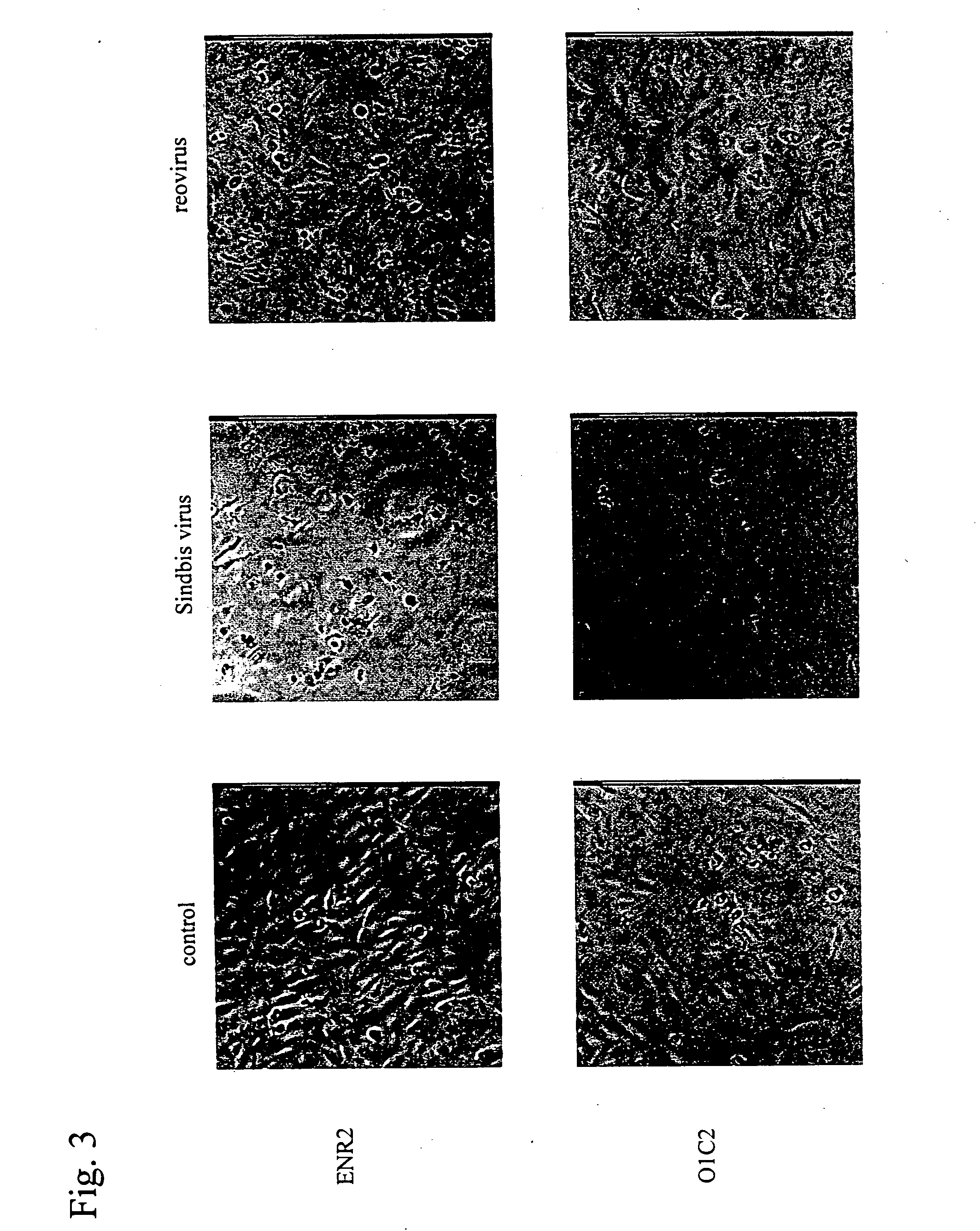
An object of the present invention is to find a new oncolytic virus and to utilize it for treatment of cancers. The present invention relates to a pharmaceutical composition for treatment of cancers containing Sindbis virus as an active ingredient, a method for treatment of cancers, which comprises administering a therapeutically effective amount of Sindbis virus to a mammal having cancers, a method for identifying cancers, which comprises introducing Sindbis virus into an animal, thereby detecting a Sindbis virus protein or Sindbis virus RNA in vital tissues of the above animal, a method of identifying cancers, which comprises introducing into an animal Sindbis virus having a reporter gene incorporated therein, thereby, detecting the product of said reporter gene in vital tissues of said animal, and a method of identifying cancers, which comprises detecting a increase in antibody titer to the Sindbis virus protein or the product of the reporter gene in the aforementioned animals. It is confirmed that Sindbis virus has cytopathic effects upon various cancer cell lines in vitro, and that reduction in tumor size or disappearance of tumor takes place in vivo. Therefore, Sindbis virus is effective for treatment of cancers. In addition, Sindbis virus shows selective proliferation in a tumor, and is effective for identification or diagnosis for cancers within the living body.
Owner:SHINO YUJI +3
Short-chain dehydrogenase CPE (Cytopathic Effect) gene, coding enzyme, carrier, recombination engineering bacteria and application
The invention discloses a short-chain dehydrogenase CPE (Cytopathic Effect) gene, a gene coding enzyme, a carrier, a recombination engineering bacteria and an application of the engine coding enzyme in an asymmetric reductive carbonylation compound. The short-chain dehydrogenase CPE has relatively high conversion rate and e.e. value; and the reductase with relatively low Km value can keep enzyme activity for a longer time under a condition that the concentration of substrate is relatively low; and the quantity of added enzyme can be reduced in the actual industrial production; and the cost can also be reduced; and moreover, the maximum reaction speed can be reached when the substrate with low concentration is added, so that the production period of unit weight products can be reduced in the actual industrial production, and the energy consumption and the labor cost can be decreased.
Owner:HANGZHOU NORMAL UNIVERSITY
Use of pyridoheterocyclic ester compounds in manufacture of anti coxsackievirus B3 drugs
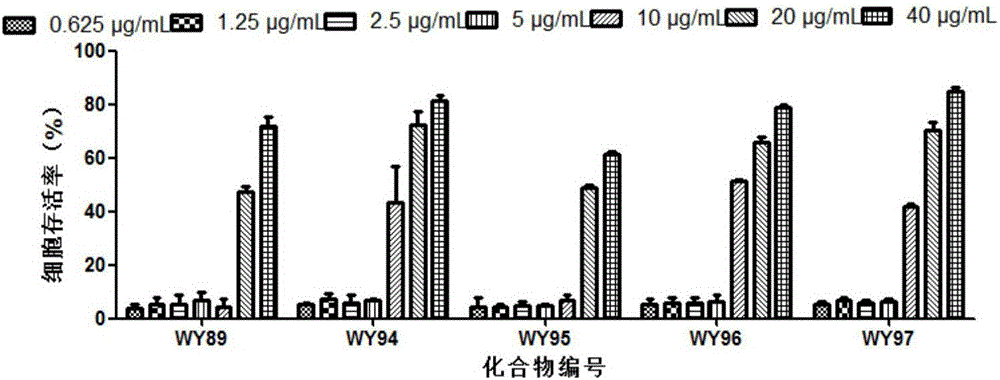
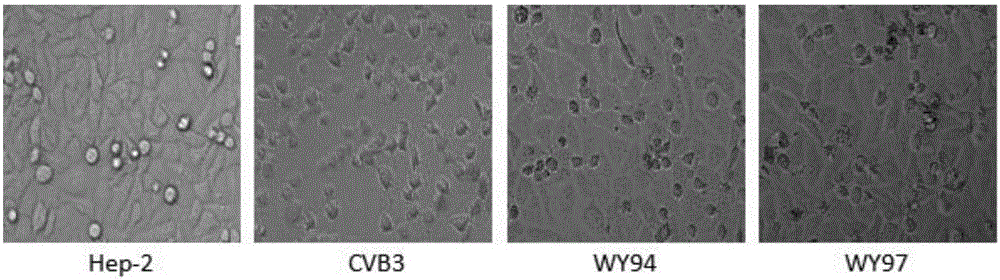
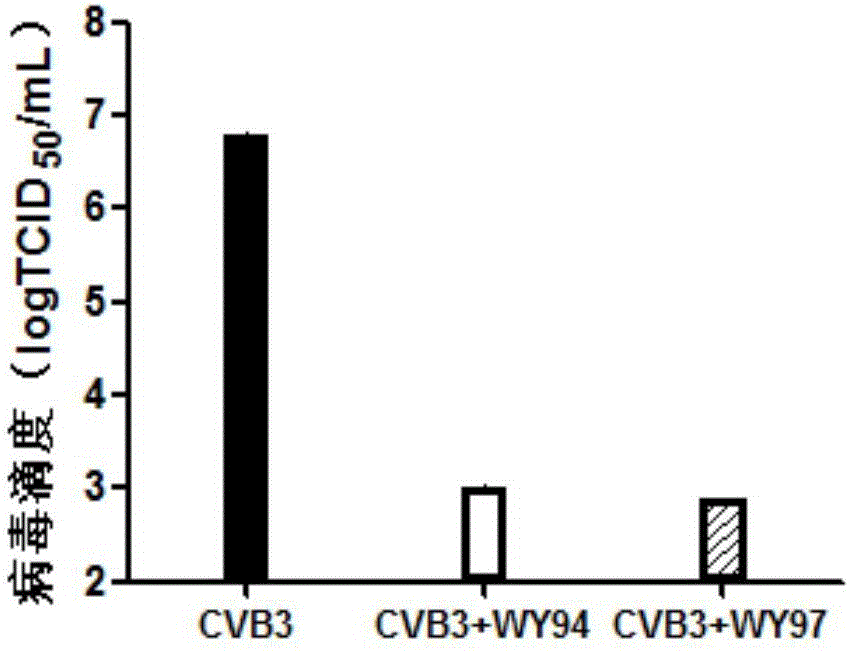
The invention belongs to the technical field of antiviral drugs, and relates to use of novel pyridoheterocyclic ester compounds in manufacture of anti coxsackievirus B3 (CVB3) drugs. The pyridoheterocyclic ester compounds are as shown in formulas WY89, WY94, W95, WY96 and WY97. Through anti CVB3 activity study experiments, the WY89, the WY94, the W95, the WY96 and the WY97 can inhibit cytopathic effect (CPE) produced by CVB3 in host cell Hep-2, can enhance cell viability, reduces progeny virus production, and inhibits of host cell apoptosis caused by CVB3 infection. The anti CVB3 activity study experiments show that the novel pyridoheterocyclic ester compounds have potential in preparation of anti CVB3 infection specific therapeutic drugs, and the novel pyridoheterocyclic ester compounds are simple, rapid and economic in synthesis process and easy in mass production, and have prospects of clinical application.
Owner:HUBEI UNIV OF TECH
Preparation method and application of EV71 type full length infectious clone with luciferase tag
InactiveCN102559754AEffective generationEfficiently obtainedMicrobiological testing/measurementMicroorganism based processesCytopathic effectTotal rna
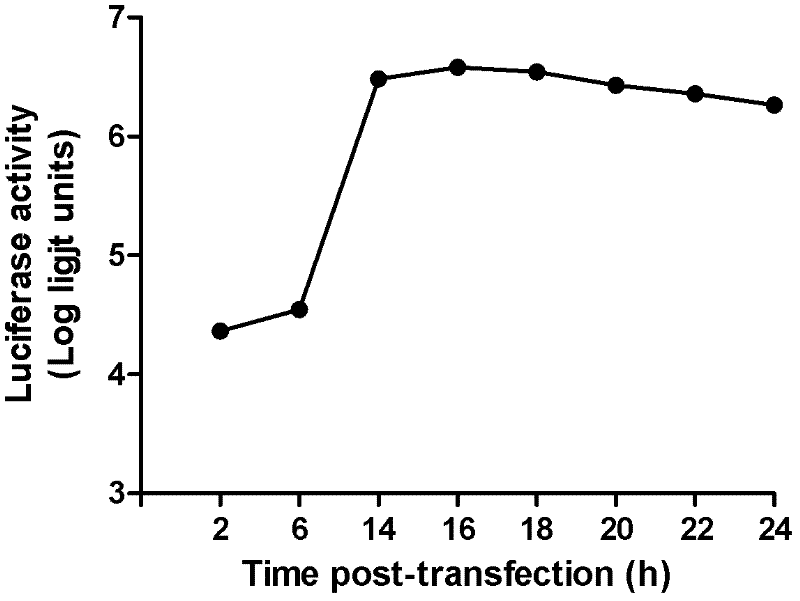
The invention discloses a preparation method and application of EV71 full length infectious clone with a luciferase tag, belonging to the field of biotechnology. The preparation method comprises the steps of: (1) extracting total RNA; (2) amplifying EV71 through RT-PCR; (3) constructing an EV71 subclone; (4) constructing a recombinant clone containing an EV71 gene; and (5) constructing an EV 71 full length infectious clone with a Rluc tag. Experiments of cytopathic effect, Rluc activity detection, virus plaque, drug inhibition and the like show that the EV71 full length infectious clone with a tag is successfully obtained. The invention has wide application values in the aspects of animal models, virus replication and pathogenesis, drug screening and mechanism of drug action, development of vaccines and diagnostic reagents and the like.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
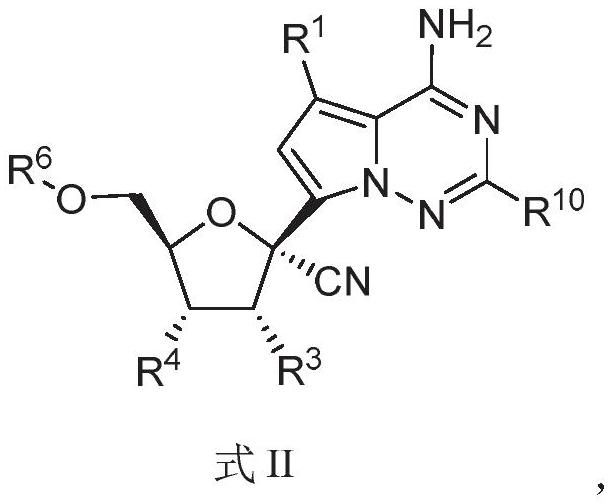
Nucleoside compound and application thereof
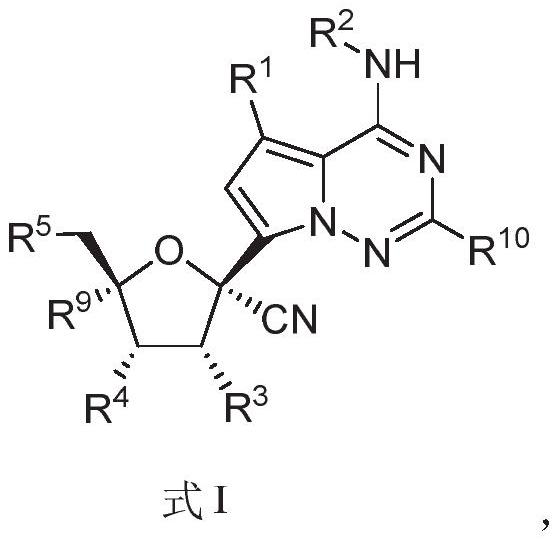
PendingCN114292272AInhibition of replicationAntibacterial agentsOrganic chemistryCytopathic effectVariant virus
The invention relates to a nucleoside compound and application thereof, in particular to a compound shown in a formula I, a prodrug thereof and / or pharmaceutically acceptable salt thereof, and a preparation method, a composition and application thereof. The compound and the composition have the application of preventing, relieving and / or treating coronavirus infection, or replication or reproduction of homologous variant viruses of the coronavirus infection, and cytopathic effects generated by the coronavirus infection or the replication or reproduction of the homologous variant viruses of the coronavirus infection.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA +1
Seneca valley virus SVV-ZM-201801 and application thereof
ActiveCN109554352APromote rapid proliferationHigh titerSsRNA viruses positive-senseViral antigen ingredientsCytopathic effectCulture cell
The invention provides a Seneca valley virus SVV-ZM-201801 and an application thereof. Separation is performed from pig tissues, and sub-culture and plaque purification are performed, so that the Seneca valley virus is obtained. The separated strain can be stably proliferated on sub-culture cells, typical cytopathic effects are formed, and the virus titer is as high as 108.5-1010.5TCID50 / mL. The separated strain does not have obvious pathogenicity on piggies. The Seneca valley virus separated strain has good proliferating properties, and is good in immunogenicity. Vaccines prepared from the separated strain can induce the piggies to generate high-level neutralizing antibodies, and powerful technical support is provided for effective prevention and control of the Seneca valley virus.
Owner:CHINA ANIMAL HUSBANDRY IND
Recombinant virus of chimeric IBV H120 S1 gene ectodomain suitable for cell culture and construction method and application thereof
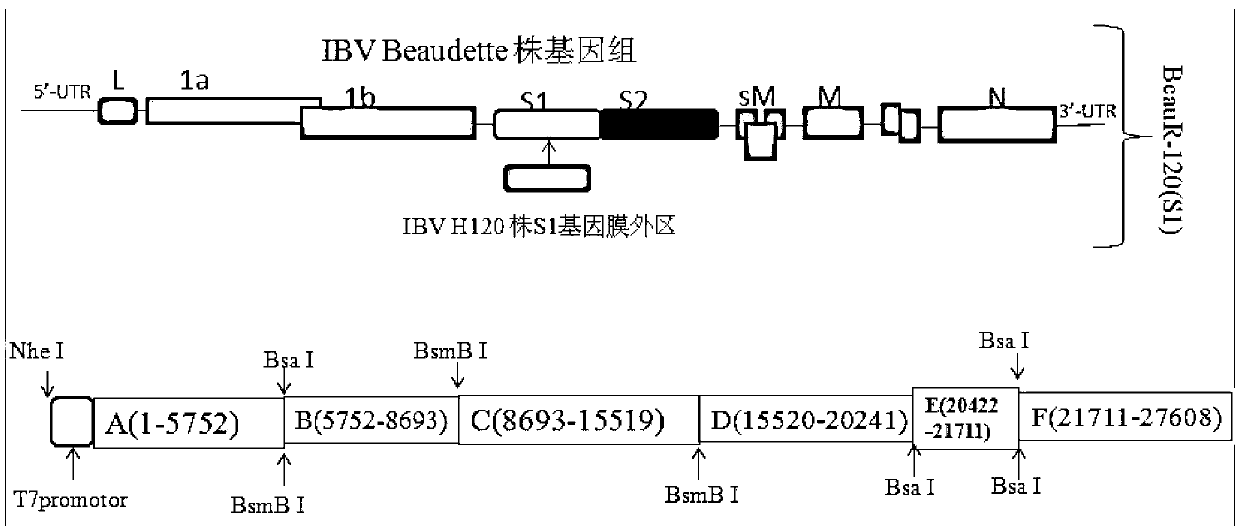
ActiveCN103275939AViral antigen ingredientsMicroorganism based processesCytopathic effectFreeze and thaw
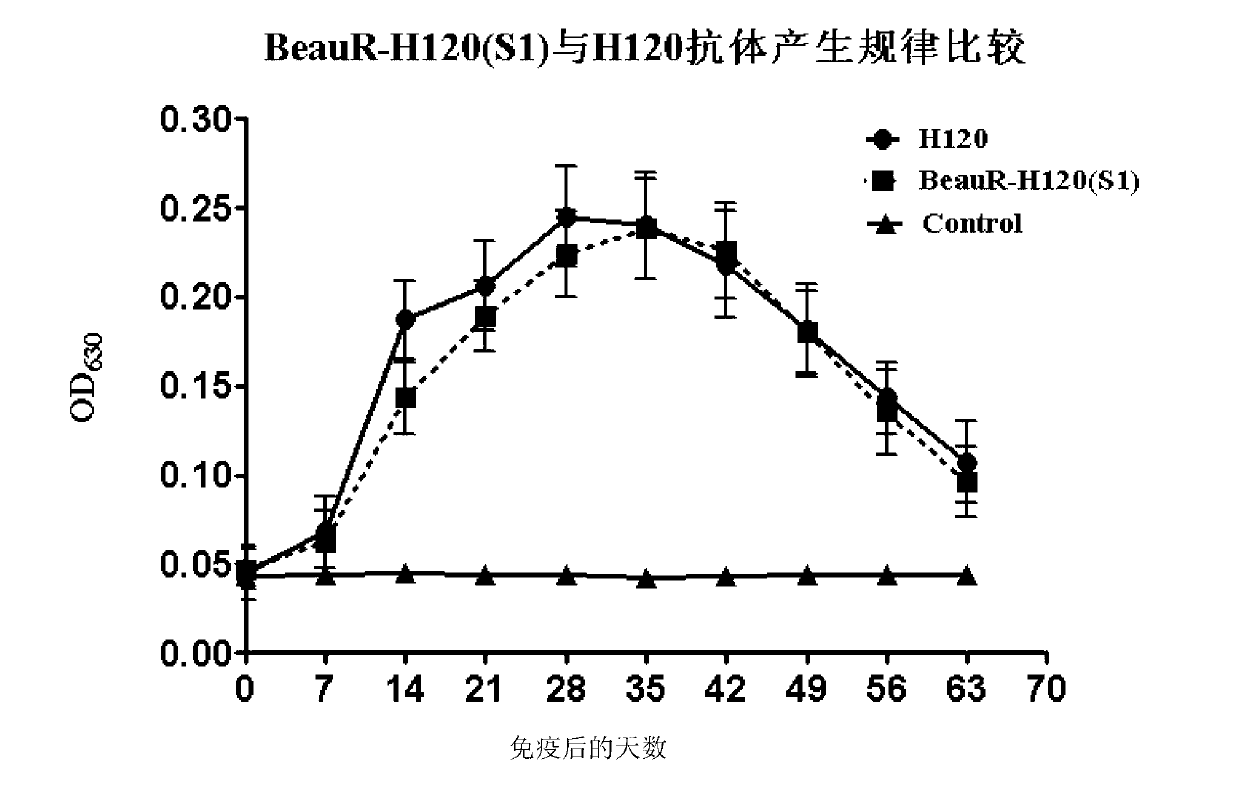
The invention discloses a recombinant virus of a chimeric IBV H120 S1 gene ectodomain suitable for cell culture and a construction method and application thereof. The recombinant virus is constructed by the following steps of: replacing the S1 gene ectodomain segment of an IBV Beaudette strain with an S1 gene ectodomain segment of an IBV H120 strain to construct the recombinant virus; after successful virus rescue, passing from the Vero cell; collecting toxicity when the cell cytopathic effect area is over 90%; and repeatedly freezing and thawing for three times, and continuing passage to obtain the recombinant virus of a chimeric IBV H120 S1 gene ectodomain suitable for cell culture. The recombinant virus disclosed by the invention is used as a vaccine strain to immunize SPF chicken; after counteracting toxicity, the chicken flocks of an immunity group and a control group are continuously observed to discover that the recombinant virus disclosed by the invention can serve as a vaccine strain to provide good immunity protection to the inoculated chicken; and the recombinant virus is safe to various kinds of chicken and avoids side reaction.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com