Nucleoside compound and application thereof
A compound, selected technology, applied in the field of prodrugs and/or pharmaceutically acceptable salts thereof, nucleoside compounds and derivatives thereof, capable of solving the problems of low bioavailability orally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] Embodiment 1. Synthesis of compound 1
[0237]
[0238] Dissolve 5.62g of compound GS-441524 in 30mL of acetone, add 11.50mL of 2,2-dimethoxypropane and 1.34mL of 98% sulfuric acid, stir at 45°C for half an hour, cool to room temperature, and remove by rotary evaporation Organic solvents. Extract with 100 mL of ethyl acetate and 100 mL of saturated sodium bicarbonate solution, repeat the extraction three times, combine the ethyl acetate layers, add anhydrous sodium sulfate to dry, and filter to remove sodium sulfate. The organic solvent was removed by rotary evaporation, and separated by column chromatography (eluent: petroleum ether / ethyl acetate (V / V)=1 / 2) to obtain 6.20 g of compound 1 (white solid, yield 97%). Get obtained compound 1 to detect proton spectrum, the result is as follows:
[0239] 1 H NMR (400MHz, Chloroform-d) δ7.95(s, 1H), 7.11(d, J=4.7Hz, 1H), 6.69(dd, J=4.8, 2.4Hz, 1H), 5.77(s, 2H) , 5.42 (d, J=6.6Hz, 1H), 5.24 (dd, J=6.6, 2.4Hz, 1H), 4.67 (...
Embodiment 2
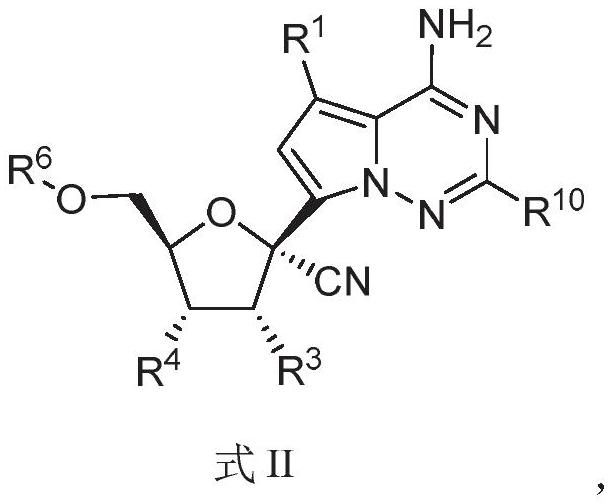
[0240] Embodiment 2. Synthesis of compound 2
[0241]
[0242] Dissolve 1.50g of compound 1 in 15ml of dichloromethane, then add 0.42mL of tetrahydro-2H-pyran-4-carboxylic acid and 0.27g of 4-dimethylaminopyridine, stir for 10min, then add 1.1g of Dicyclohexylcarbodiimide, stirred at room temperature for 4h. After separation by column chromatography (eluent: petroleum ether / ethyl acetate (V / V) = 1 / 1), 1.9 g of compound 2 (white solid, yield 95%) was obtained, ESI-MS: m / z 444.5[M+H] + .
Embodiment 3
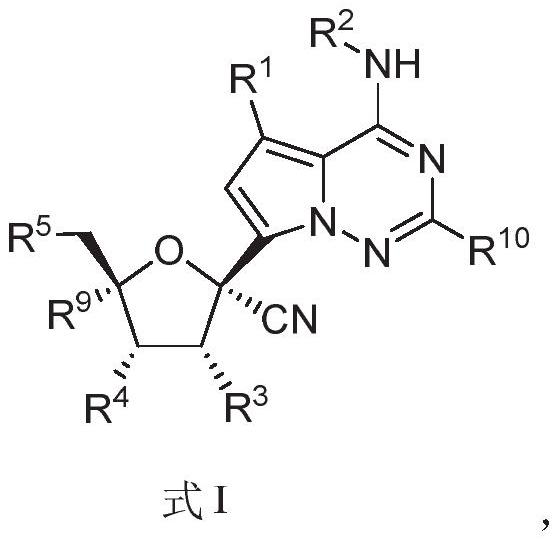
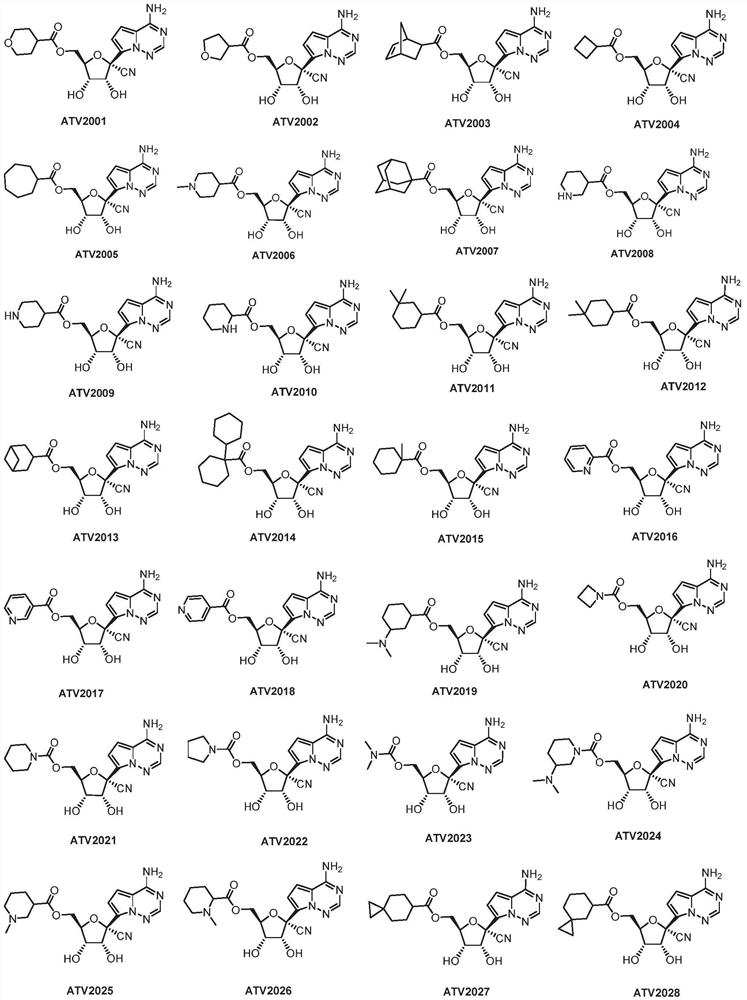
[0243] Example 3. Compound ((2R, 3S, 4R, 5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4 Synthesis of -dihydroxytetrahydrofuran-2-yl)methyl tetrahydro-2H-pyran-4-carboxylate (ATV2001)
[0244]
[0245] Dissolve 1.50 g of compound 2 in 10 mL (6.7 V) of formic acid and 5 mL (3.3 V) of water, stir at room temperature for 30 hours, evaporate the excess formic acid to dryness, dissolve the residue in ethyl acetate, and wash with saturated aqueous sodium carbonate Adjust the pH to 8, separate the organic layer, extract the aqueous layer twice with EA, combine the organic layers, wash with saturated brine, dry over sodium sulfate, filter and evaporate to dryness, and the crude product is separated by column chromatography (the eluent is : DCM / MeOH (V / V)=10 / 1), to obtain 0.96 g of compound ATV2001 (white solid, yield 70%). The obtained compound ATV2001 detects hydrogen spectrum and carbon spectrum, and the results are as follows:
[0246] 1 H NMR (600MHz, DMSO-d 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com