Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about How to "Large dosage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Green biological active feed
InactiveCN101978850AImprove balancePromote growthFood processingClimate change adaptationBiotechnologyDisease
The invention discloses a green biological active feed, which relates to the technical field of feeds. The green biological active feed is prepared from the following materials in part by weight: 40 to 80 parts of straw, 5 to 30 parts of corn four, 5 to 25 parts of cake dregs, 5 to 25 parts of wheat bran, 0.1 to 0.2 part of complex bacteria and 0.1 to 0.2 part of complex enzyme. During preparation, the straw is crushed and then is uniformly mixed with the corn flour, the cake dregs and the wheat bran; the complex bacteria and the complex enzyme are mixed into the mixture; water is added into the mixture to ensure that the water content reaches 45 to 50 percent, and the mixture is filled in a big tank, sealed and stored for later use; or the mixture is baked or dried by natural wind for storage and later use. The green biological active feed has the following advantages of: (1) improving the balance of intestinal flora, and improving the feed utilization rate; (2) promoting the growth of meat livestock and poultry, and shortening the breeding cycle; (3) strengthening the body conditions of laying hens, ducks and geese, and having a significant strengthening action on prolonging theegg-laying peak period; (4) strengthening the body condition of female domestic animals, and improving the yielding rate; (5) strengthening the body conditions of the livestock and poultry, enhancingthe disease prevention and disease resisting capacities, and reducing the sickness rate; and (6) purifying the culture environment, and improving the meat quality due to no public nuisances and no residues.
Owner:徐贵阁
Magnetic covalent bond type chitosan-based modified flocculant and preparation method and application thereof
ActiveCN113121840AGood chemical stabilityGood mechanical strengthWater/sewage treatment by flocculation/precipitationLipophilicitySuperparamagnetism
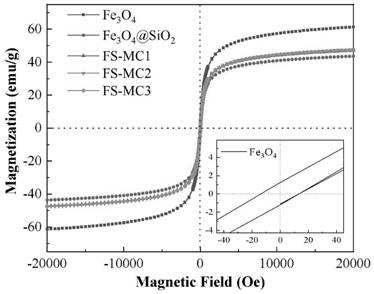
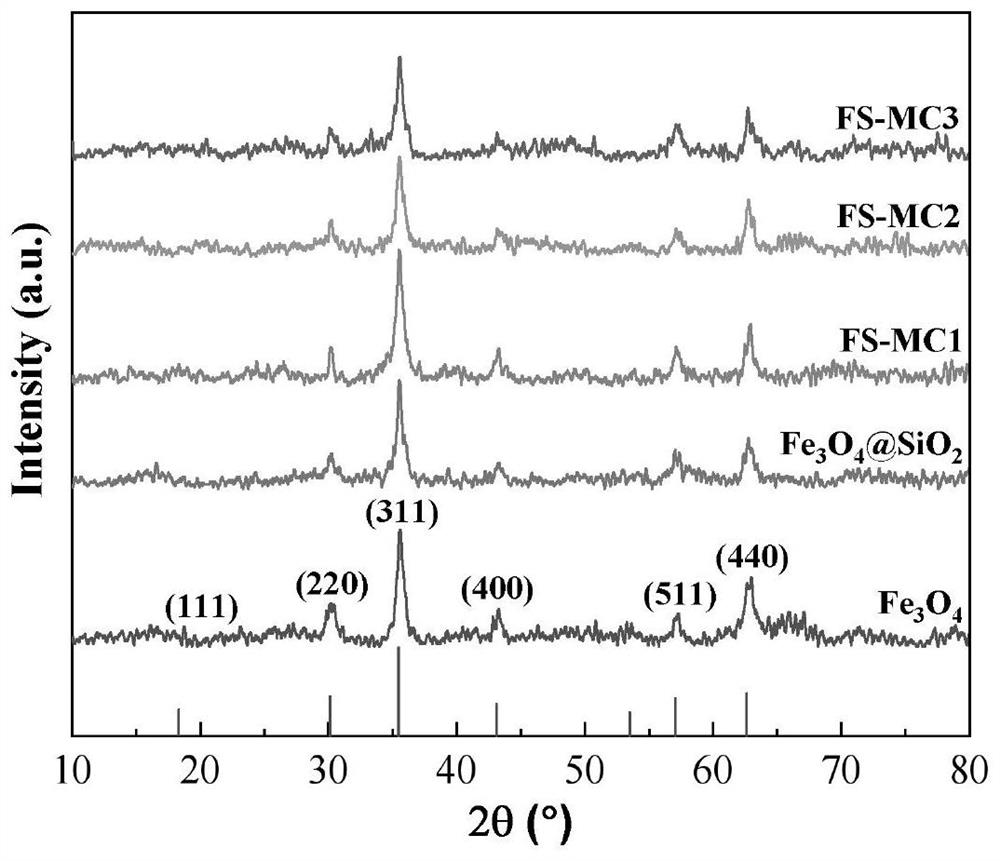
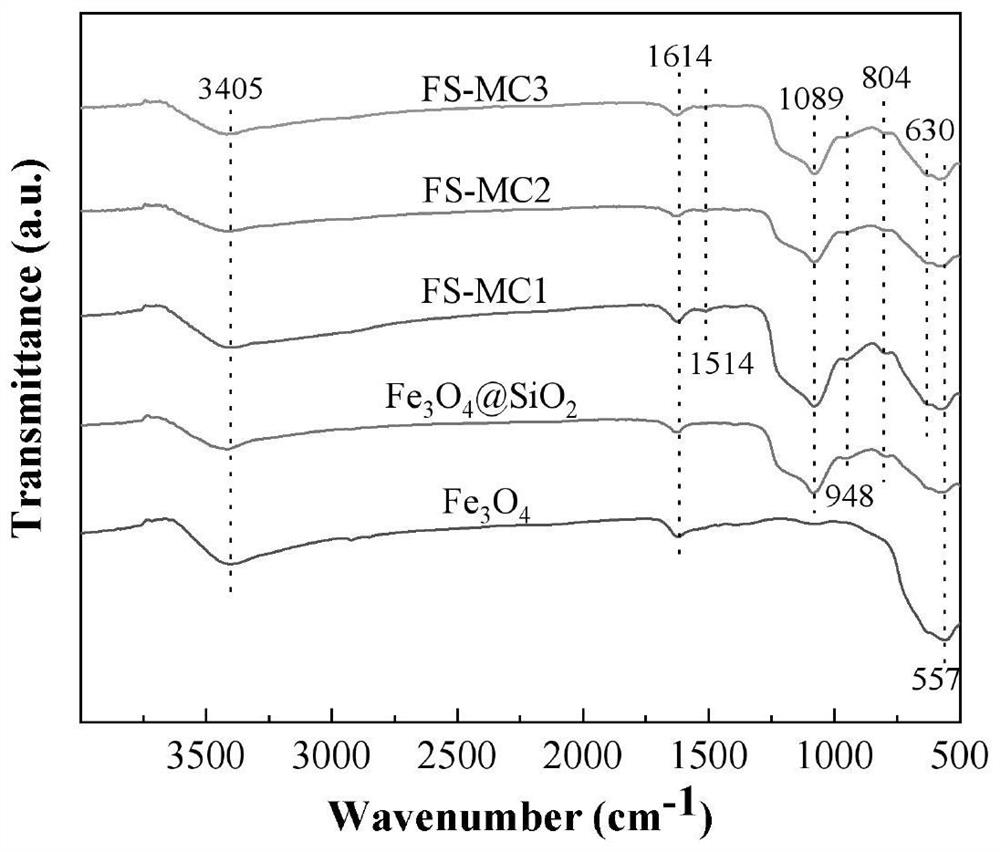
The invention belongs to the field of water treatment, and particularly relates to a magnetic covalent bond type chitosan-based modified flocculant and a preparation method and application thereof. According to the invention, a cationic monomer and a hydrophobic monomer are introduced into chitosan molecules through a graft copolymerization reaction, so that the flocculant is ensured to have good stability, the electricity neutralization capability and the demulsification capability on oil droplets of the flocculant in a flocculation process are improved, the solubility of the flocculant is increased, the pH application range of the flocculant is widened, the dosage is reduced, meanwhile, the nano Fe3O4 has superparamagnetism, the settling performance of floc can be remarkably improved under the action of an external magnetic field when the nano Fe3O4 is introduced into the flocculating agent, and the settling time is shortened. Results of the embodiment show that the flocculant provided by the invention is good in lipophilicity and excellent in flocculation performance, and has efficient oil-water separation capacity, wider pH application range and excellent recoverability.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
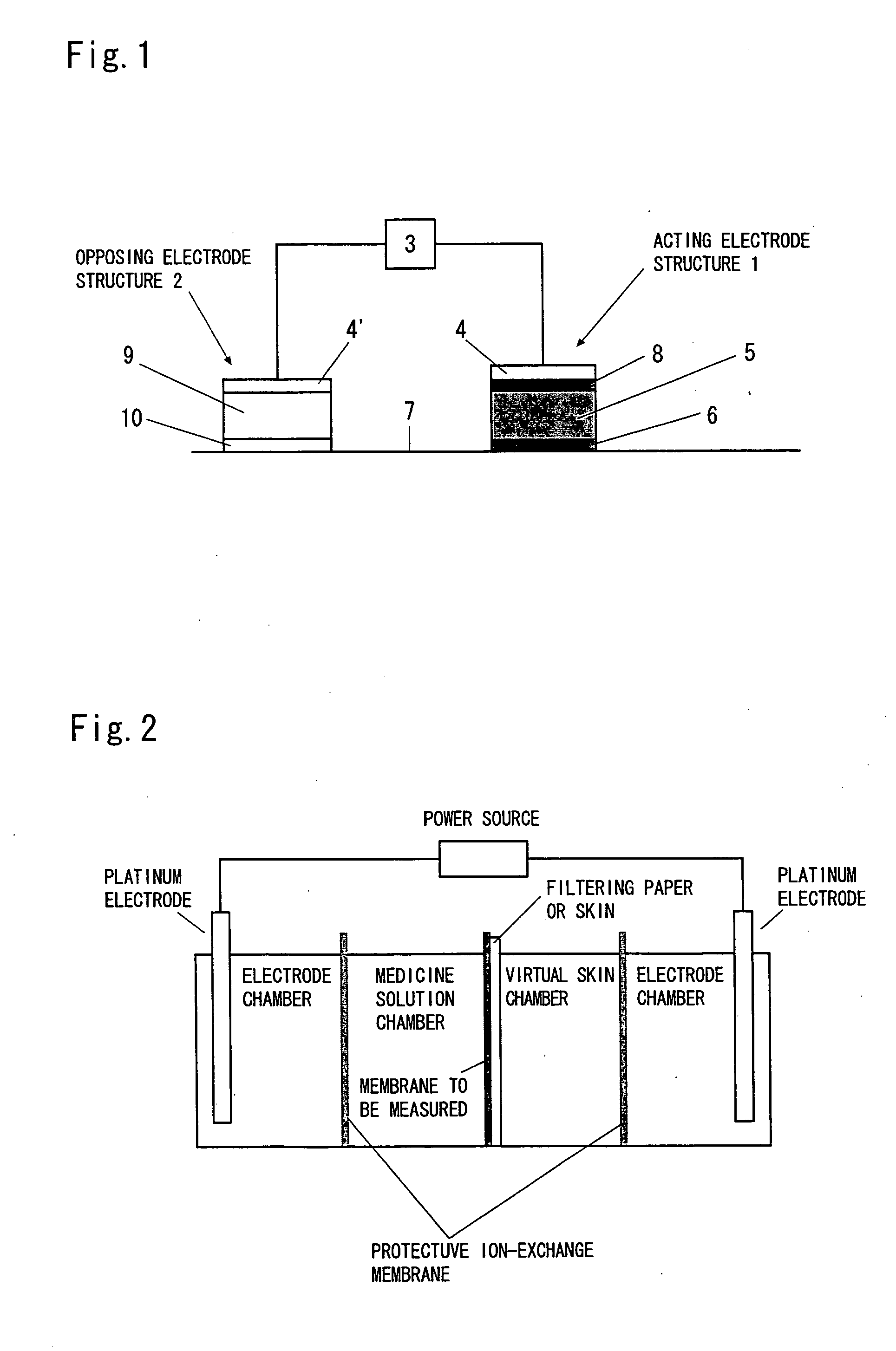
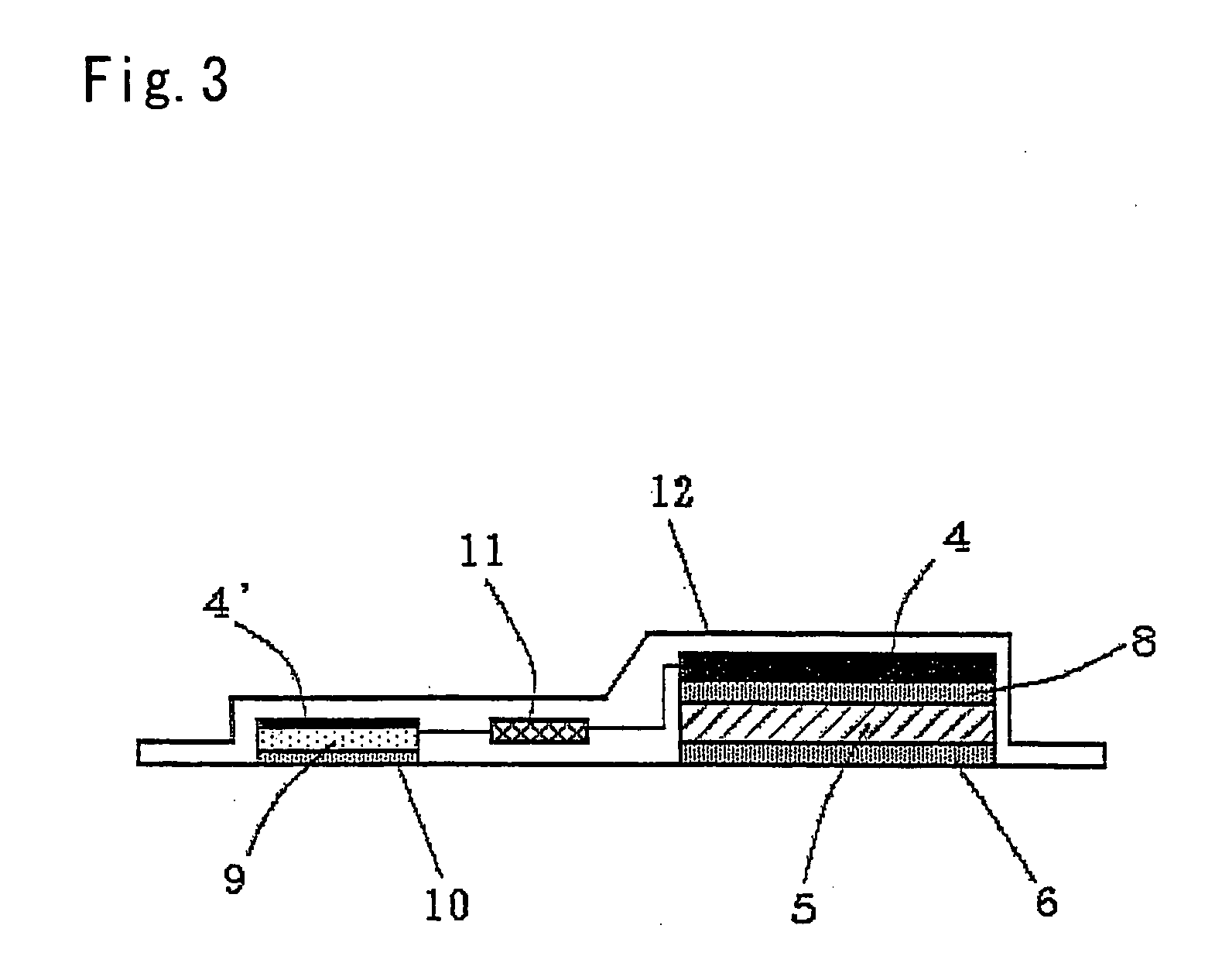
Iontophoresis apparatus
InactiveUS20060241548A1Large dosageLarge doseElectrotherapySheet deliveryIon-exchange membranesBiomedical engineering
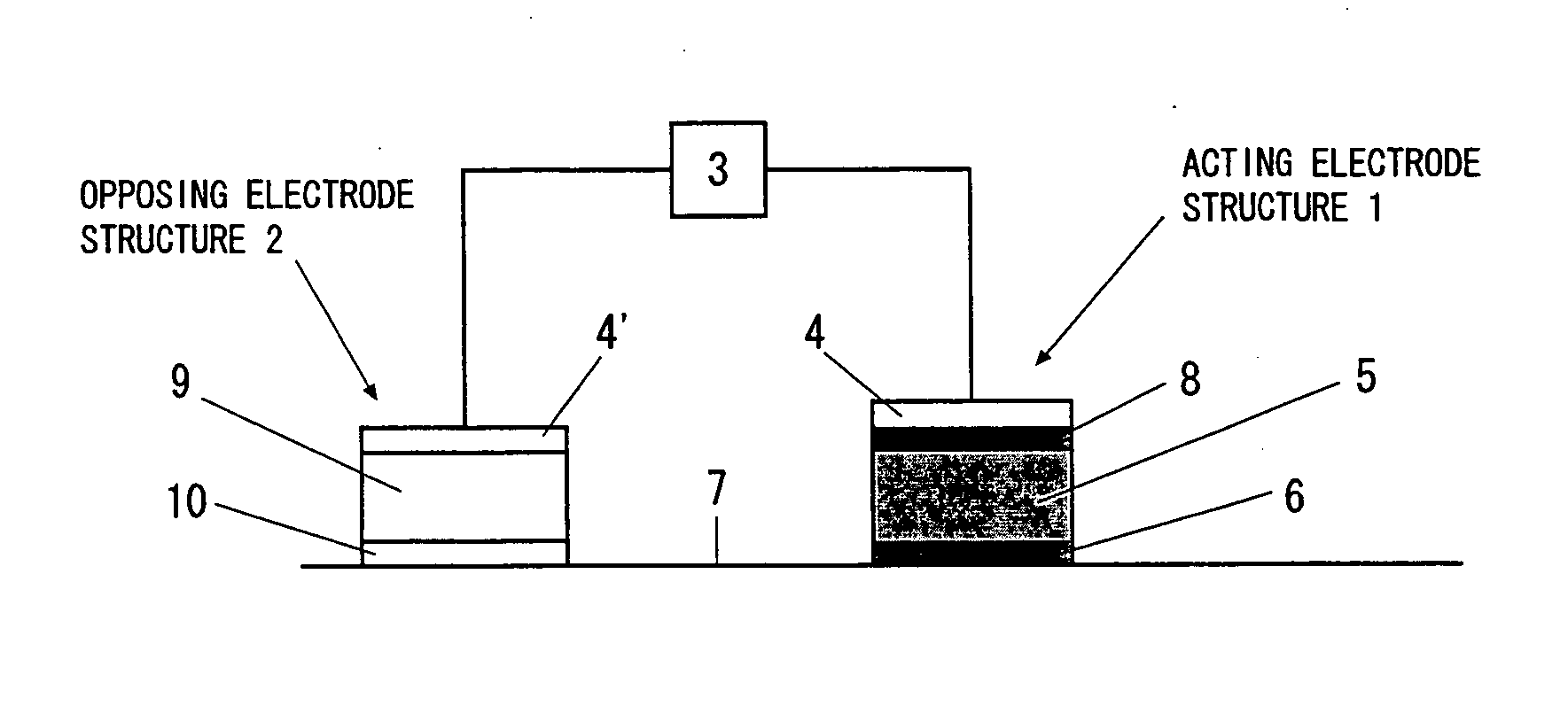
An iontophoresis device comprising (A) a working electrode assembly having a working electrode, a medicine-containing portion and an ion-exchange membrane, (B) a counter electrode assembly having an electrode which opposes the working electrode, and (C) a power source unit electrically connected to the working electrode assembly and to the counter electrode assembly, enabling an ionic medicine contained in the medicine containing portion to be permeated into a living body by the electrophoresis through the ion-exchange membrane, wherein the ion-exchange membrane has a structure in which voids of a porous film are filled with an ion-exchange resin. The iontophoresis device using the above ion-exchange membrane makes it possible to administer the medicine in amounts larger than those accomplished by using the conventional devices.
Owner:TOKUYAMA CORP
Nano coagulant for swimming pool
InactiveCN1626452ALarge specific surface areaHuge surface energyWater/sewage treatment by flocculation/precipitationAluminium sulfateSuspended matter
Owner:曾智勇 +1
Polyethylene glycol-cactus oligopeptide bonded rapamycin derivative
ActiveCN104448295AHigh drug loadIncrease diversityOrganic active ingredientsNervous disorderImmunologic disordersDisease
The invention provides a compound shown in the formula I in the specification and pharmaceutically acceptable salts of the compound, the preparation method of the compound and pharmaceutically acceptable salts of the compound and a medicine composition containing the compound shown in the formula I or the pharmaceutically acceptable salts of the compound. In the compound provided by the invention, each end group of the polyethylene glycol molecule is capable of bonding with a plurality of rapamycin molecules through cactus oligopeptide, and the medicine loading rate is improved. The compound can be used for inducing immunosuppression and treating transplant rejection, self-immune diseases, solid tumors, fungal infection and cardiovascular and cerebrovascular diseases.
Owner:JENKEM TECH
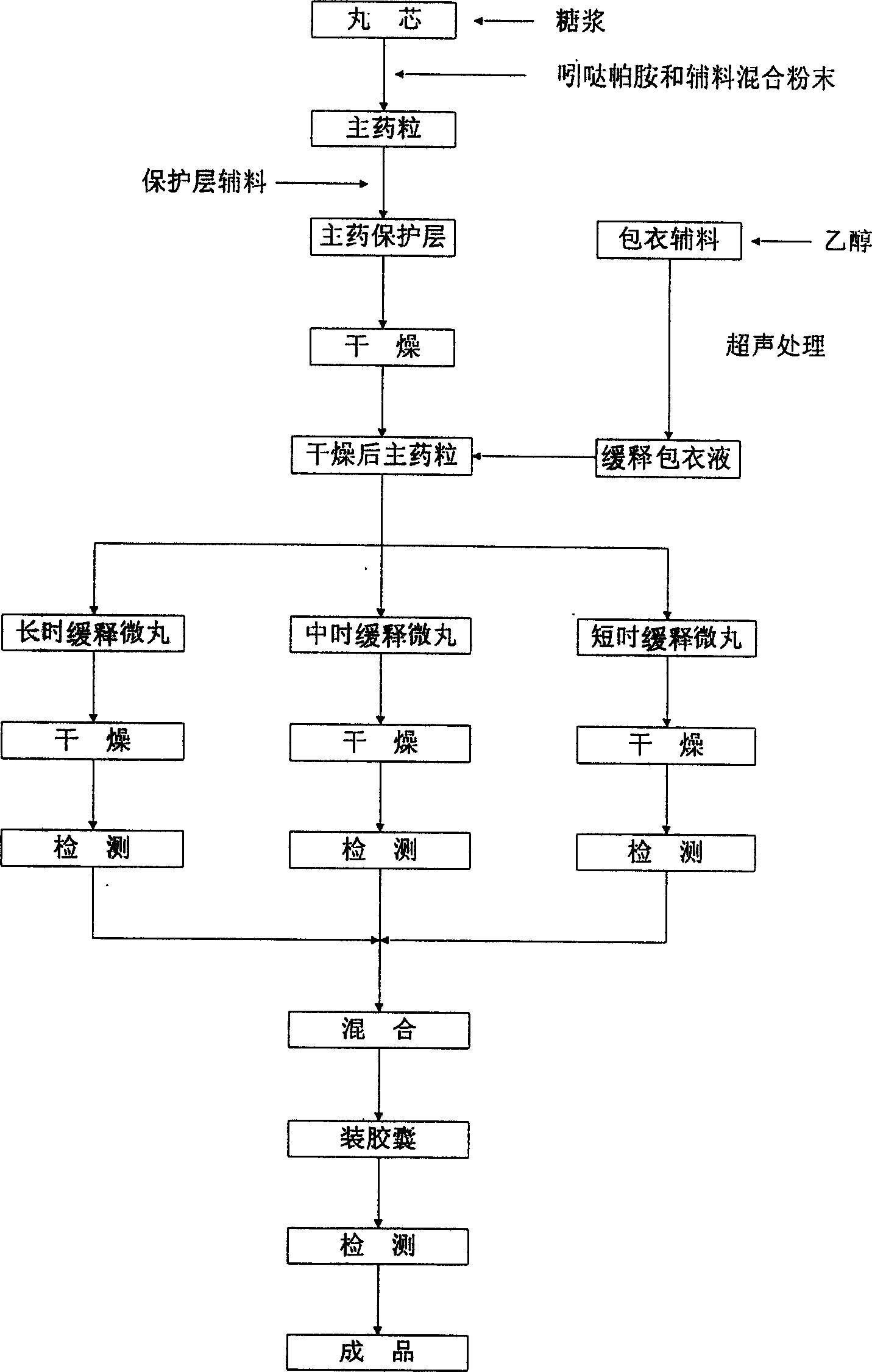
Gingko leaf sustained release formulation and preparation process thereof
InactiveCN1631395AReduce the amount addedLittle side effectsPharmaceutical delivery mechanismUnknown materialsAdjuvantCellulose acetate
The invention provides a gingko leaf sustained release formulation and preparation process, wherein the formulation comprises total flavone glucoside extracted from ginkgo leaves as active components, slow release material coated on the outer layer of the reactive component and adjuvant by the weight ratio of 40-50:50-70:290-300, and is prepared into slow release micro-pellet with different releasing rate, the slow release material can be any one to four of acrylic acid resin, methyl hydroxypropylcellulose, ethyl cellulose, cellulose acetate phthalate, and polyvinylpyrrolidone.
Owner:天津米克莱特生物技术有限公司
Nano-drug carrier particles capable of controlling drug release and preparation method thereof
ActiveCN105902516AGood drug encapsulation abilityReduce leakageOrganic active ingredientsInorganic non-active ingredientsElectricityMedicine
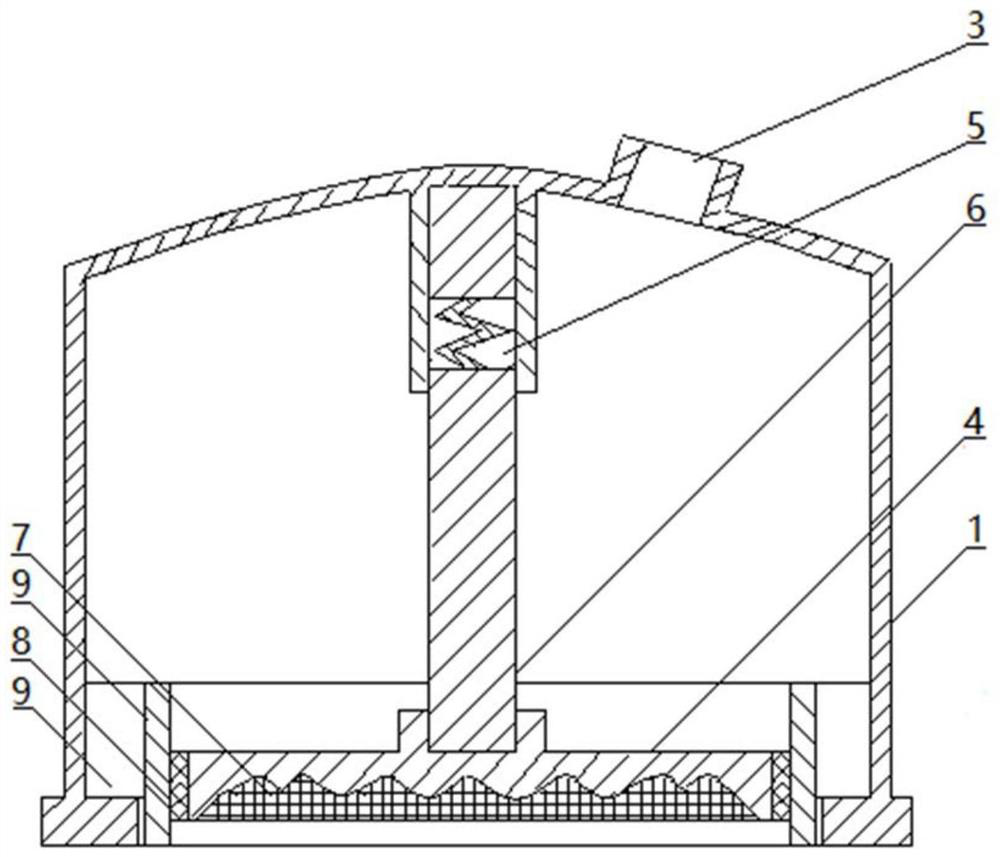
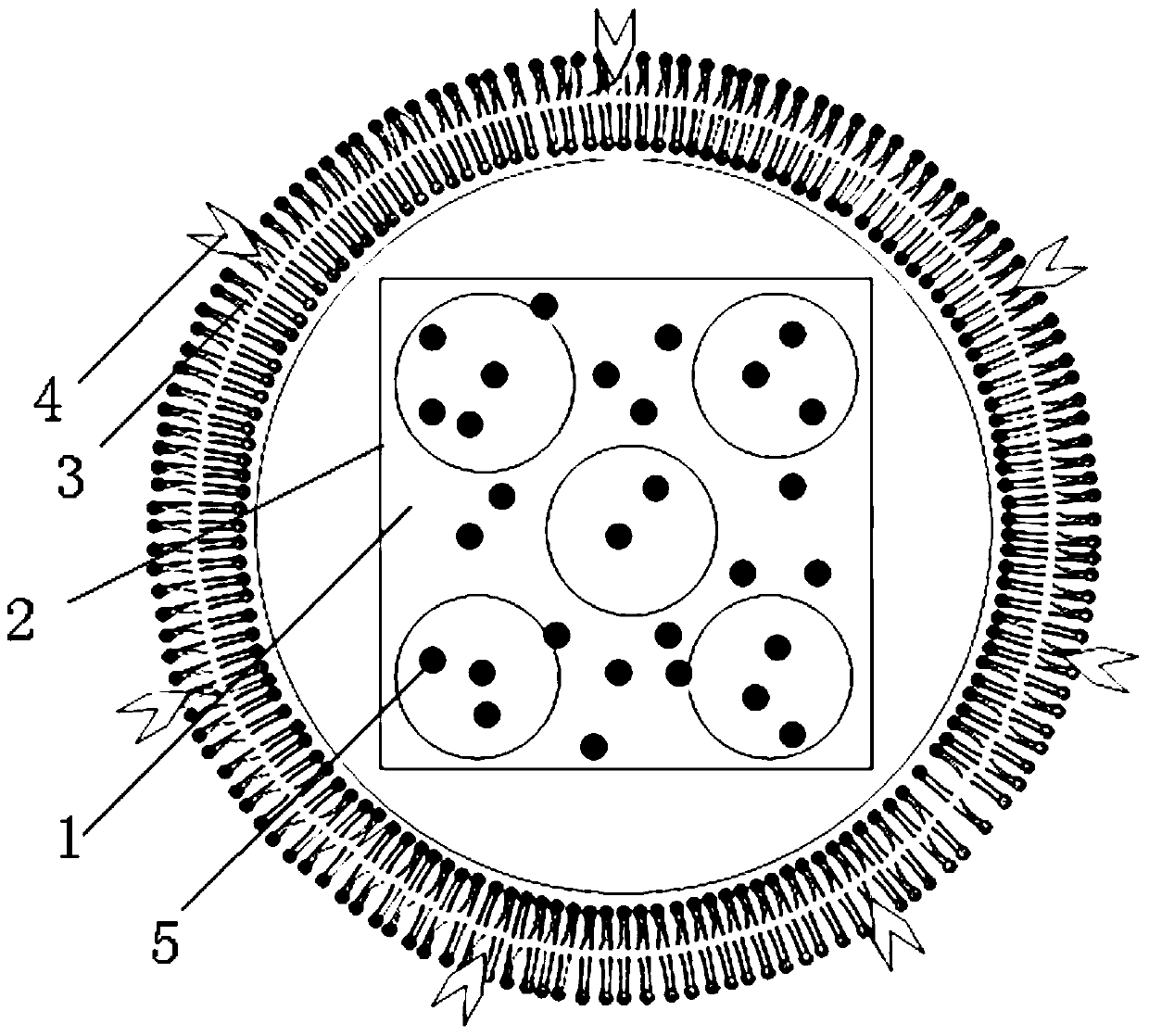
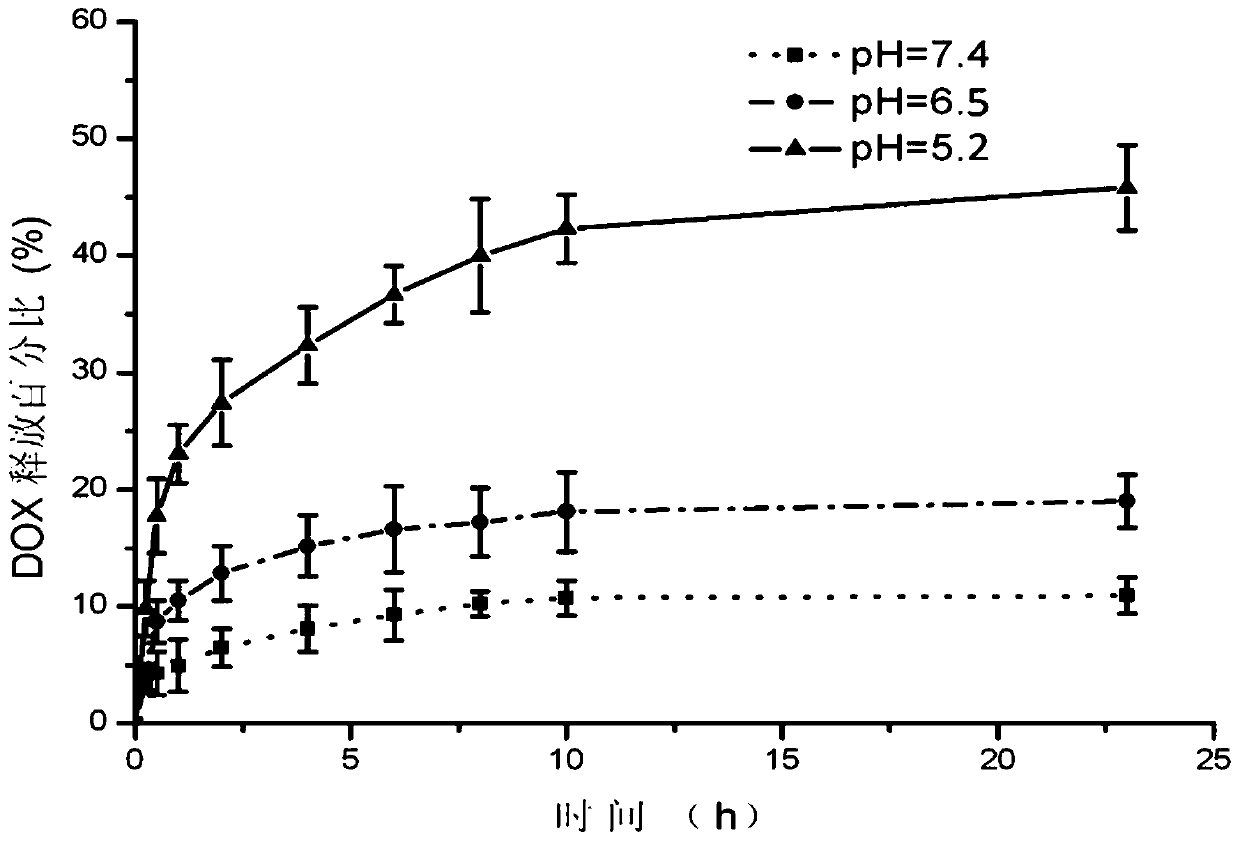
The invention discloses nano-drug carrier particles capable of controlling drug release. The particles have a core-shell structure, wherein a gold nano-cage (1) which is provided with a mesoporous surface and has a hollow structure is arranged in the innermost layer; a polymer PAH layer (2) carrying positive electricity is used for modifying the surface of the gold nano-cage (1); a pH sensitive lipid layer (3) is arranged on the outer surface coating layer of the polymer PAH layer (2). Under pH and light external excitation trigger, gating is switched from a 'closed' state to an 'open' state, and drug molecules are released. The carrier particles can be used for effectively improving the efficiency of cancer chemotherapy.
Owner:SOUTHEAST UNIV
Use of PNAS-4 gene in preparing antineoplastic and antineoplastic auxiliary medicament
InactiveCN101130081AImprove the quality of lifeGood effectPeptide/protein ingredientsAntineoplastic agentsSide effectLife quality
The invention relates to a use of PNAS-4 gene in aspects of preparing antineoplastic in the tumour gene treatment field. The invention with PNAS-4 recombinant vector can limit the growth and the migration of the endocytosis effectively, which can limit the growth of various tumors and extend the survive time of mouse. The antineoplastic of the invention with PNAS-4 recombinant vector liposome and chemotherapy drug cisplatin and magnolol as the active component has better effect than the single application and can reduce the usage of both parties. The product has the appreciable effect, the little toxic and side effect and the easy preparing method, which compensates the defect of protein infusion drug, increases the time interval of medicine, reduces the usage, reduces the economic burden of the patient, improves the life quality of patient and has the wide market prospect.
Owner:SICHUAN UNIV
Benzamide analog mediated brain-targeting delivery system
ActiveCN102160894AStrong complianceLarge dosagePowder deliveryNervous disorderBrain disorder diagnosisIn vivo
The invention belongs to the field of medicinal preparations, and relates to a brain-targeting delivery system. The system comprises a mediating molecule, a vector and a medicament, wherein the mediating molecule is a benzamide analog, the vector is a polycation macromolecule, and the mediating molecule and the vector are combined by a covalence mode; and the medicament is carried through an entrapping or adsorption mode. In the system, in-vivo and in-vitro brain-targeting is characterized through a tracer technique to prompt that the delivery system can span a blood brain barrier, and a genemedicament and a diagnosis medicament are delivered into a brain, so the system can be used for brain disease diagnosis and treatment. The system can avoid potential risks of an enteroinvasive administration mode and a complicated administration process, and has the advantages of large administration quantity, simple administration mode and the like; and the brain-targeting mediated molecule has the advantages of small molecular weight, low price and availability and industrialization convenience.
Owner:SHANGHAI WHITTLONG PHARMA INST
Slow-released indapamide capsule and its prepn process
InactiveCN1468598ALittle side effectsFully absorbedOrganic active ingredientsPharmaceutical delivery mechanismSide effectCellulose acetate
The slow-released Indapamide capsule consists of Indapamide, slow-releasing material and supplementary material in the ratio of 0.5-3.0 to 5-20 to 131-165. The slow-releasing material is one to four of polyacrylic resin, hydroxypropyl methyl cellulose, ethyl cellulose, acetyl phthalate cellulose and PVP, and each capsule contains Indapamide in 0.5-3.0 mg. The slow-released Indapamide capsule may be prepared via rolling process. The preparation form of the present invention has lasting effective time, less side effect and high biological utilization, and through the different combination of the micro pellet, ideal release rate may be reached.
Owner:TIANJIN GUOYAO BOHAI BIOMEDICAL
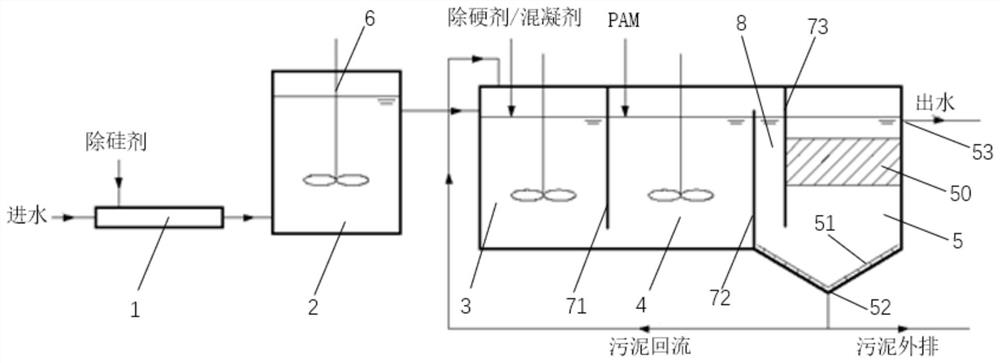
Silicon removal agent and silicon removal and hardness removal sewage treatment system and method
PendingCN111892142AAvoid hydrolysisImprove stabilityWater contaminantsWater softeningAluminium chlorideActive agent
The invention discloses a silicon removal agent, which comprises the following components in percentage by mass: 70-85% of an active agent, 10-30% of a modifier, and 3-5% of a stabilizer, wherein theactive agent comprises sodium metaaluminate and further comprises aluminum chloride and / or aluminum sulfate, the modifier is magnesium oxide powder and / or calcium powder, and the stabilizer is sodiumhydroxide and / or sodium carbonate. The invention also provides a sewage treatment system for removing silicon and hardness, and provides a sewage treatment method for removing silicon and hardness. When the silicon removal agent is used for sewage treatment, the operation is simple and convenient, the sludge yield is low, the wastewater hardness is not increased, and the silicon removal efficiencyis improved; and in combination with the hardness removal step, wastewater with high hardness can be synchronously treated, and the hardness removal efficiency is improved.
Owner:SHANGHAI LANKE PETROCHEM ENG & TECH
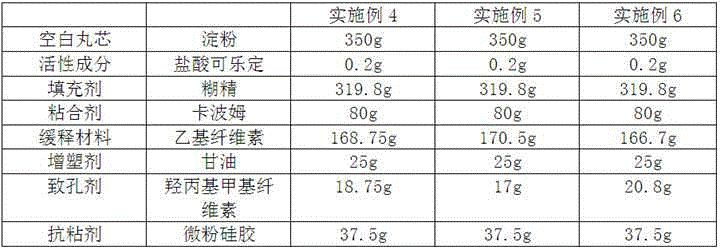
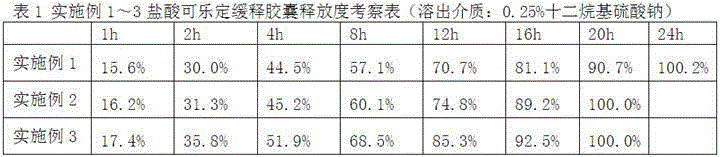
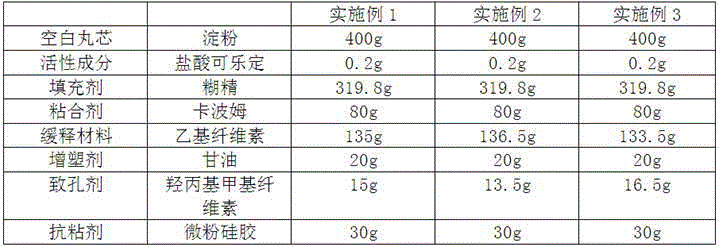
Clonidine hydrochloride sustained-release capsule
ActiveCN105395518ARelease evenlyStable blood concentrationOrganic active ingredientsNervous disorderDrugClonidine Hydrochloride
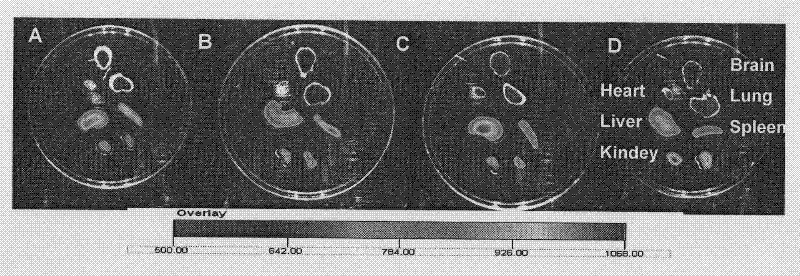
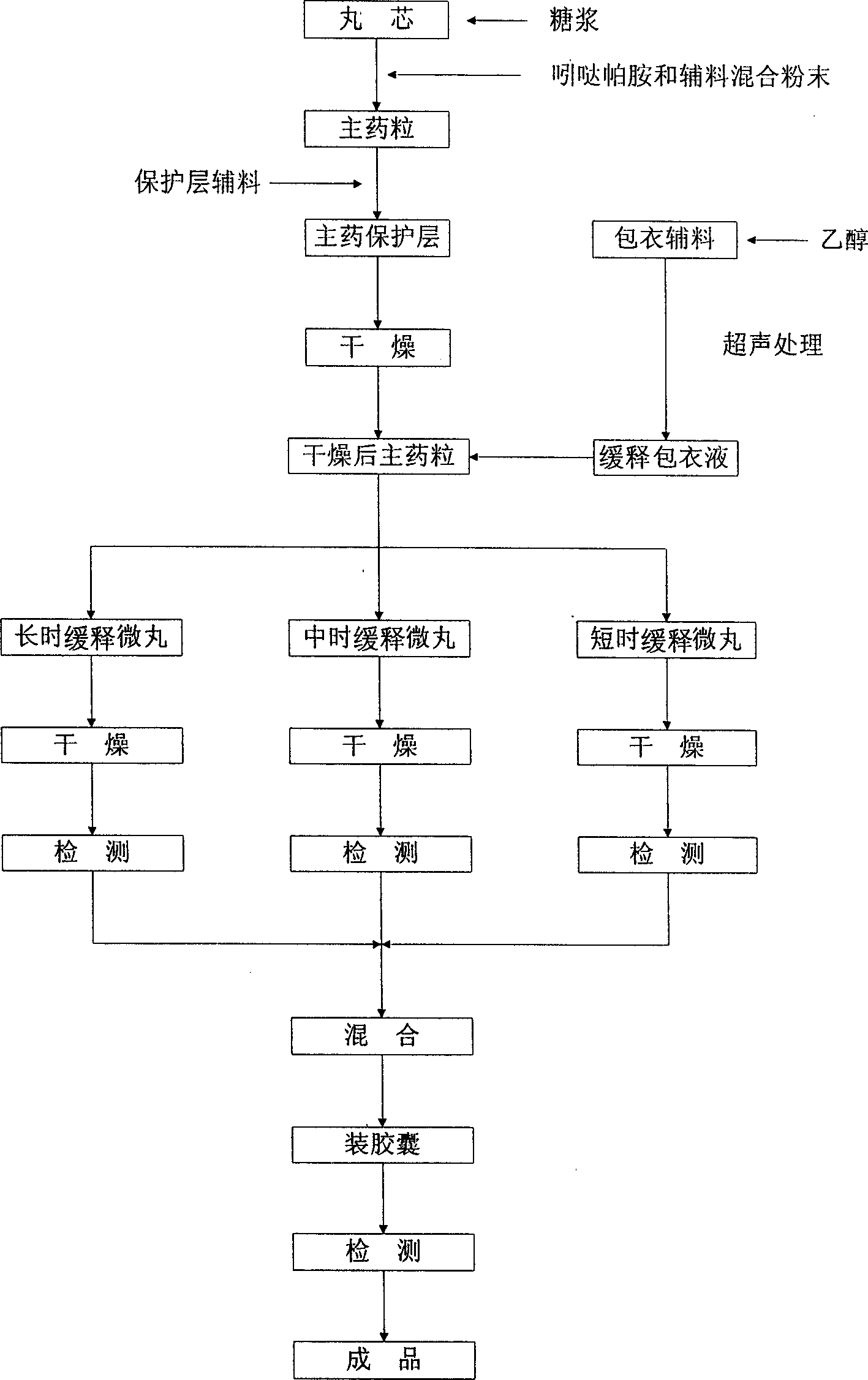
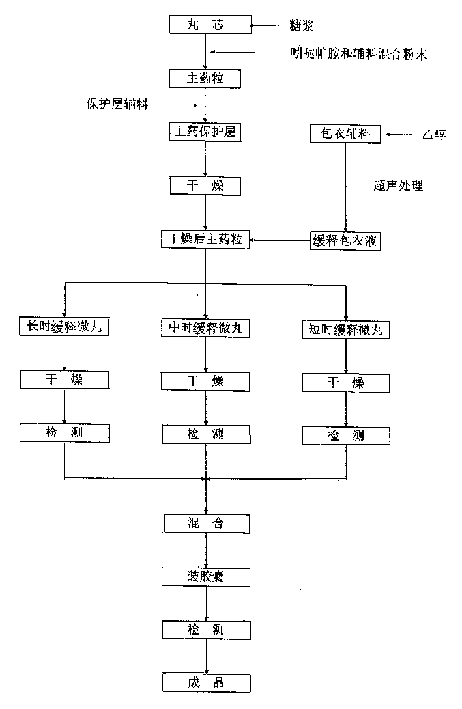
The invention discloses a clonidine hydrochloride sustained-release capsule, which is formed by filling clonidine hydrochloride sustained-release pellets in a capsule shell; during preparing, drug-containing layers and coating layers cover blank pellet cores, so that the clonidine hydrochloride sustained-release pellets are obtained, and then the clonidine hydrochloride sustained-release pellets are filled into the capsule shell, so that the clonidine hydrochloride sustained-release capsule is prepared. The clonidine hydrochloride, as a major ingredient in the clonidine hydrochloride sustained-release capsule disclosed by the invention, has an effect of relieving muscle spasm as well as accompanied severe pain in skeletal muscle and the like; the sustained-release preparation can develop effects of stopping pain and relieving spasm stably; and the sustained-release capsule is safe and effective, stable in quality, low in cost and low in administration efficiency, and patient compliance is enhanced.
Owner:CP PHARMA QINGDAO CO LTD
Method for preparing digitalis floating tablets and use thereof
InactiveCN101468100ALittle side effectsFully absorbedOrganic active ingredientsPharmaceutical non-active ingredientsSide effectRetention time
The invention disclosed floating film stomach digitalis preparation method and its application, the invention of new technologies are the drug, involving a new form of digitalis for the treatment of congestive heart failure and certain arrhythmias such as atrial fibrillation, atrial flutter and paroxysmal tachycardia in preparation methods and pharmaceutical preparations. The characteristics of the new formulations is that the gastric floating tablet containing a wide range of hydrophilic polymer material, in contact with gastric fluid temperature, the surface water into a gel, by volume expansion. At this point the weight of tablets is less than the buoyancy gastric juice to pills floating on the gastric juice, gastric retention time has been extended. The present invention has been floating piece of digitalis stomach with a simple, good stability and high bioavailability, compared with the general formulations to reduce the side effects of drug characteristics.
Owner:BEIJING HOPE HUGE PHARM SCI
Recombinant human esoderma colyone adenovirus, and its preparing method and use
ActiveCN1935999AInhibit growth and migrationGrowth and metastasis inhibitionPeptide/protein ingredientsGenetic material ingredientsSignal peptideDrug
The invention supplies a new recombination endostatin gene that connects the 3' end of IL-2 gene signal peptide sequence with the 5' end of endostatin coding sequence, and the gene could be constructed into adenovirus and make anti-tumor injection. The injection would restrain the growth and transferring of tumor in body and keep entire bioactivity. It has the advantages of lowering medicine cost, improving life quality of patients and good market prospect.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Reverse osmosis concentrated water treatment process for Fenton reagent oxidation enhanced adsorption
InactiveCN113415924ALarge specific surface areaHigh operating costsWater treatment compoundsSpecific water treatment objectivesIndustrial wastewater treatmentActivated carbon
The invention discloses a reverse osmosis concentrated water treatment process for Fenton reagent oxidation enhanced adsorption, and relates to the technical field of industrial wastewater treatment. An activated carbon adsorption technology can effectively adsorb organic matters in reverse osmosis concentrated water, and a Fenton reagent oxidation technology utilizes high-activity free radicals to attack macromolecular organic matters and react with the macromolecular organic matters, so that organic matter molecular structures are destroyed; According to the process, Fenton reagent oxidation and activated carbon adsorption technologies are combined, the organic matters in reverse osmosis concentrated water are diffused and adsorbed into a microporous structure of activated carbon by the activated carbon, and then the hydroxyl radicals generated by Fenton reagent oxidation can efficiently degrade organic pollutants in the microporous structure of the activated carbon. The Fenton reagent oxidation enhanced activated carbon adsorption can achieve a good synergistic effect, the saturated adsorption capacity and the adsorption efficiency of the activated carbon are improved, and the deep treatment efficiency of the reverse osmosis concentrated water is improved.
Owner:SHANGHAI HONESS ENVIRONMENTAL TECH CORP
A brain-targeted drug delivery system
InactiveCN101897669BStrong complianceLarge dosagePowder deliveryGenetic material ingredientsPatient complianceCvd risk
The invention belongs to the field of pharmaceutic preparations, and relates to a brain targeting drug delivery system which comprises mediated molecules, carriers and drugs, wherein the mediated molecule is fatty acid, the carrier is polycation macromolecule, the fatty acid and the polycation macromolecule are combined to form nano particles or micelles in a covalence mode, and drug loading is completed in an entrapment or absorption mode. The invention utilizes a brain targeting tracing system to perform in-vivo and in-vitro characterization, and the result shows that the drug delivery system in the invention can obviously improve the amount of the drugs entering in the brain by permeating blood brain barriers, delivery gene drugs and diagnostic drugs in the brain by spanning the blood brain barriers and perform prevention and treatment as well as diagnosis on brain diseases. The invention can avoid potential risks and complicated administration process of an invasive administration mode, has small molecular weight and no immunogenicity, and has the advantages of large administration amount, simple administration mode, strong patient compliance and the like compared with a nasal administration mode.
Owner:FUDAN UNIV
Sustained release high calcium formulation and preparation process thereof
InactiveCN1634129ALittle side effectsFully absorbedMetabolism disorderPharmaceutical non-active ingredientsCellulose acetate phthalateAdjuvant
Disclosed is a sustained release high calcium formulation and preparation process, wherein the formulation comprises calcium as active component, slow release material coated outside the active component and adjuvant by the weight ratio of 90-110 : 5-20 : 131-165, the slow release material includes any one to four from acrylic resin, methyl hydroxypropylcellulose, ethyl cellulose, cellulose acetate-phthalate, and polyvinylpyrrolidone.
Owner:天津开发区渤海医药贸易公司
Musk precordial pain relieving drop pills and preparation method thereof
InactiveCN1634483AQuality improvementDefinite curative effectUnknown materialsPill deliverySide effectDrug content
The invention relates to a medicinal oral preparation of musk drop pill with the functions of arousing coma, relieving pain and treating precordial pain, chest pain, the drop pill has the advantages of high biological availability, quick-speed medicine release, quick-speed effect, less toxic and side effects, higher medicinal content, smaller amount of administration, accurate administration dosage, easy administering, low price, and facilitated carrying. The medicine is prepared through the conventional drop pill preparing process.
Owner:北京博智绿洲医药科技有限公司
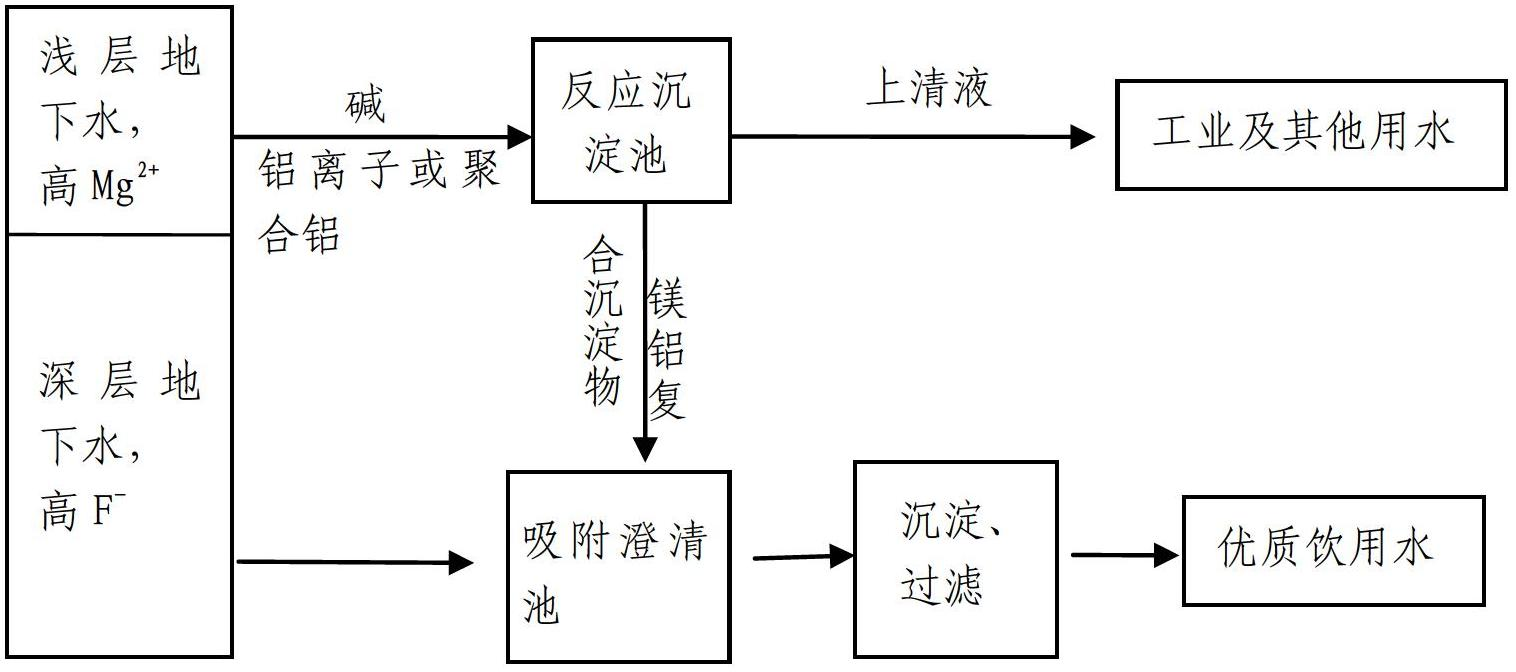
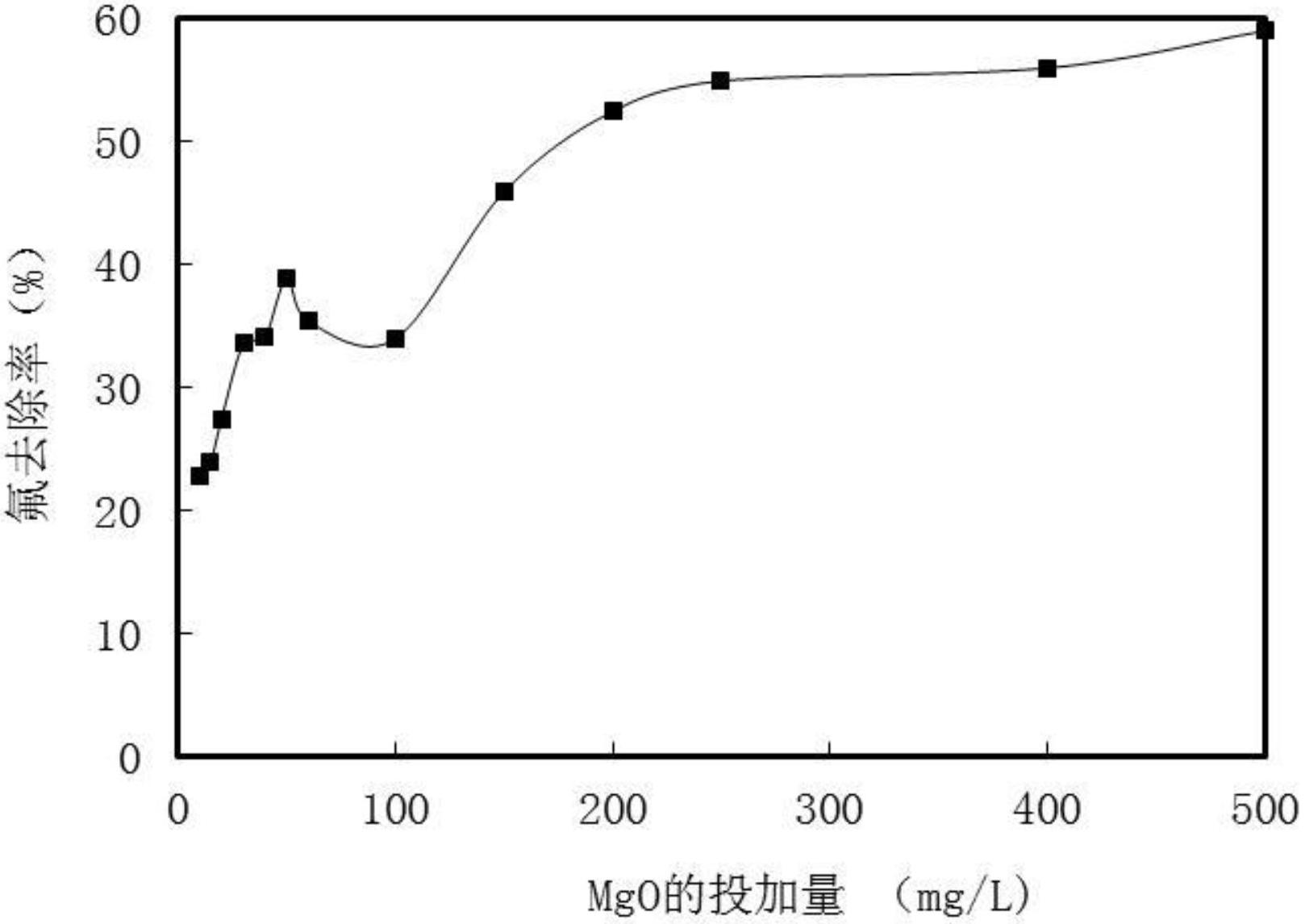
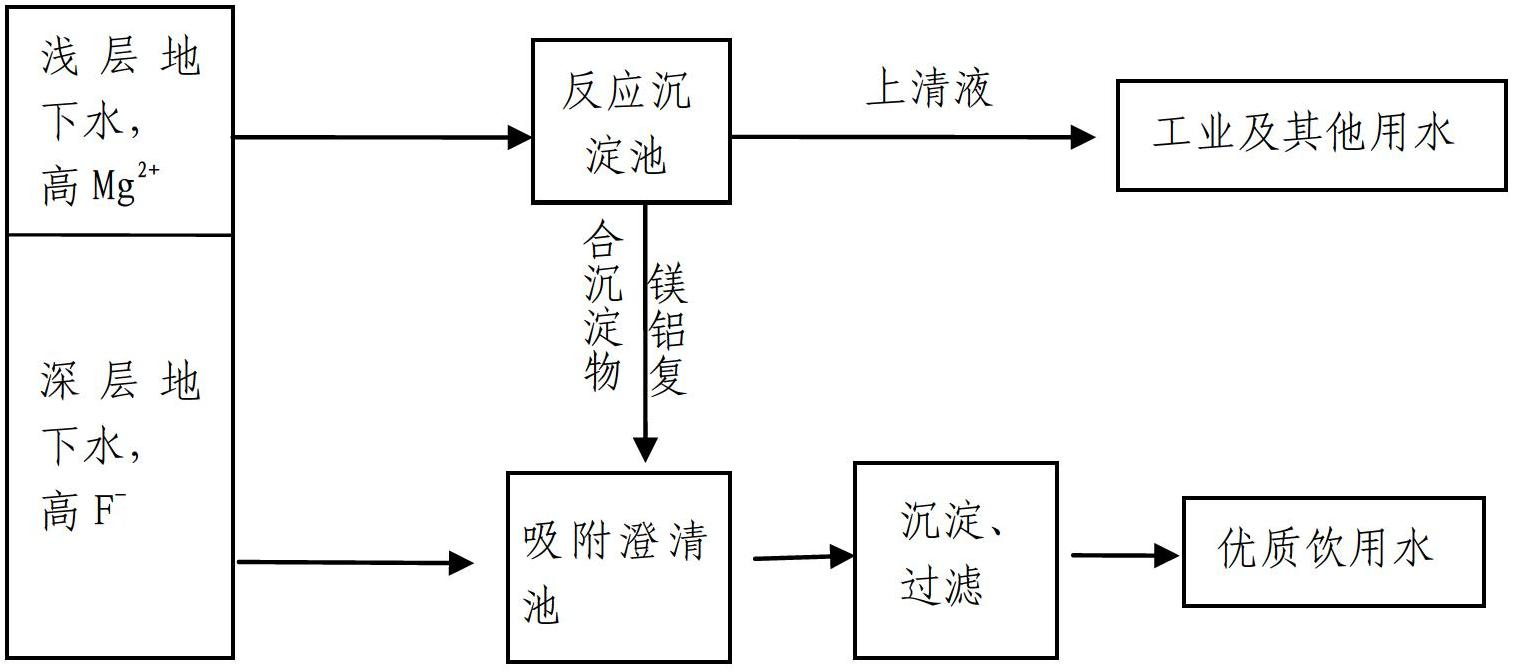
Method for removing fluorions in drinking water by strengthened magnesium precipitations
InactiveCN102659254AGood removal effectGood defluoridation effectWater contaminantsWater softeningAlkalinityALUMINUM STEARATES
The invention relates to a method for removing fluorions in drinking water by strengthened magnesium precipitations. The method includes two means of comprehensive purification of high-hardness superficial water and high-fluorine water and purification of strengthened co-precipitation, and the two means are referred in details in instructions. The method for removing fluorion in drinking water by strengthening magnesium precipitations has the advantages that firstly, in the conventional fluorion removal by neutralizing and softening, fine fluorion removal effect can be realized only by requiring the pH value to be 11 around and a great quantity of magnesium precipitations to be generated; while in the method for removing fluorinion, magnesium ion precipitations can be generated at the lower pH value, and better removal effect of fluorinions is achieved; and secondly, compared with the existing fluorion removal method by the coagulation technology, the method for removing fluorions is high in fluorion removal efficiency. In the existing fluorion removal method by the coagulation technology, fine removal effect can be realized only at the partial acid or neutral pH conditions due to utilization of aluminum stearate and ferric salt type coagulant; coagulation efficiency is limited, feed quantity of the coagulant is large and residual capacity of water outlet coagulant is high due to high alkalinity and pH value of underground water. Besides, the method for removing fluorions in drinking water by strengthened magnesium precipitations can realize better removal effect with the lower chemical feed quantity.
Owner:PEKING UNIV
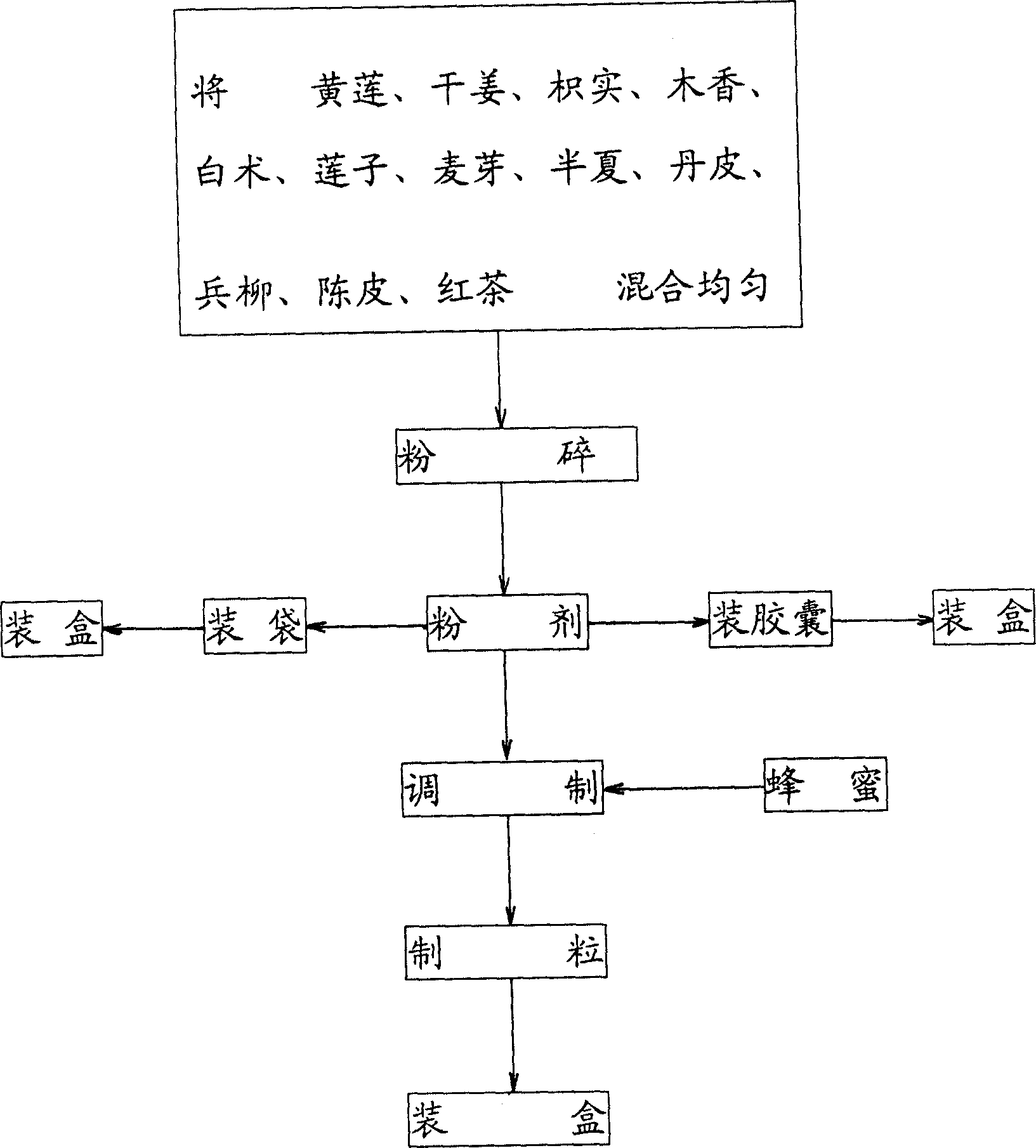
Chinese medicine formulation for treating gastropathy and its preparation
The invention discloses a Chinese formulation for treating gastropathy and process for preparation, which comprises water content 8-18%, coptis root 45-55g, dried ginger 40-50g, citrus auranlium 30-40g, banksia rose 30-40g, white atractylodes rhizome 10-30g, lotus seed 50-60g, malt 20-25g, pinellia tuber 10-20g, root bark of tree peony 25-45g, dried orange peel 10-15g, black tea 50-60g.
Owner:荣玉明
Green biological active feed
InactiveCN101978850BImprove palatabilityPromote digestionFood processingClimate change adaptationDiseaseBiotechnology
The invention discloses a green biological active feed, which relates to the technical field of feeds. The green biological active feed is prepared from the following materials in part by weight: 40 to 80 parts of straw, 5 to 30 parts of corn four, 5 to 25 parts of cake dregs, 5 to 25 parts of wheat bran, 0.1 to 0.2 part of complex bacteria and 0.1 to 0.2 part of complex enzyme. During preparation, the straw is crushed and then is uniformly mixed with the corn flour, the cake dregs and the wheat bran; the complex bacteria and the complex enzyme are mixed into the mixture; water is added into the mixture to ensure that the water content reaches 45 to 50 percent, and the mixture is filled in a big tank, sealed and stored for later use; or the mixture is baked or dried by natural wind for storage and later use. The green biological active feed has the following advantages of: (1) improving the balance of intestinal flora, and improving the feed utilization rate; (2) promoting the growth of meat livestock and poultry, and shortening the breeding cycle; (3) strengthening the body conditions of laying hens, ducks and geese, and having a significant strengthening action on prolonging theegg-laying peak period; (4) strengthening the body condition of female domestic animals, and improving the yielding rate; (5) strengthening the body conditions of the livestock and poultry, enhancingthe disease prevention and disease resisting capacities, and reducing the sickness rate; and (6) purifying the culture environment, and improving the meat quality due to no public nuisances and no residues.
Owner:徐贵阁
Method for recovering phosphorus from phosphorus-containing sludge by using glycine as phosphorus releasing agent
InactiveCN113698057ALarge dosagePhosphate release rate is controllableWater contaminantsWater/sewage treatment by neutralisationPolyphosphateP phosphate
The invention discloses a method for recovering phosphorus from phosphorus-containing sludge by using glycine as a phosphorus releasing agent. The method comprises the following steps: adding exogenous glycine into phosphorus-rich excess sludge under an anaerobic condition, and exciting and realizing decomposition of polyphosphate and release of phosphate in sludge microbial cells under the condition of not consuming glycine, so as to obtain a phosphorus-released mixed solution; performing standing on the phosphorus-released mixed solution to realize solid-liquid separation; adjusting the pH value of the phosphorus-rich supernate obtained after solid-liquid separation or adding a precipitator to realize precipitation of phosphate so as to obtain a phosphate precipitate; after the phosphate precipitate is dried, obtaining a phosphorus recovery product; and recycling the glycine-containing supernate after phosphate recovery for next batch of sludge phosphate release. According to the method, the release rate of phosphate can be regulated and controlled by adjusting the concentration of glycine in the mixed solution, efficient, rapid and sufficient release of phosphorus can be ensured, and glycine in the solution is extremely low in consumption and can be recycled.
Owner:SOUTH CHINA UNIV OF TECH
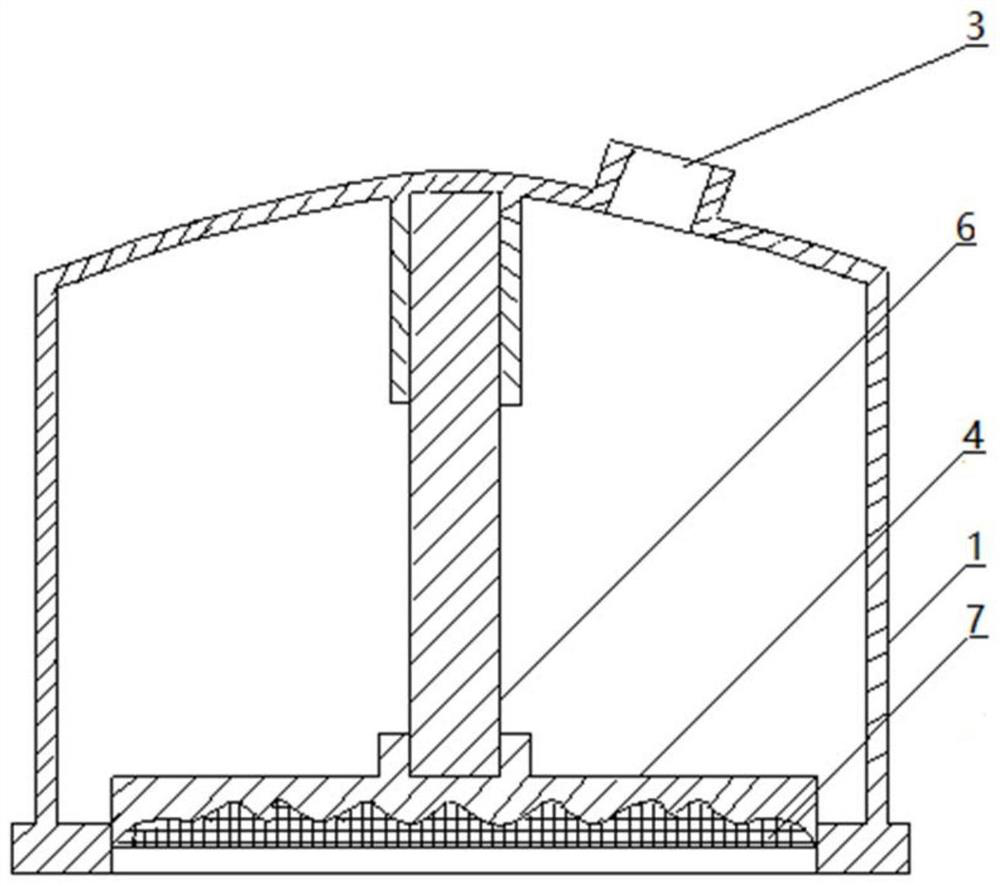
Pressure drug delivery device and drug storage core layer
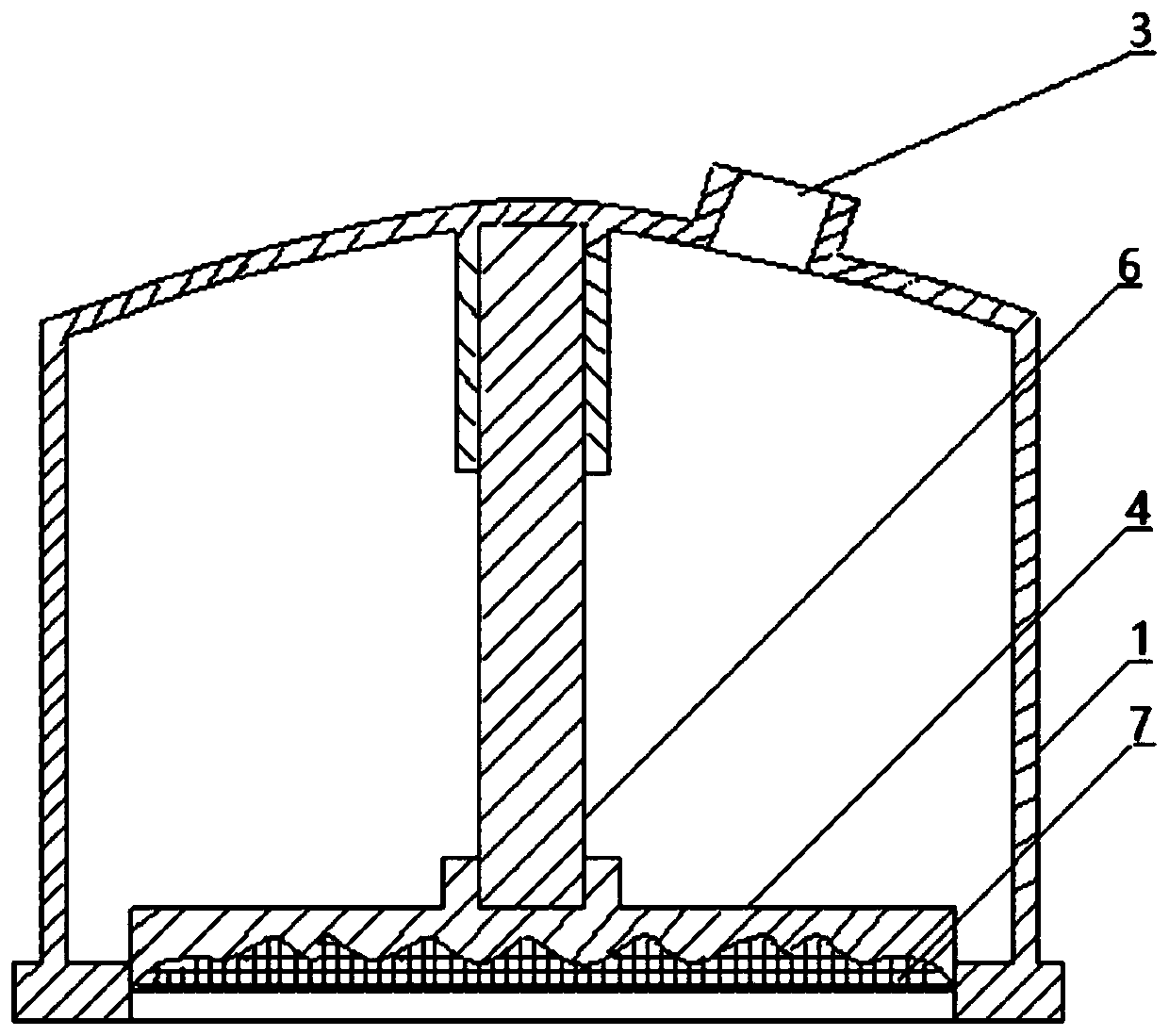
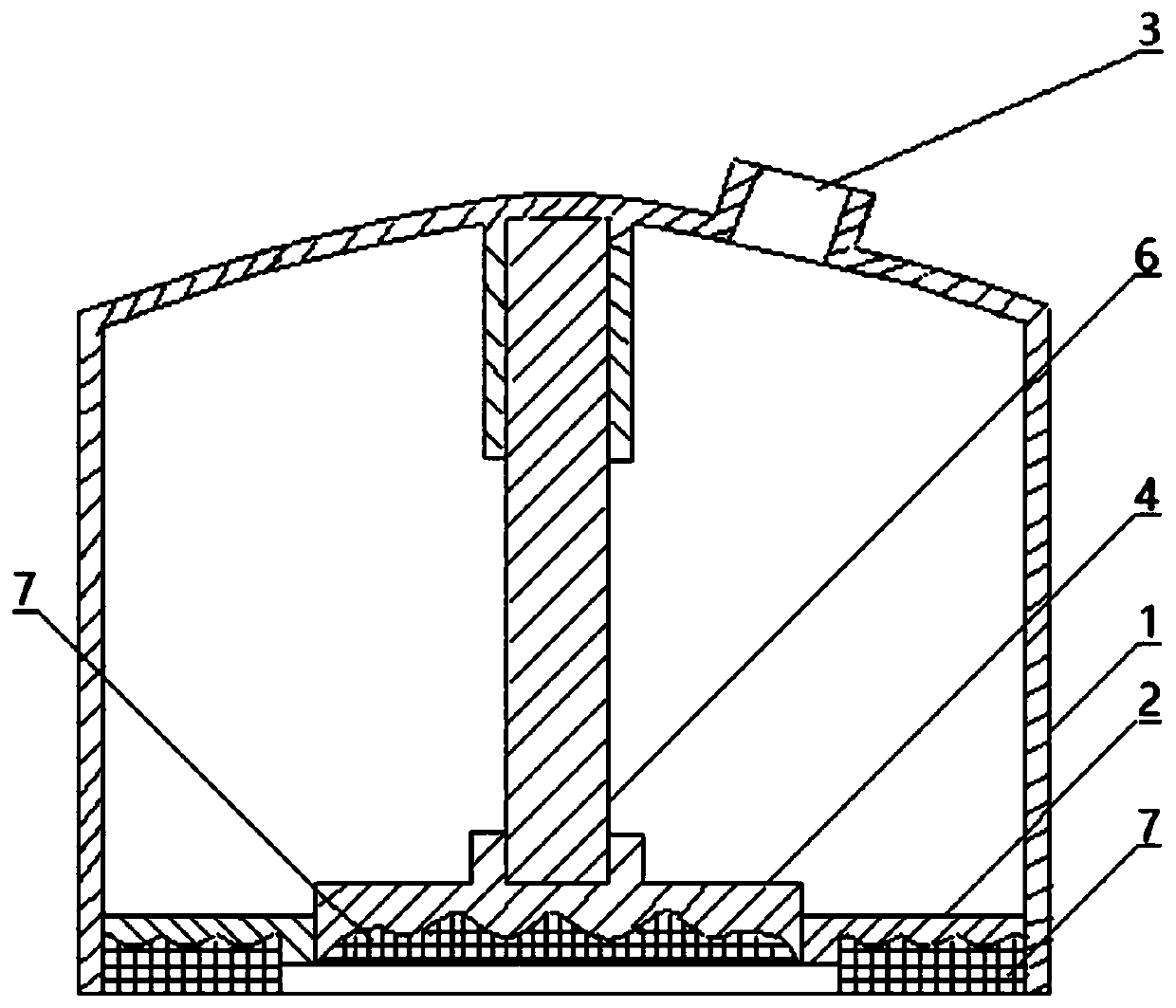
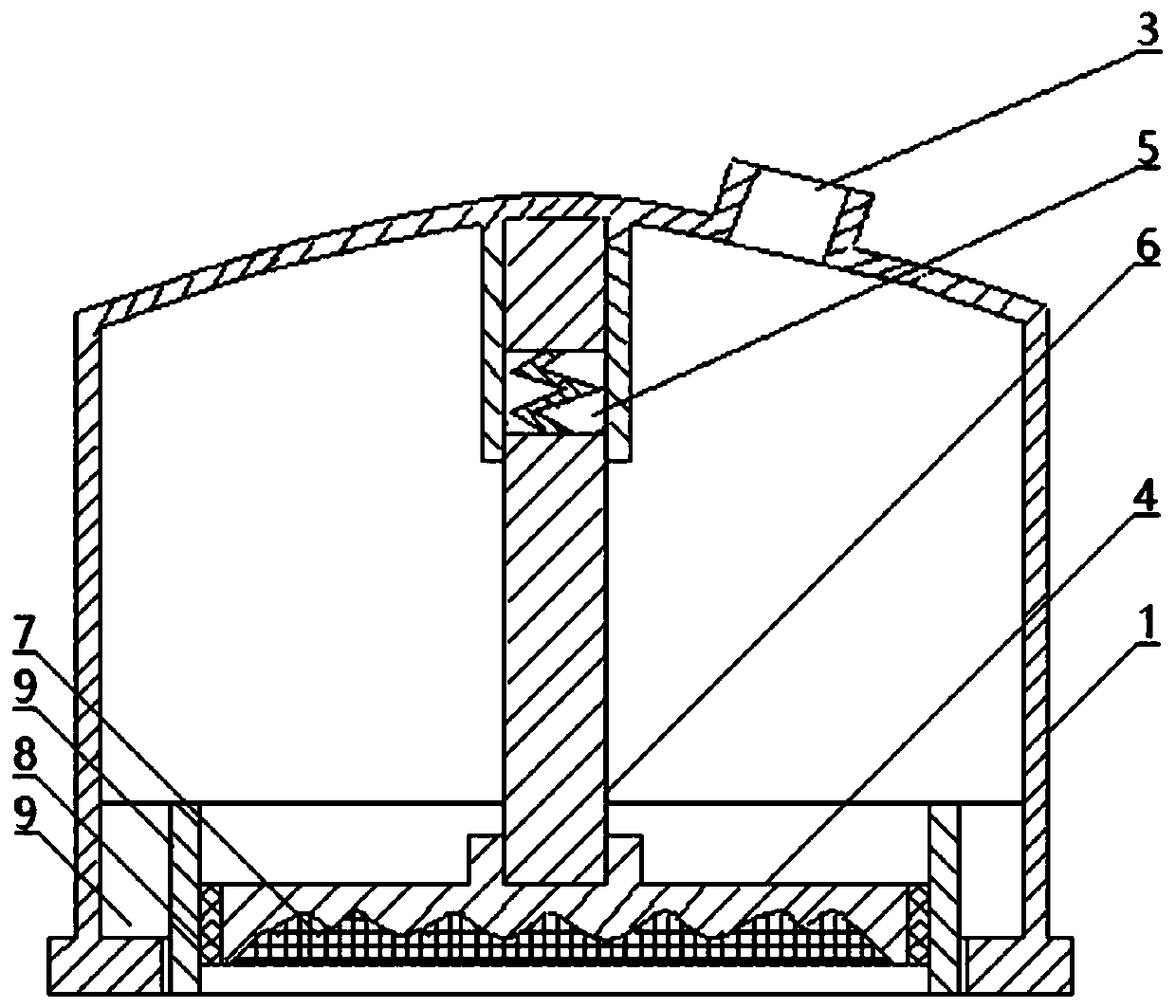
ActiveCN110841185AIncrease surface areaThe overall thickness is thinMedical devicesSkin InjuryStratum corneum
The invention relates to a pressure drug delivery device and a drug storage core layer, and is suitable for external and transdermal drug delivery. The pressure drug delivery device comprises a negative-pressure shell. One side of the negative-pressure shell is of an enclosed shell with a negative-pressure air outlet, and the other side of the negative-pressure shell is of an open end. At least one open drug delivery end with one or more recessed drug delivery surfaces on the cross section line, or at least one middle drug delivery plate with one or more recessed drug delivery surfaces on thecross section line, is mounted on the side of the open end of the negative-pressure shell. The drug storage core layer with one or more matched protrusions on the cross section is mounted on the sideof the drug delivery surface. The pressure drug delivery device has the advantages that the surface area of stratum corneum is increased, the thickness is reduced, skin injuries are small, the drug delivery pressure is uniform and high, the drug delivery amount, depth and area are large, the drug delivery distribution is uniform, there is a step-by-step drug delivery process based on infiltration,requirements on the size of delivered particles are low, the dosage form range is wide, the cost is low, and operations are simplified.
Owner:张子辰
Recombinant human esoderma colyone adenovirus, and its preparing method and use
ActiveCN100494376CGrowth inhibitionInhibit migrationPeptide/protein ingredientsGenetic material ingredientsPharmaceutical drugEndostatin Gene
The invention supplies a new recombination endostatin gene that connects the 3' end of IL-2 gene signal peptide sequence with the 5' end of endostatin coding sequence, and the gene could be constructed into adenovirus and make anti-tumor injection. The injection would restrain the growth and transferring of tumor in body and keep entire bioactivity. It has the advantages of lowering medicine cost, improving life quality of patients and good market prospect.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Benzamide analog mediated brain-targeting delivery system
ActiveCN102160894BStrong complianceLarge dosagePowder deliveryNervous disorderBrain disorder diagnosisIn vivo
The invention belongs to the field of medicinal preparations, and relates to a brain-targeting delivery system. The system comprises a mediating molecule, a vector and a medicament, wherein the mediating molecule is a benzamide analog, the vector is a polycation macromolecule, and the mediating molecule and the vector are combined by a covalence mode; and the medicament is carried through an entrapping or adsorption mode. In the system, in-vivo and in-vitro brain-targeting is characterized through a tracer technique to prompt that the delivery system can span a blood brain barrier, and a gene medicament and a diagnosis medicament are delivered into a brain, so the system can be used for brain disease diagnosis and treatment. The system can avoid potential risks of an enteroinvasive administration mode and a complicated administration process, and has the advantages of large administration quantity, simple administration mode and the like; and the brain-targeting mediated molecule has the advantages of small molecular weight, low price and availability and industrialization convenience.
Owner:SHANGHAI WHITTLONG PHARMA INST
Use of PNAS-4 gene in preparing antineoplastic and antineoplastic auxiliary medicament
InactiveCN101130081BImprove the quality of lifeGood effectPeptide/protein ingredientsAntineoplastic agentsSide effectLife quality
The invention relates to a use of PNAS-4 gene in aspects of preparing antineoplastic in the tumour gene treatment field. The invention with PNAS-4 recombinant vector can limit the growth and the migration of the endocytosis effectively, which can limit the growth of various tumors and extend the survive time of mouse. The antineoplastic of the invention with PNAS-4 recombinant vector liposome andchemotherapy drug cisplatin and magnolol as the active component has better effect than the single application and can reduce the usage of both parties. The product has the appreciable effect, the little toxic and side effect and the easy preparing method, which compensates the defect of protein infusion drug, increases the time interval of medicine, reduces the usage, reduces the economic burdenof the patient, improves the life quality of patient and has the wide market prospect.
Owner:SICHUAN UNIV
A biological and chemical coupling sludge conditioning method
ActiveCN105417915BImprove dehydration effectEnergy-enhancing substancesSludge treatment by de-watering/drying/thickeningRunoff/storm water treatmentChemical couplingWater content
The invention discloses a biology and chemistry coupled sludge conditioning method and belongs to the technical field of environment engineering. The invention aims to solve the problem that the conventional municipal sludge processing technology has the disadvantages of bad dehydration effect, high cost, and long period under a low temperature condition. The method comprises the following steps: mixing processed materials, returned sludge, and a mixed solution of medicament A in a contact area, carrying out reactions in a reaction area; returning part of mixed sludge to the contact area, mixing the other part of mixed sludge with a mixed solution of medicament B in next reaction area, and carrying out biochemical reactions. Two-point medicament addition technology is adopted, the addition amount of medicament is little, and the cost is low. The efficiency of biological reactions is high. The reaction period of biological acidification required by bioleaching is shortened by 25% or more. The sludge dehydration effect under a low temperature condition is improved. The water content of sludge is less than 60% after deep hydration at a temperature less than 10 DEG C and the sludge conditioning time is shorter than 36 hours. The provided method is beneficial for the subsequent recycling of dehydrated sludge and the treatment of press-filtered acidic liquid.
Owner:HARBIN INST OF TECH
Slow-released indapamide capsule and its preparation process
InactiveCN1175812CLittle side effectsFully absorbedOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSide effect
An indapamide sustained-release capsule and a preparation method thereof. It is composed of indapamide, slow-release materials and auxiliary materials in a weight ratio of 0.5-3.0:5-20:131-165 to form slow-release pellets with different release degrees. The slow-release materials are acrylic resin, hydroxyl Any one to four of propyl methylcellulose, ethyl cellulose, cellulose acetate phthalate and polyvinylpyrrolidone; the content of indapamide in a single capsule is 0.5-3.0 mg. The preparation method adopts the method of rolling into pellets. The capsule can maintain a balanced and long-lasting effective blood drug concentration within the same dosage and time interval, or reduce the dosage and reduce side effects while maintaining the same drug effect; especially it is a dispersed preparation, which can not only improve the The contact area of the channel can make the drug absorb completely and have high bioavailability, and different combinations of pellets can be used to obtain the ideal drug release rate, and the cost is low. The adopted preparation method has simple process and is easy to operate and control.
Owner:TIANJIN GUOYAO BOHAI BIOMEDICAL
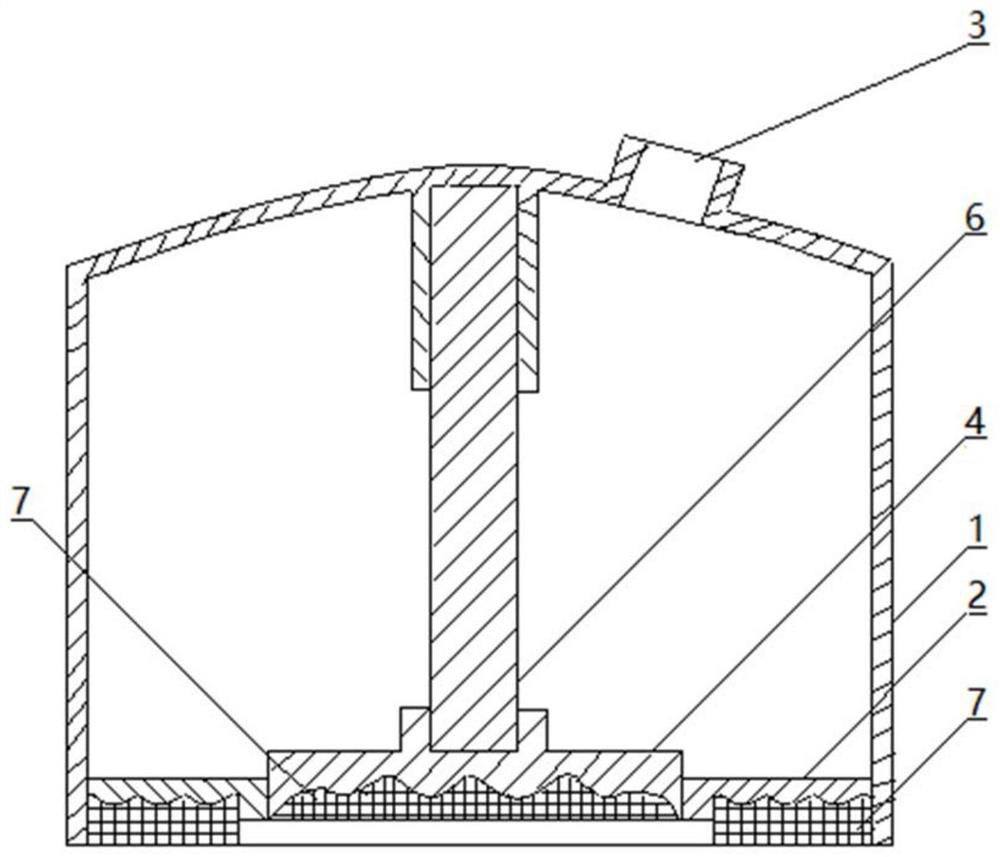
Stress administration device and drug storage core layer
ActiveCN110841185BIncrease surface areaThe overall thickness is thinMedical devicesSkin InjuryStratum corneum
The present invention involves a pressure supply device and drug storage core layer, which is suitable for external use and meridian skin administration.It has a negative pressure shell. On one side of the negative pressure shell is a closed shell equipped with a negative pressure exhaust port, and the other side is the open end. On one side of the open end of the negative pressure shell, at least one cross -section line is installed.For 0 to multiple concave types, the dating end or a cross -section line is 0 to multiple concave feed on the middle of the administration plate, and the cross -side side is equipped with an cross -section of 0 to multiple matching convex types.Drug storage core layer.The beneficial effect of the present invention is the increase in the surface area of the stratum corneum, thinning thickness, small skin damage, uniform pressure on dose, large administration, deep administration, large administration area, uniform distribution of administration, uniform distribution of administration, gradually improving, gradually given.Drug processes, low dose particle size requirements, large dosage range, low cost, and simple operation.
Owner:张子辰
A kind of nano drug carrier particles with controllable drug release and its preparation method
ActiveCN105902516BGood drug encapsulation abilityReduce leakageOrganic active ingredientsInorganic non-active ingredientsDrug releaseChemo therapy
Owner:SOUTHEAST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com