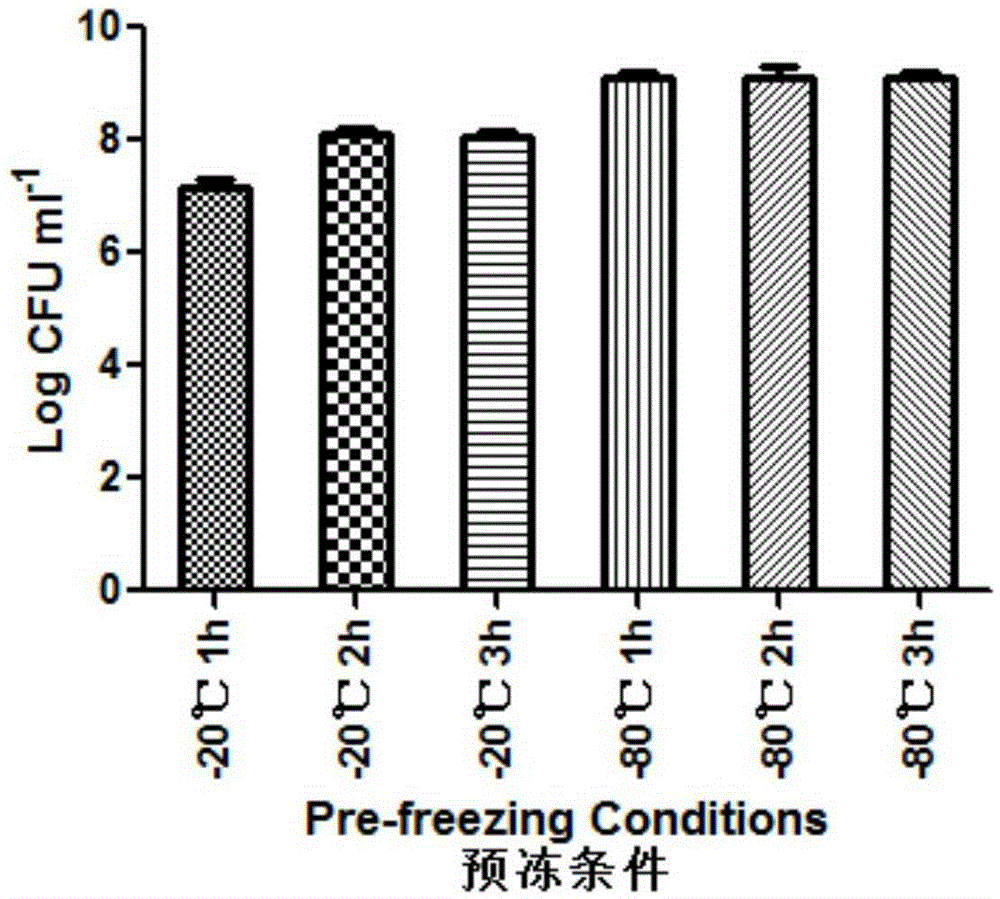
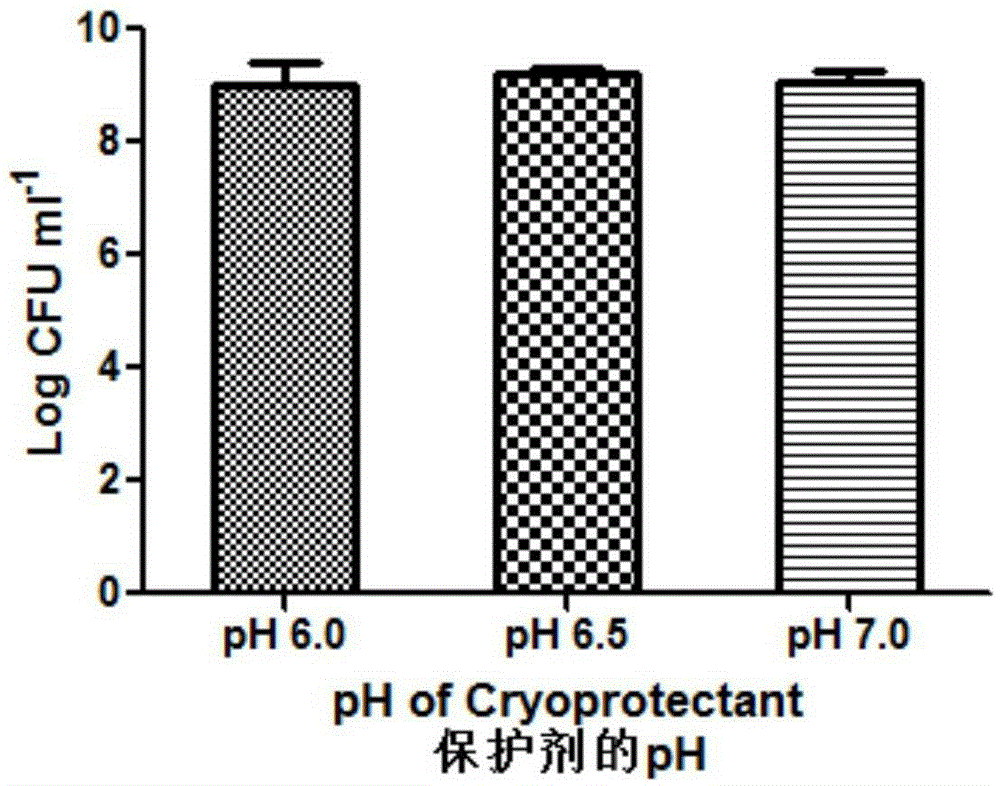
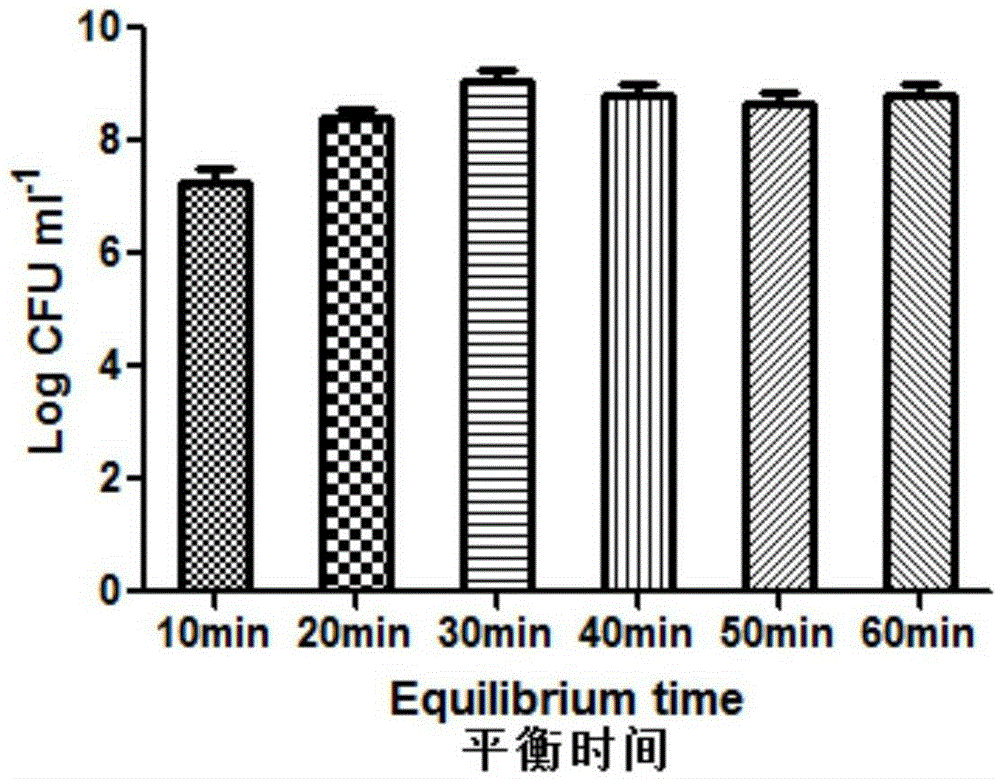
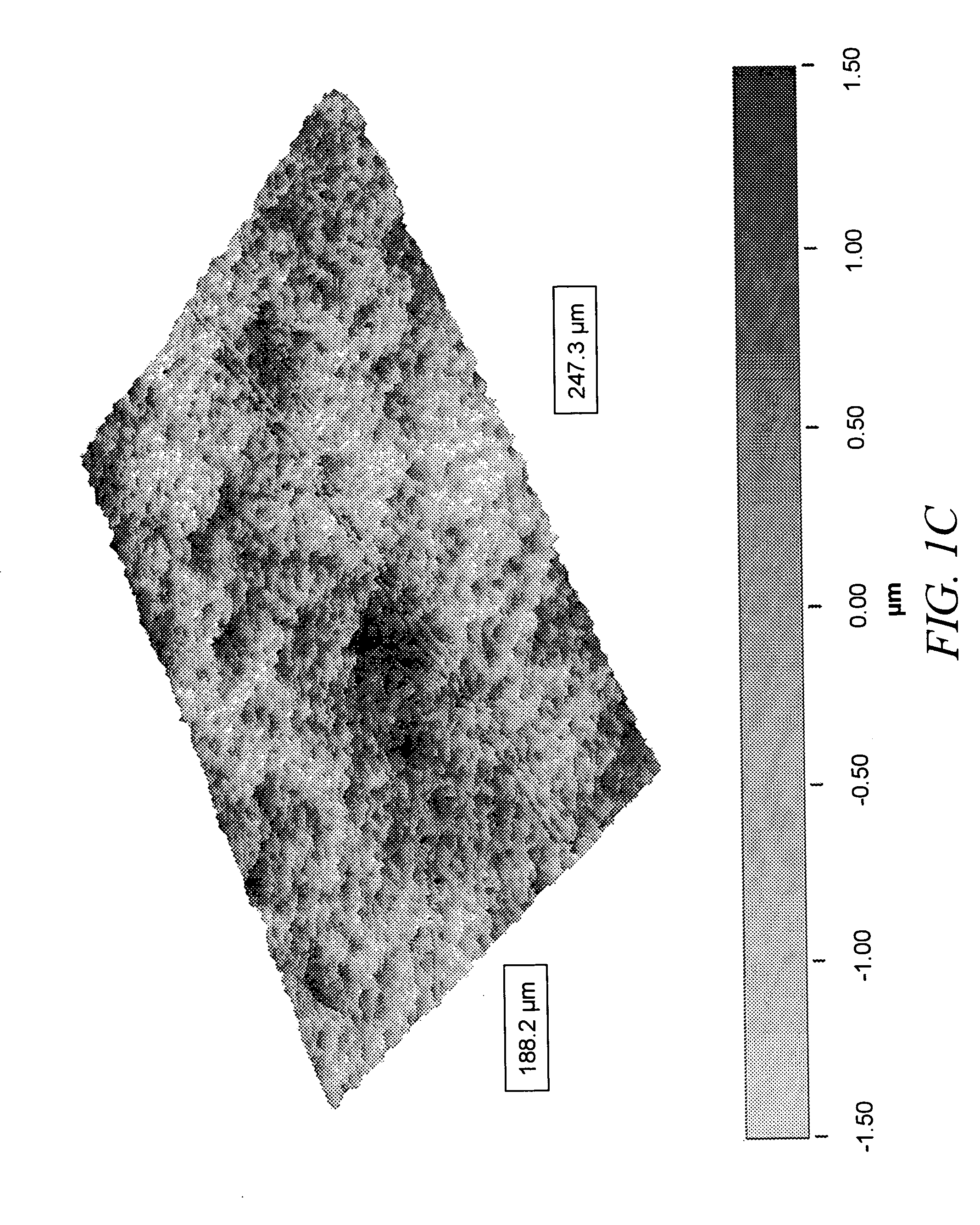
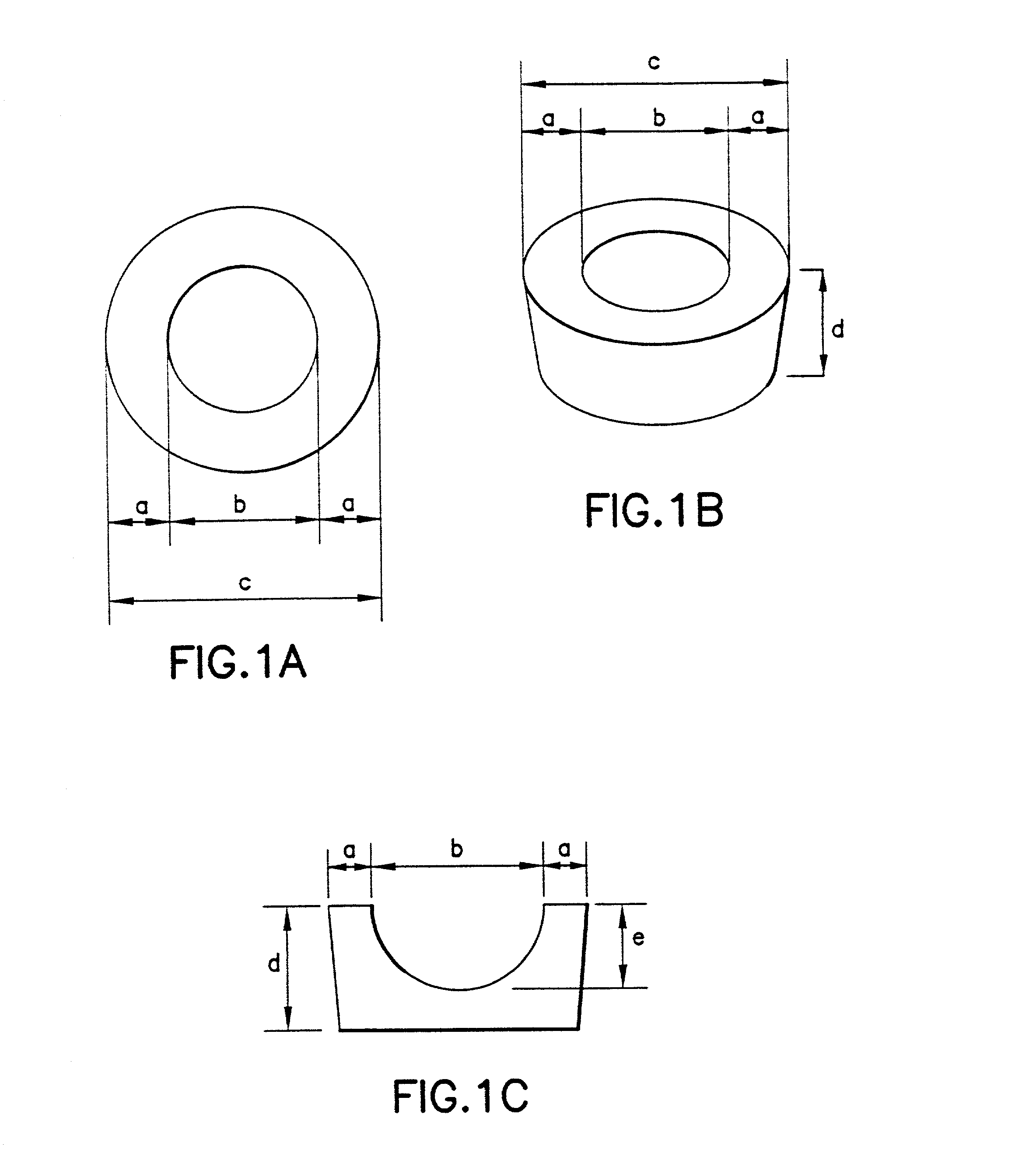
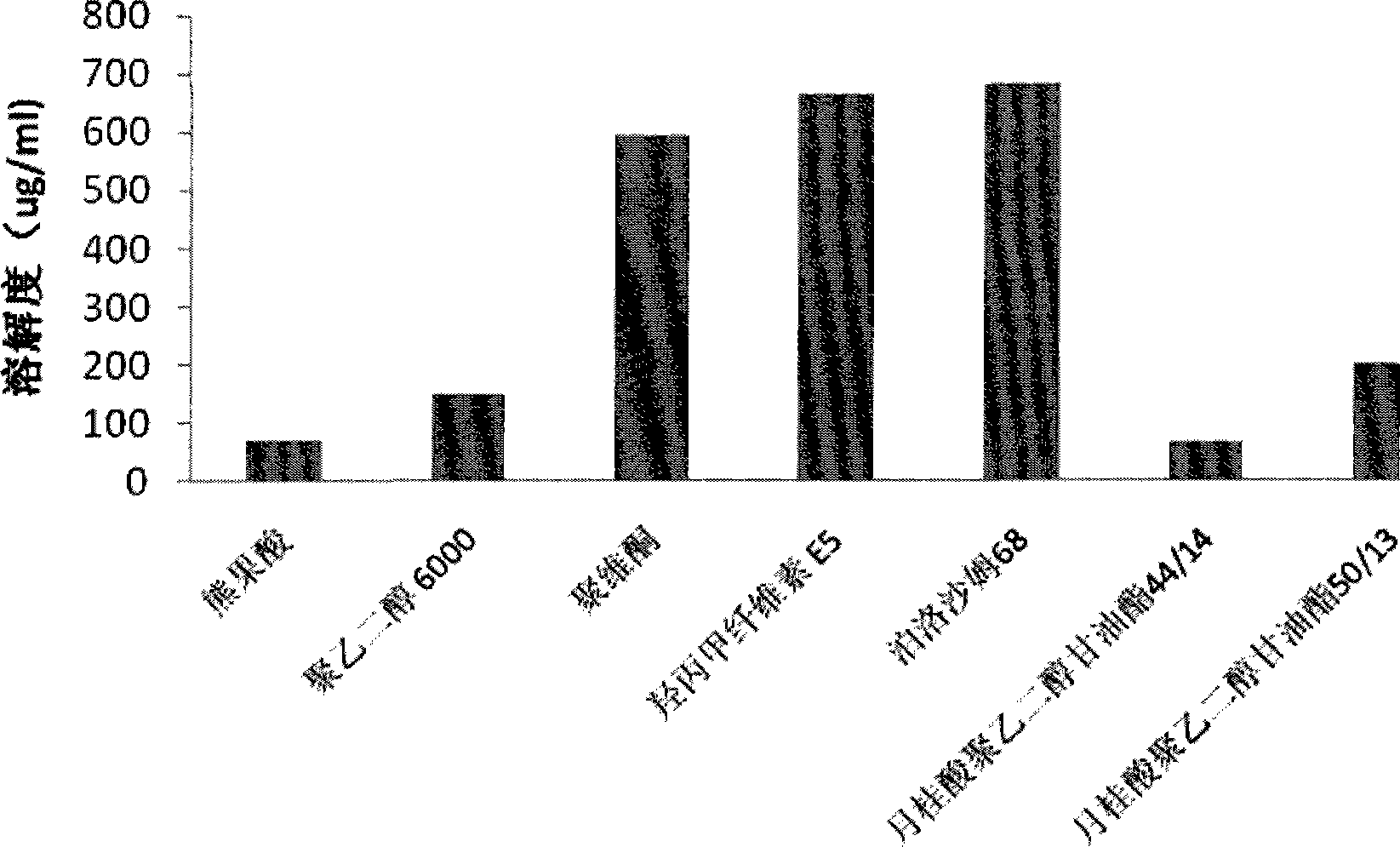
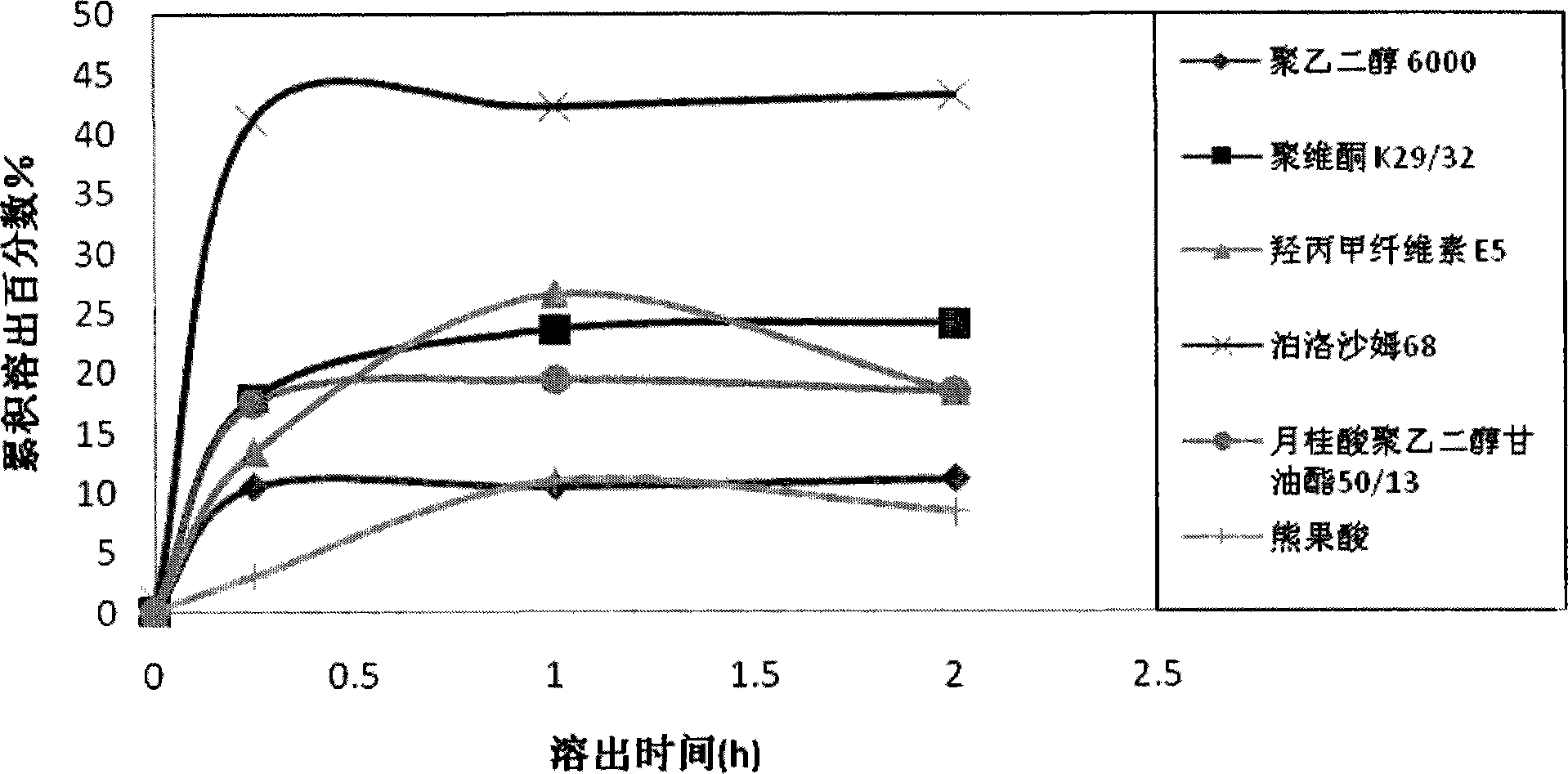
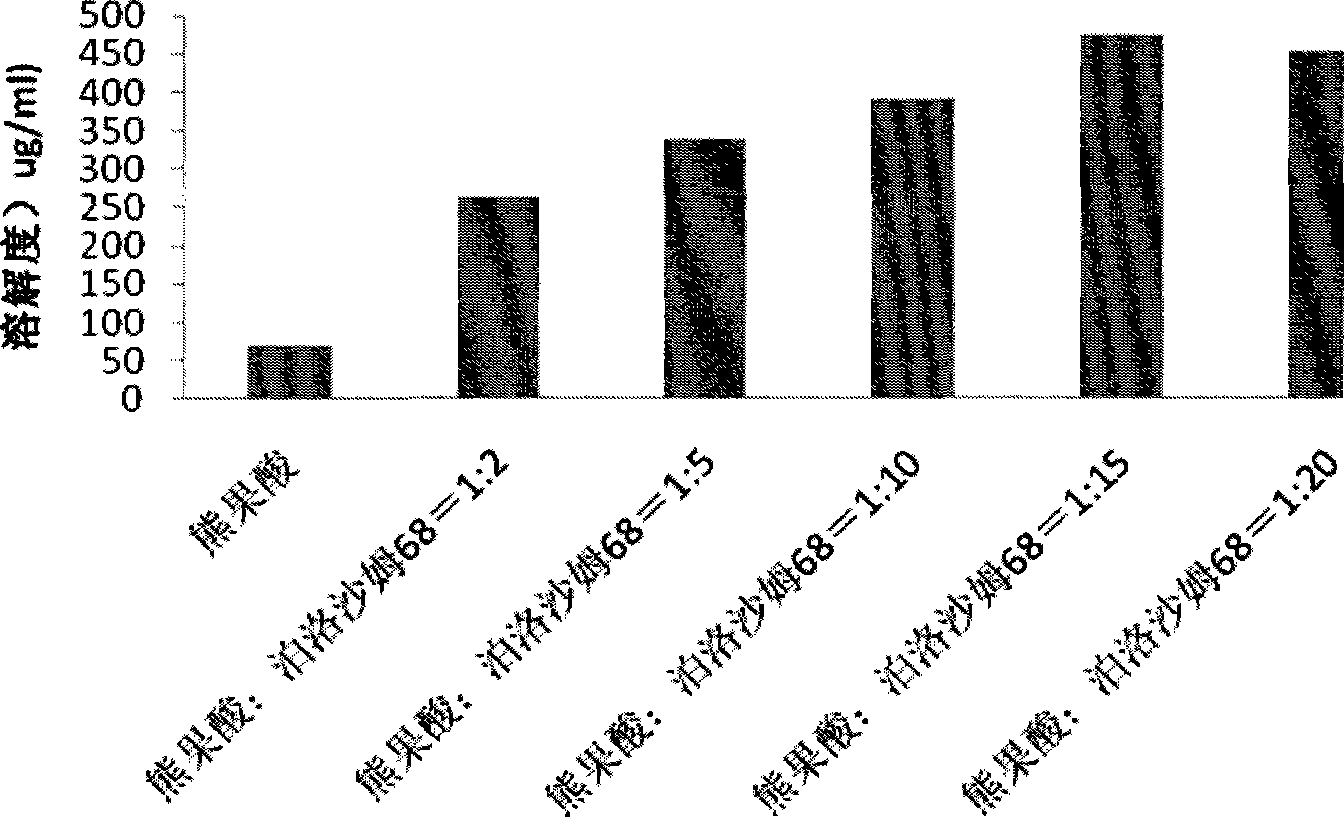
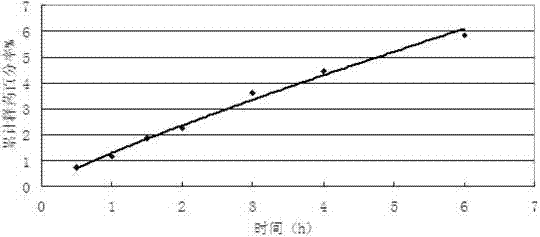
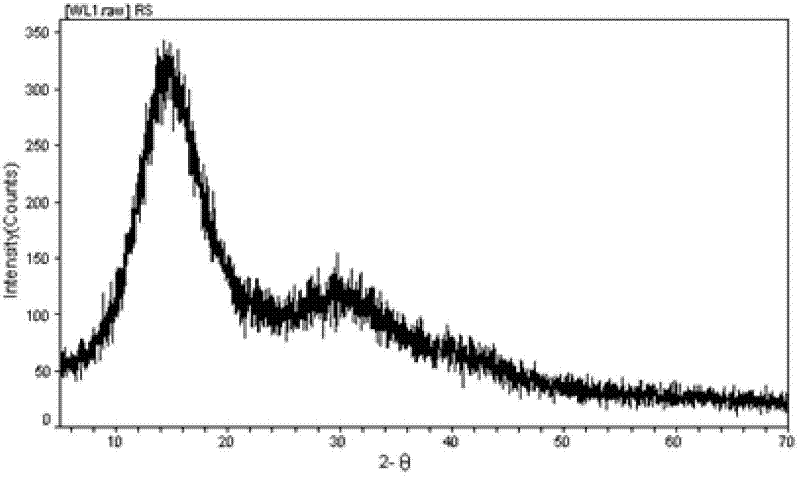
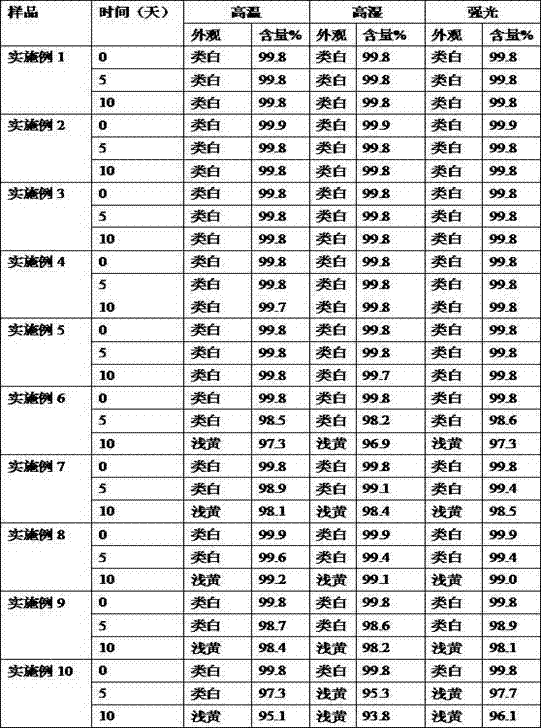
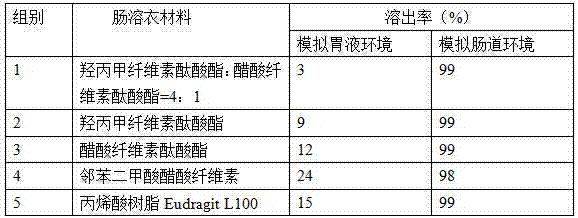
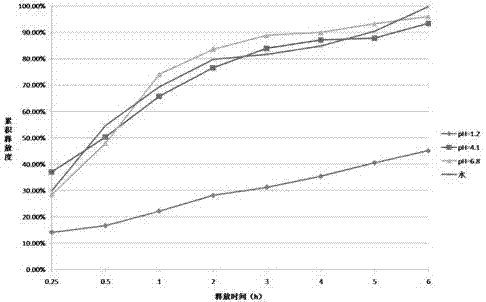
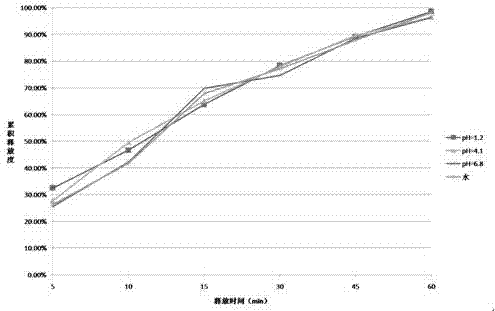
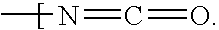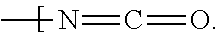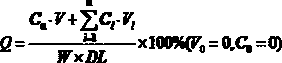Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95 results about "Cellulose acetate phthalate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
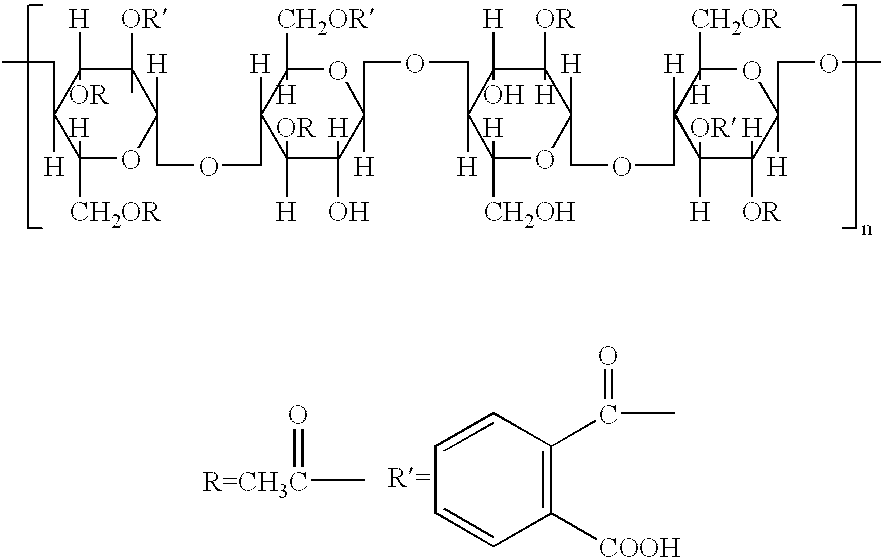
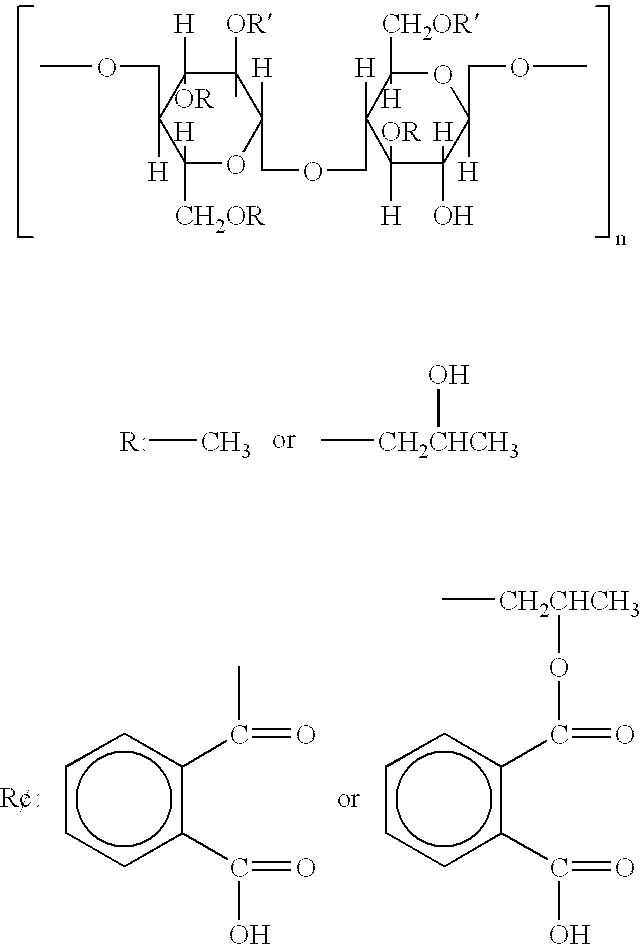
Cellulose acetate phthalate (CAP), also known as cellacefate (INN) and cellulosi acetas phthalas, is a commonly used polymer phthalate in the formulation of pharmaceuticals, such as the enteric coating of tablets or capsules and for controlled release formulations. It is a cellulose polymer where about half of the hydroxyls are esterified with acetyls, a quarter are esterified with one or two carboxyls of a phthalic acid, and the remainder are unchanged. It is a hygroscopic white to off-white free-flowing powder, granules, or flakes. It is tasteless and odorless, though may have a weak odor of acetic acid. Its main use in pharmaceutics is with enteric formulations. It can be used together with other coating agents, e.g. ethyl cellulose. Cellulose acetate phthalate is commonly plasticized with diethyl phthalate, a hydrophobic compound, or triethyl citrate, a hydrophilic compound; other compatible plasticizers are various phthalates, triacetin, dibutyl tartrate, glycerol, propylene glycol, tripropionin, triacetin citrate, acetylated monoglycerides, etc.
Probiotic microcapsules as well as preparation method and application thereof
ActiveCN105310080AImprove the situation of low freeze-drying survival rateImprove stabilityFood freezingFood shapingFreeze-dryingK carrageenan
The invention relates to probiotic microcapsules as well as a preparation method and application thereof. The probiotic microcapsules comprise a core material and a wall material, wherein the core material is probiotics; the outer layer of the wall material is coated with chitosan; the wall material is prepared from an aqueous solution containing a natural polymer material and a freeze-drying protection agent; the freeze-drying protection agent comprises one or more of glucose, fructose, sucrose, lactose, trehalose, soluble starch, glycerin, mannitol, Arabic gum, dextran 40 and skim milk; the natural polymer material comprises one or more of gellan gum, xanthan gum, k-carrageenan, sodium alginate, cellulose acetate phthalate or gelatin; in the aqueous solution, the volume fraction of the freeze-drying protection agent is 4.0%-20.0% and the volume fraction of the polymer material is 0.5%-5.0%. The probiotic microcapsules can keep excellent acid resistance and storage stability before and after being freeze-dried.
Owner:SUN YAT SEN UNIV
Controlled release composition
InactiveUS20080254124A1Easy for to follow prescribed regimenConstant of releasePowder deliverySolution deliveryEthyl(hydroxyethyl)celluloseCellulose acetate
A composition for controlled delivery of at least one active substance into an aqueous medium by erosion at a preprogrammed rate of at least one surface of the composition, comprising a matrix comprising the active substance, the matrix being erodible in the aqueous medium in which the composition is to be used, and a coating having at least one opening exposing at least one surface of said matrix, the coating comprising a first cellulose derivative which has thermoplastic properties and which is substantially insoluble in the aqueous medium in which the composition is to be used, and at least one of a second cellulose derivative which is soluble or dispersible in water, a plasticizer, and a filler. The coating is a coating which crumbles and / or erodes upon exposure to the aqueous medium such as a body fluid. The first cellulose derivative may be, e.g., ethylcellulose, cellulose acetate, cellulose propionate or cellulose nitrate, and the second cellulose derivative may be, e.g. methylcellulose, carboxymethylcellulose or salts thereof, cellulose acetate phthalate, microcrystalline cellulose, ethylhydroxyethylcellulose, ethylmethylcellulose, hydroxyethylcellulose, hydroxyethylmethylcellulose, hydroxypropylcellulose, hydroxypropylmethylcellulose, hydroxymethylcellulose or hydroxymethylpropylcellulose.
Owner:EGALET LTD
Controlled release composition
InactiveUS20050019405A1Easy for to follow prescribed regimenConstant of releasePharmaceutical non-active ingredientsCoatingsEthyl(hydroxyethyl)celluloseCellulose acetate
A composition for controlled delivery of at least one active substance into an aqueous medium by erosion at a preprogrammed rate of at least one surface of the composition, comprising a matrix comprising the active substance, the matrix being erodible in the aqueous medium in which the composition is to be used, and a coating having at least one opening exposing at least one surface of said matrix, the coating comprising a first cellulose derivative which has thermoplastic properties and which is substantially insoluble in the aqueous medium in which the composition is to be used, and at least one of a second cellulose derivative which is soluble or dispersible in water, a plasticizer, and a filler. The coating is a coating which crumbles and / or erodes upon exposure to the aqueous medium such as a body fluid. The first cellulose derivative may be, e.g., ethylcellulose, cellulose acetate, cellulose propionate or cellulose nitrate, and the second cellulose derivative may be, e.g., methylcellulose, carboxymethylcellulose or salts thereof, cellulose acetate phthalate, microcrystalline cellulose, ethylhydroxyethylcellulose, ethylmethylcellulose, hydroxyethylcellulose, hydroxyethylmethylcellulose, hydroxypropylcellulose, hydroxypropylmethylcellulose, hydroxymethylcellulose or hydroxymethylpropylcellulose.
Owner:EGALET LTD
Water dispersible film
InactiveUS20050070501A1Avoid difficultyAntibacterial agentsOrganic active ingredientsDiseaseComposite film
Owner:NEW YORK BLOOD CENT
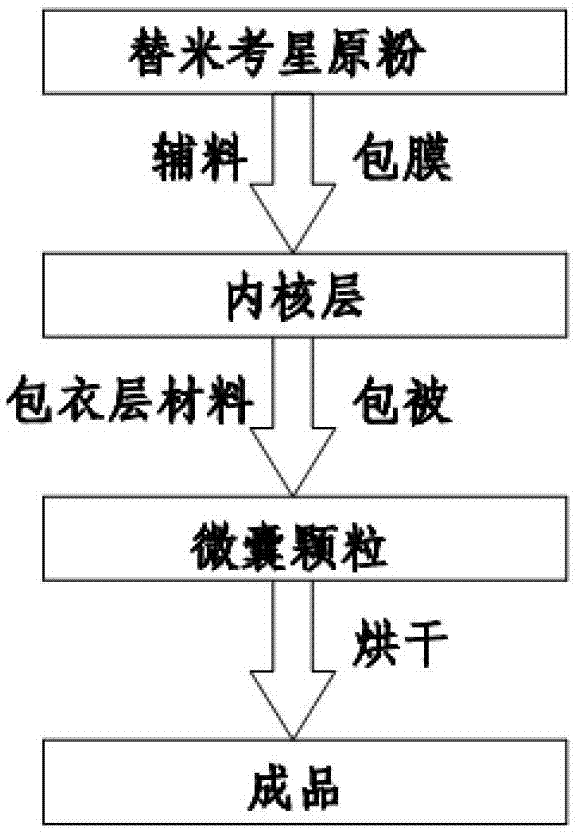
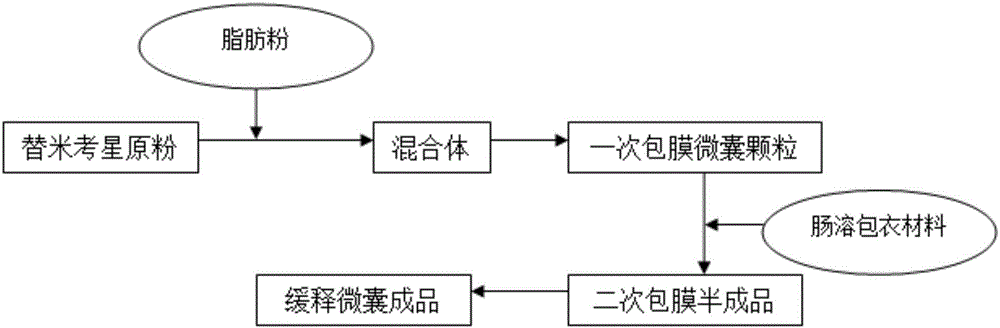
Enteric-coated tilmicosin slow-release micro-capsule preparation and preparation method thereof
InactiveCN103083281AProlong the action timeTo achieve the purpose of sustained releaseAntibacterial agentsOrganic active ingredientsMonoglycerideAcrylic resin
The invention relates to an enteric-coated tilmicosin slow-release micro-capsule preparation and a preparation method thereof and belongs to the field of tilmicosin preparations. The enteric-coated tilmicosin slow-release micro-capsule preparation provided by the invention comprises an inner core layer and a coating layer, wherein the inner core layer comprises tilmicosin raw powder and an auxiliary material; the auxiliary material comprises one or more than one of stearic acid, glycerin monostearate, stearyl alcohol, saturated triglyceride, monoglyceride and paraffin; and the coating layer is made from one or more than one of cellulose acetate phthalate, hydroxypropyl methyl cellulose phthalate, acrylic resin, polyvinyl acetate phthalate and acetic hydroxypropyl methylcellulose succinate. The preparation method comprises the following steps of: carrying out primary coating on the tilmicosin raw powder and the auxiliary material, carrying out secondary coating by using the materials of the coating layer, and drying to obtain the finished product. According to the invention, the tilmicosin is coated by using high polymer materials and the coated tilmicosin micro-capsule is undissolved in acid environment and slowly dissolved in alkaline environment of enteric canal, so that the purpose of slow release is achieved and the action time of the tilmicosin is prolonged.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
Method for compounding polymer pellets with functional additives
New methods of forming compounded cellulose esters are provided. The methods comprise mixing a cellulose ester, functional additive, and a swelling agent and subsequently removing at least a portion of the swelling agent. The swelling agent is one that assists in causing the functional additive to penetrate into the cellulose ester, while not acting significantly as a solvent for the cellulose ester. Preferred cellulose esters include, but are not limited to, cellulose acetates, cellulose triacetates, cellulose acetate phthalates, and cellulose acetate butyrates. The functional additive can be a plasticizer, stabilizer, or other additive selected to modify a particular property of the cellulose.
Owner:EASTMAN CHEM CO
Biodegradable microbicidal vaginal barrier device
An intravaginal bio-erodible microbicidal barrier device. The device comprises (a) at least one micronized compound selected from the group consisting of cellulose acetate phthalate and hydroxypropylmethylcellulos- e phthalate, and (b) at least one water soluble or water dispersible cellulose compound selected from the group consisting of hydroxypropylmethylcellulose, methylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, hydroxyethylmethylcellulose, hydroxyethylethylcellulose and hydroxypropylethylcellulose; or a pectin, such as an apple pectin. The device is prepared by a combination of foaming, freezing and freeze-drying processes.
Owner:NEW YORK BLOOD CENT
Ursolic acid solid dispersion and preparation method thereof
ActiveCN102871950AImprove solubilityIncrease dissolution rateAntibacterial agentsOrganic active ingredientsSolubilityUrsolic acid
The invention discloses an ursolic acid solid dispersion and a preparation method thereof. The formula of the ursolic acid solid dispersion comprises ursolic acid and a carrier material; the mass ratio of the ursolic acid to the carrier material is 1:2 to 1:20; and the carrier material is one or more of PEG6000 (polyethylene glycol 6000), povidone k29 / 33, hypromellose E5, polyving alcohol, poloxamer 68, GELUCIRE 50 / 13, polyacrylic resin EPO, polyacrylic resin L 100-55, povidone-vinyl alcohol, hypromellose AS-HG, hypromellose AS-LG, hypromellose AS-MG, low substituted hypromellose, hypromellose phthalate, cellulose acetate phthalate, glucan, polyoxyethylene, polyoxyethylene stearates and polyvinyl acetate phthalate. The preparation method comprises the following steps: dissolving the ursolic acid and the carrier material into an organic solvent according to the formula; removing the organic solvent; and grinding. The ursolic acid solid dispersion has good solubility and has high bioavailability.
Owner:CHINA GATEWAY PHARMA DEV CO LTD
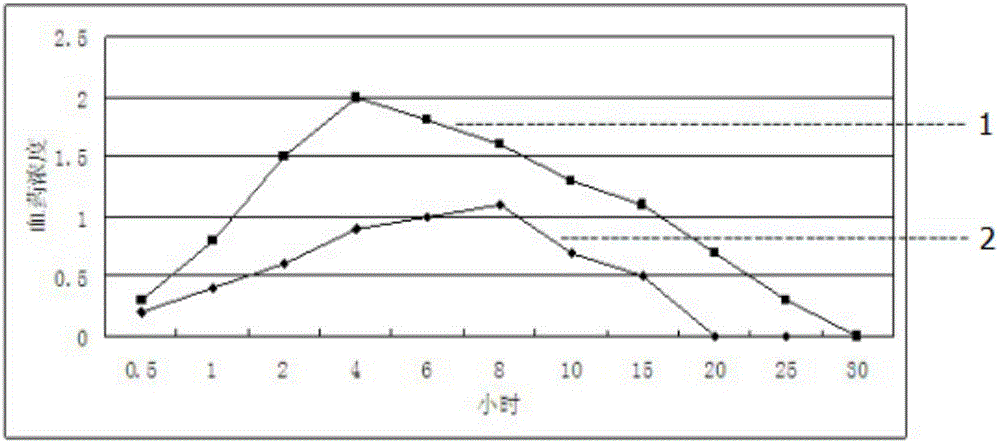
Enteric-coated tilmicosin sustained release microcapsule and preparation method thereof
InactiveCN106176680AProlong the action timeTo achieve the purpose of sustained releaseAntibacterial agentsOrganic active ingredientsDispersityAcrylic resin
The invention discloses an enteric-coated tilmicosin sustained release microcapsule and a preparation method thereof, and belongs to the field of tilmicosin preparations. The enteric-coated tilmicosin sustained release microcapsule is prepared from 10wt%-50wt% of raw tilmicosin powder, 40wt%-88wt% of an auxiliary fatty powder material and 2wt%-10wt% of an enteric coating material, wherein the enteric coating material is prepared from one or more of cellulose acetate phthalate, hydroxypropyl methylcellulose phthalate, L-type acrylic resin, S-type acrylic resin, polyvinyl acetate phthalate and hydroxypropyl methylcellulose acetate succinate; the diameter of the prepared microcapsule is 50-200 mu m. The preparation method comprises the steps as follows: the raw tilmicosin powder and the auxiliary material are subjected to primary coating, are subjected to secondary coating with the enteric coating material and then are dried, and a finished product is obtained. According to the enteric-coated tilmicosin sustained release microcapsule and the preparation method thereof, the sustained release purpose is achieved, the acting time of tilmicosin is prolonged, the fluidity and the dispersity of a drug are improved, the pharmacodynamical function is remarkably improved, and the dosage of the drug is reduced.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
Hemostatic device
ActiveUS20090156711A1Impression capsSurgical adhesivesCellulose acetate phthalateHydroxypropylmethylcellulose phthalate
A hemostatic device comprising (i) a carrier comprising at least one component selected from the group consisting of hydroxypropyl methylcellulose phthalate, hydroxypropyl methylcellulose acetate succinate, cellulose acetate phthalate; polyvinylacetate phthalate, cellulose acetate phthalate, acetaldehyde dimethylcellulose acetate, polymethacrylate-based polymers, and derivatives, salts, copolymers or combinations thereof; and (ii) thrombin
Owner:ETHICON INC
Crosslinkable, cellulose ester compositions and films formed therefrom
ActiveUS20060286397A1Improve stabilityOvercome problemsSynthetic resin layered productsCellulosic plastic layered productsTectorial membraneCellulose acetate phthalate
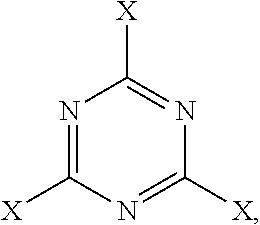
New compositions for forming films for use in optical devices are provided. The compositions comprise a cellulose and a crosslinking agent dissolved or dispersed in a solvent system. Preferred celluloses are cellulose esters such as cellulose acetates, cellulose triacetates, cellulose acetate phthalates, and cellulose acetate butyrates. Preferred crosslinking agents are triazines such as those derived from melamine and benzoguanamine. The inventive compositions can be used to form, for example, protective and / or compensation films for use in polarizing plates.
Owner:EASTMAN CHEM CO
Method for compounding polymer pellets with functional additives
New methods of forming compounded cellulose esters are provided. The methods comprise mixing a cellulose ester, functional additive, and a swelling agent and subsequently removing at least a portion of the swelling agent. The swelling agent is one that assists in causing the functional additive to penetrate into the cellulose ester, while not acting significantly as a solvent for the cellulose ester. Preferred cellulose esters include, but are not limited to, cellulose acetates, cellulose triacetates, cellulose acetate phthalates, and cellulose acetate butyrates. The functional additive can be a plasticizer, stabilizer, or other additive selected to modify a particular property of the cellulose.
Owner:EASTMAN CHEM CO
Novel enteric sustained-release pellet for feed and preparation method thereof
ActiveCN102308916AReduce weight ratioReduce diarrhea rateAnimal feeding stuffAccessory food factorsSustained release pelletsCellulose acetate
The invention relates to a novel enteric sustained-release zinc oxide-oligosaccharide composite pellet for feed and a preparation method thereof. The novel enteric sustained-release zinc oxide-oligosaccharide composite pellet for feed comprises: by weight, 38 to 75% of zinc oxide powder, 15 to 35% of oligosaccharide, 2 to 14% of starch, 1.5 to 5.5% of cellulose acetate-phthalate, 0.05 to 0.1% of sodium alginate, 4.5 to 9.5% of talcum powder, and 0.15 to 0.5% of diethyl phthalate. The novel enteric sustained-release zinc oxide-oligosaccharide composite pellet for feed has the advantages of good effects of dissolution in the enteric canal and good treatment effects.
Owner:珠海天凯生物科技有限公司
Anti-infective hygiene products based on cellulose acetate phthalate
InactiveUS20070082035A1Hinders its propagationOrganic active ingredientsBiocideCellulose acetate phthalateCellulose acetate
Owner:NEW YORK BLOOD CENT
Gingko leaf sustained release formulation and preparation process thereof
InactiveCN1631395AReduce the amount addedLittle side effectsPharmaceutical delivery mechanismUnknown materialsAdjuvantCellulose acetate
The invention provides a gingko leaf sustained release formulation and preparation process, wherein the formulation comprises total flavone glucoside extracted from ginkgo leaves as active components, slow release material coated on the outer layer of the reactive component and adjuvant by the weight ratio of 40-50:50-70:290-300, and is prepared into slow release micro-pellet with different releasing rate, the slow release material can be any one to four of acrylic acid resin, methyl hydroxypropylcellulose, ethyl cellulose, cellulose acetate phthalate, and polyvinylpyrrolidone.
Owner:天津米克莱特生物技术有限公司
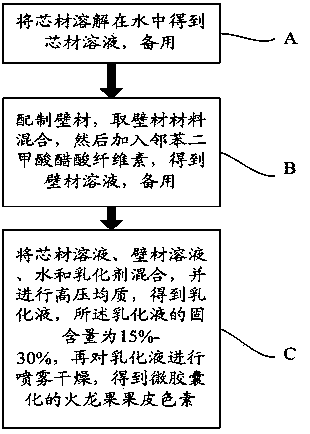
Method for microencapsulating pitaya peel pigment
ActiveCN103349287AImprove stabilityImprove solubilityFood shapingFood preparationSolubilityCellulose acetate phthalate
The invention discloses a method for microencapsulating pitaya peel pigment. The method comprises the following steps: A, dissolving a core material (namely pitaya peel pigment) into water, with that, obtaining a core material solution for standby, wherein the concentration of the core material solution is 95-98 percent; B, preparing a wall material, taking and mixing the wall material, and adding cellulose acetate phthalate into the wall material, with that, obtaining a wall material solution for standby; C, mixing the core material solution, the wall material solution, the water and an emulsifier, performing high-pressure homogenization to obtain emulsion, wherein the solid content of the emulsion is 15-30 percent; performing spray drying to the emulsion to obtain the microencapsulated pitaya peel pigment. According to the invention, via microencapsulating the pitaya peel pigment, the stability of the pitaya peel pigment and the solubility of the pitaya peel pigment in a fat-soluble medium are improved, and the technical effect of the food industry application of the pitaya peel pigment is expanded.
Owner:FOSHAN POLYTECHNIC +1
Hemostatic device
A hemostatic device comprising (i) a carrier comprising at least one component selected from the group consisting of hydroxypropyl methylcellulose phthalate, hydroxypropyl methylcellulose acetate succinate, cellulose acetate phthalate; polyvinylacetate phthalate, cellulose acetate phthalate, acetaldehyde dimethylcellulose acetate, polymethacrylate-based polymers, and derivatives, salts, copolymers or combinations thereof; and (ii) thrombin.
Owner:ETHICON INC
Polyvinylidene fluoride composite film
InactiveCN105817145AReduce interfacial resistanceImprove thermal stabilitySemi-permeable membranesComposite filmBetaine
The invention discloses a polyvinylidene fluoride composite film, the raw materials of which include by weight: 20-30 parts of polyvinylidene fluoride, 1-5 parts of poly(acrylonitrile-acrylic acid-N-vinylpyrrolidone) terpolymer, 1-3.5 parts of acrylamide, 0.5-2 parts of carboxybetaine methacrylate, 1-5 parts of cellulose acetate phthalate, 0.5-2 parts of inorganic particles, 2-3.5 parts of polyvinylpyrrolidone, 55-70 parts of solvent 0.5-1.5 parts of polyethylene glycol monostearyl ether; the inorganic particles are mesoporous silica, zeolite molecular sieve loaded with silver ions, nano-calcium carbonate, nano-zinc oxide, modified montmorillonite, and nano-zirconia. Mixture in weight ratio 1‑5:2‑6:1‑5:3‑10:1‑4:2‑5. The polyvinylidene fluoride composite membrane proposed by the invention has high strength, excellent weather resistance, antibacterial and stain resistance.
Owner:安庆市天虹新型材料科技有限公司
Controlled-release floating pharmaceutical compositions
ActiveUS20100310667A1Easy to controlImprove efficiencyPowder deliveryOrganic active ingredientsControlled releaseApparent density
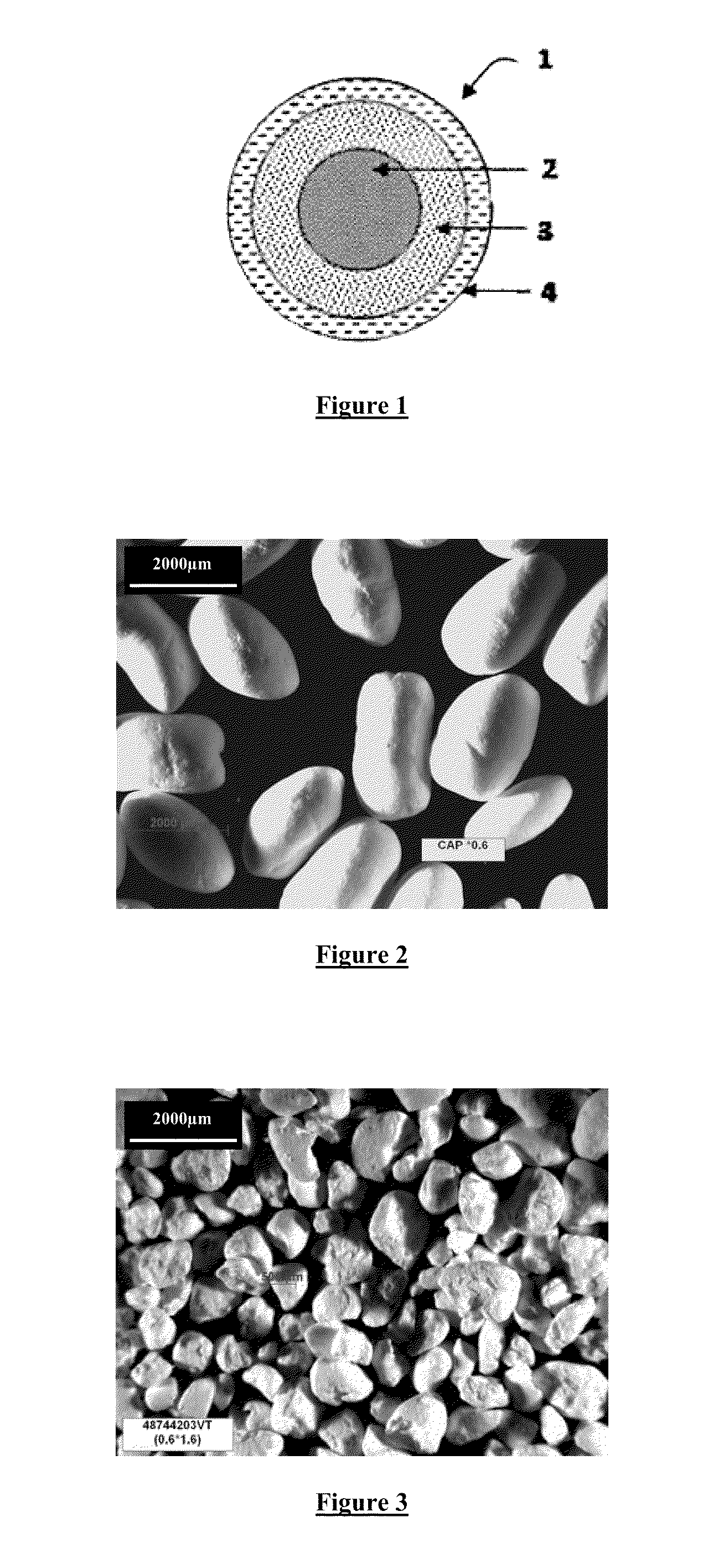
The invention relates to a pharmaceutical composition comprising a plurality of controlled-release coated microparticles each comprising a floating core, on the surface of which is deposited a layer containing at least one active principle, said layer being covered with a controlled-release coating, characterized by the fact that said floating core is composed of cellulose acetate phthalate and has an apparent density of less than or equal to 0.6 g / mL and said coated microparticles have a density of less than or equal to 0.7 g / mL.
Owner:FLAMEL IRELAND
Crosslinkable, cellulose ester compositions and films formed therefrom
InactiveUS20120222793A1Improve stabilityCellulosic plastic layered productsPolarising elementsTectorial membraneCellulose acetate phthalate
New compositions for forming films for use in optical devices are provided. The compositions comprise a cellulose and a crosslinking agent dissolved or dispersed in a solvent system. Preferred celluloses are cellulose esters such as cellulose acetates, cellulose triacetates, cellulose acetate phthalates, and cellulose acetate butyrates. Preferred crosslinking agents are triazines such as those derived from melamine and benzoguanamine. The inventive compositions can be used to form, for example, protective and / or compensation films for use in polarizing plates.
Owner:EASTMAN CHEM CO
High-plasticity wear-resistance ceramic material and preparation method thereof
The present invention discloses a high-plasticity wear-resistance ceramic material and a preparation method thereof. The preparation method comprises: weighing 3-9 parts of neodymium oxide, 5-12 parts of alumina, 20-38 parts of silica, 3-8 parts of zirconium oxide, 2-7 parts of boron oxide, and 1-6 parts of thorium oxide, and mixing in a ball mill to obtain a mixture A; adding the mixture A, 8-16 parts of cyclohexane dicarboxylic acid nonyl ester, and 23-35 parts of 2-methyl-2-nitro-1-propanol to a reactor, and carrying out a heating stirring reaction; continuously adding 12-18 parts of cellulose acetate phthalate and 15-25 parts of methyl 2-hydroxyethyl cellulose, and carrying out a stirring reaction at a temperature of 120-150 DEG C; drying in a vacuum oven to obtain mixed powder B; carrying out pressing molding on the mixed powder B under a pressure of 80-95 MPa; and placing into a muffle furnace under nitrogen protection, and calcining for 5-8 h at a temperature of 950-1000 DEG C so as to obtain the high-plasticity wear-resistance ceramic material. According to the present invention, the high-plasticity wear-resistance ceramic material has advantages of high melting point, high hardness, oxidation resistance and the like of the existing ceramic material, and further has good plasticity and good wear resistance.
Owner:YANCHENG SHENYUAN PLASTIC
Gel polymer electrolyte with blended cellulose and preparation method and application of gel polymer electrolyte
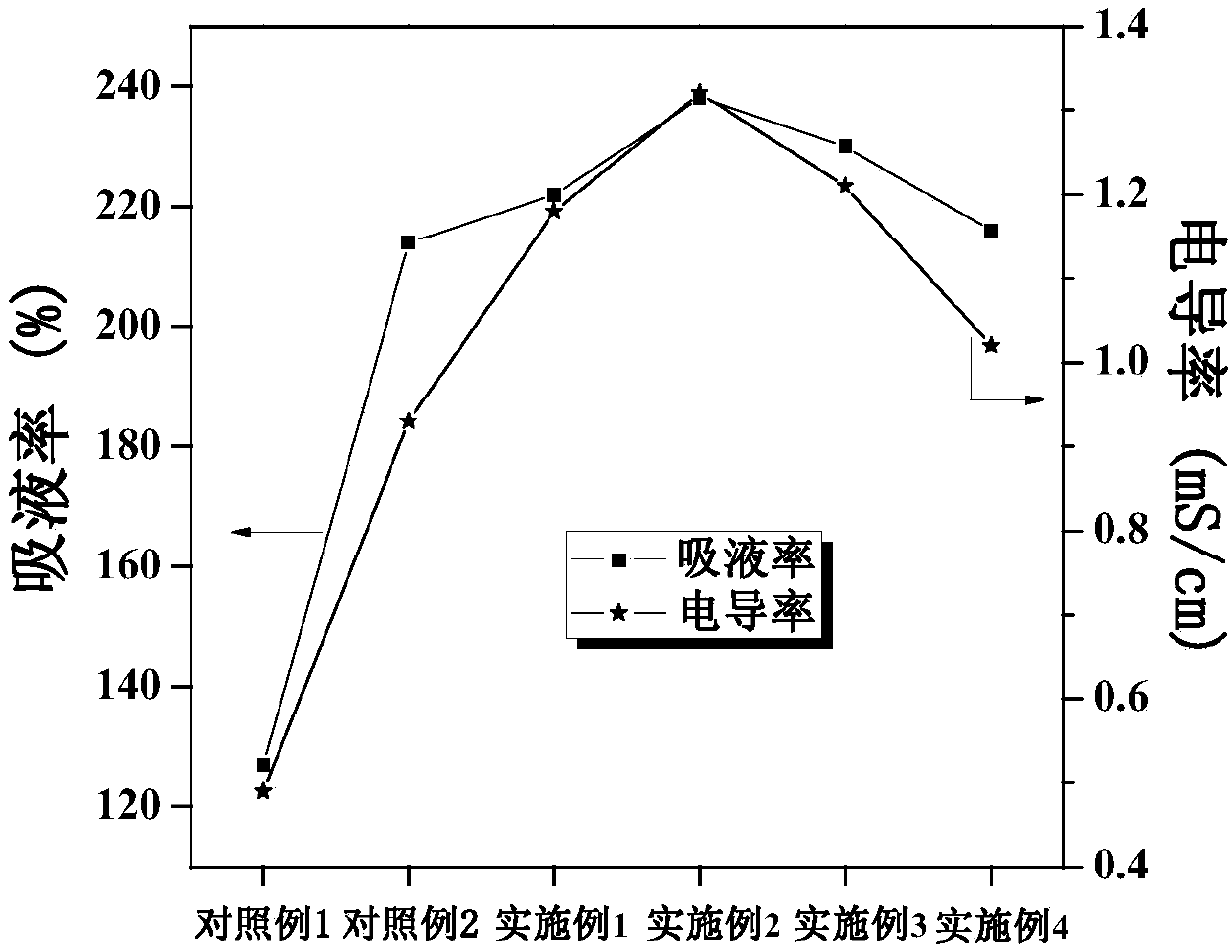
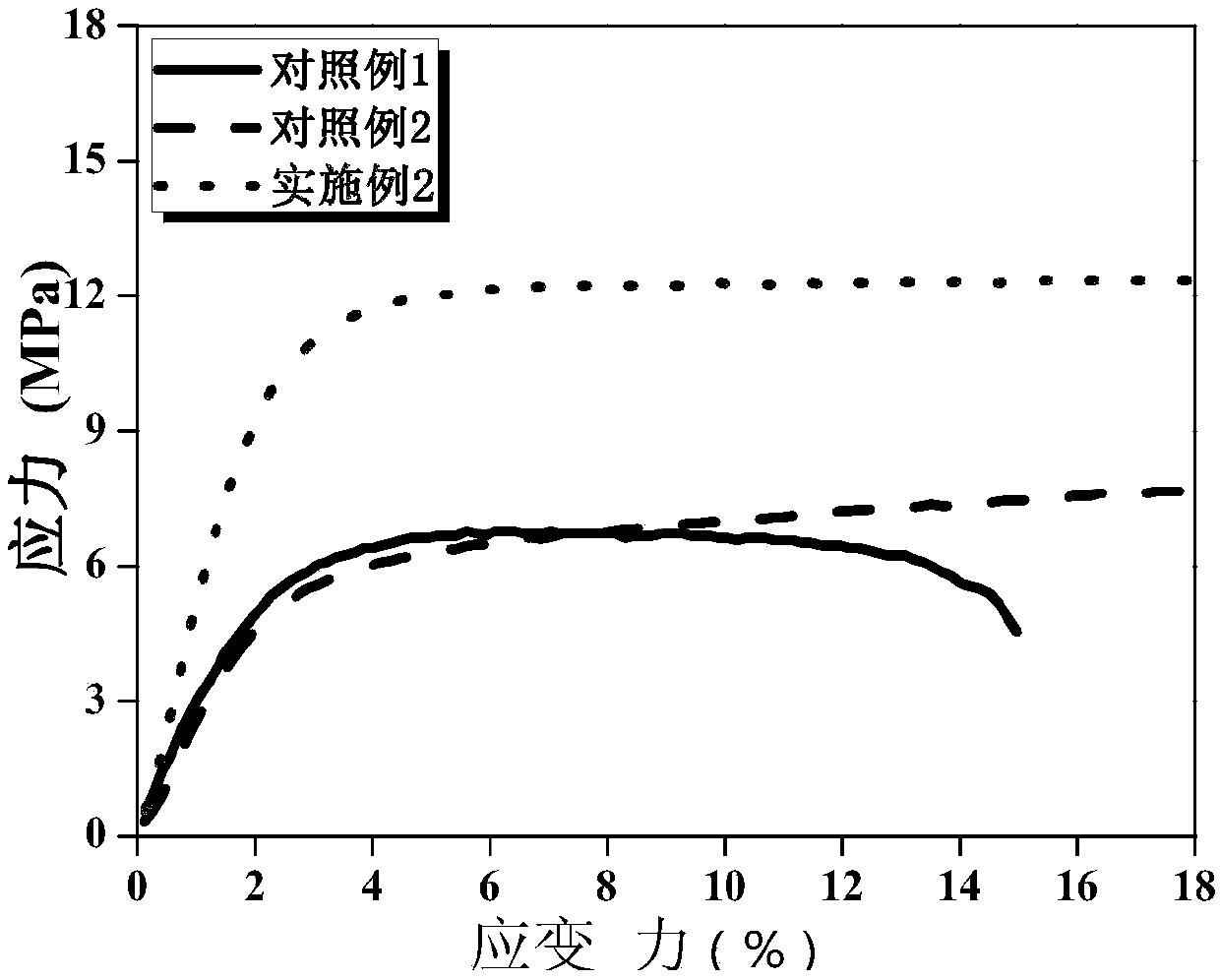
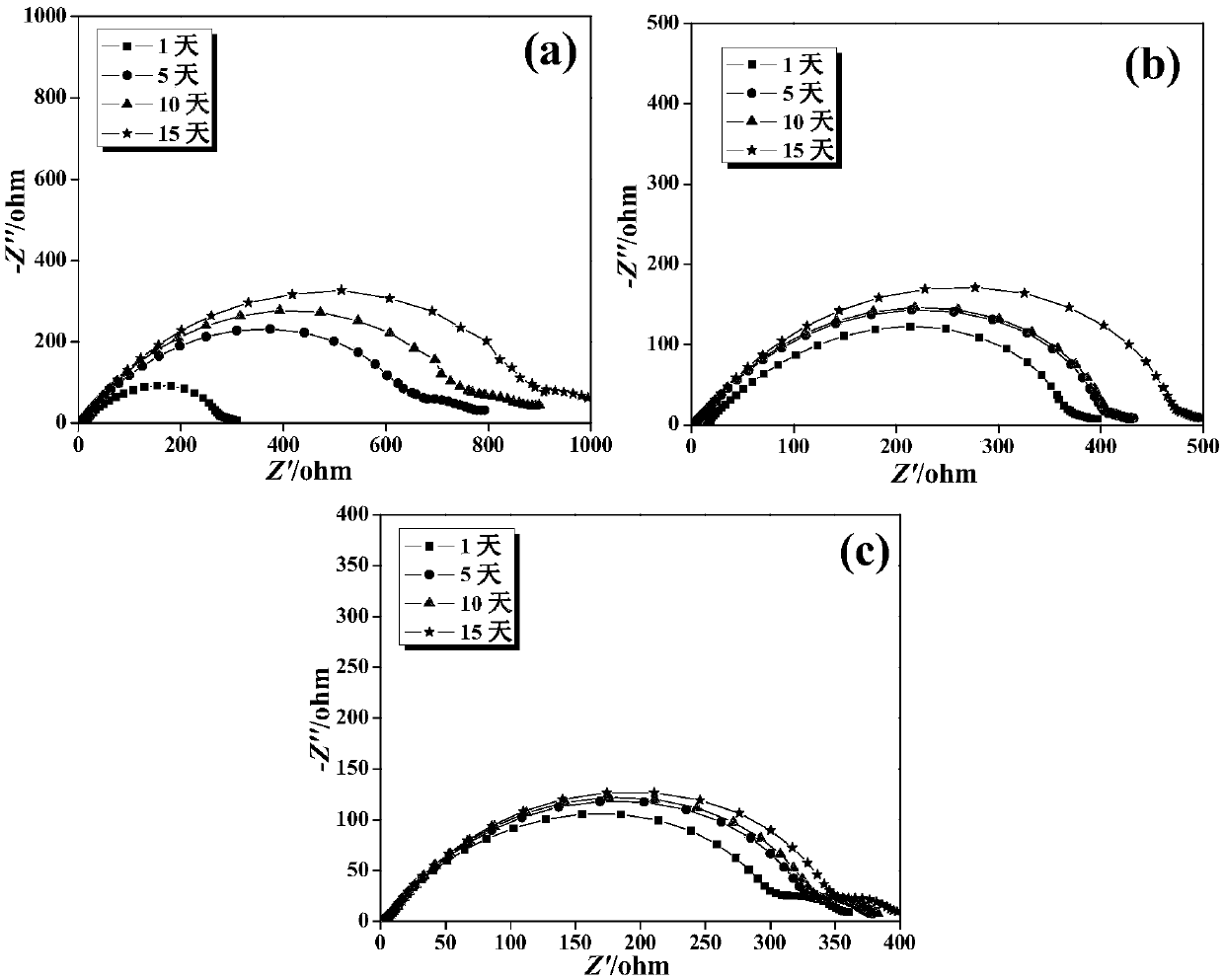
ActiveCN107565161AGood liquid absorptionGood liquid retentionSecondary cellsCellulosePolymer science
The invention discloses a gel polymer electrolyte with blended cellulose and a preparation method and application of the gel polymer electrolyte and belongs to the field of lithium-ion batteries. Themethod comprises the following steps of dissolving poly(vinylidene fluoride-hexafluoropropylene) and cellulose acetate phthalate into acetone and stirring until a polymer is completely dissolved and forms a colorless transparent gel solution, namely a blended polymer solution; immersing a blank non-woven fabric into the blended polymer solution, quickly pulling up the blank non-woven fabric aftera few seconds, drying in the air and then drying in vacuum; preparing a porous gel polymer membrane with the blended cellulose; and dropwise adding an electrolyte solution on the upper and lower surfaces of the gel polymer membrane to obtain the gel polymer electrolyte with the blended cellulose when the battery is assembled. The gel polymer electrolyte is simple in preparation technology, short in time, high in production efficiency, high in mechanical strength and excellent in cycling stability, and the obtained polymer membrane has excellent absorption performance and liquid holding capacity, and has good ionic conductivity and interface stability.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Cellulose long chain fatty acid phthalate and synthesis preparation method therefor
The present invention provides a cellulose long chain fatty acid phthalate and a synthesis preparation method therefor. The cellulose long chain fatty acid phthalate is prepared by carrying out reaction on a fatty acylating agent and a homogeneous cellulose DMAc / LiCl solution at 50 DEG C to 80 DEG C for 2-12 hours to prepare a cellulose long chain fatty acid ester; and then carrying out reaction on the cellulose long chain fatty acid ester and phthalic anhydride and a catalyst at room temperature, and washing and drying the product. Presence of long chain fatty ester groups in the cellulose long chain fatty acid phthalate greatly improves the workability of cellulose acetate phthalate (CAP) and overcomes the dependency of the existing cellulose acetate phthalate (CAP) on plasticizers in terms of workability. The technical scheme of the present invention further has the technical advantages of mild process condition, easy implementation, no to-be-separated intermediates during the reaction, short preparation time, recyclable cellulose DMAc / LiCl solution and the like.
Owner:EASTERN LIAONING UNIV
Method for preparing aceclofenac enteric microcapsules
InactiveCN102688221AThe preparation process is stableEase of industrial productionOrganic active ingredientsAntipyreticCellulose acetateAceclofenac
Disclosed is a method for preparing aceclofenac enteric microcapsules, comprising dissolving eudragit II, hydroxypropylmethylcellulose phthalate (HPMCP) or cellulose acetate phthalate in acetone to acquire a solution A; then adding aceclofenac powder into the solution A and fully dissolving the aceclofenac powder to obtain a solution B, and acquiring a primary emulsion by placing the solution B in a flask and stirring; adding span 80 into liquid paraffin and fully stirring; adding the primary emulsion into the well-stirred liquid paraffin, heating up to 75 DEG C gradually while stirring, and acquiring microcapsules by stirring continuously while preserving the temperature; and washing the microcapsules by using n-hexane three times after filtering and drying to acquire the finished microcapsules. According to the invention, the aceclofenac slow-release enteric microcapsules are prepared by using a property that decomposition of a capsule wall material is influenced by pH values. Through controlling the capsule wall material, the following effects can be achieved: drugs are sent directionally to the small intestine and released slowly to gentle the plasma concentration, prolong the action time, and improve the curative effect; the dosing frequency can be reduced and the plasma concentration is maintained in the body; and an adverse reaction in the gastrointestinal tract is reduced effectively and patient compliance is also improved effectively.
Owner:SHAANXI UNIV OF SCI & TECH
Ivermectin controlled-release capsule and preparation method and application thereof
InactiveCN107773554ARelease fullyHigh protection rateOrganic active ingredientsAntiparasitic agentsTherapeutic effectPhospholipid
The invention discloses an ivermectin controlled-release capsule and a preparation method and application thereof. The ivermectin controlled-release capsule comprises, by mass, 0.1-1% of ivermectin raw material, 20-60% of water-soluble carrier and 30-70% of enteric-soluble wrapping material, the water-soluble carrier is one or multiple of hydroxypropyl-beta-cyclodextrin, methyl-beta-cyclodextrin,HPMC, PVP, PEG, poloxamer 188, mannitol, D-alpha-tocopherol PEG 1000 succinate, cholate / phospholipid mixed micelle, polyethylenediamine dendritic polymer, phospholipid or cholesterol, and the enteric-soluble wrapping material is one or multiple of hydroxypropyl methyl cellulose phthalic acid, acrylic resin II, acrylic III, cellulose acetate phthalate, polyethylene diacetate phthalate or hydroxypropyl methyl cellulose acetate succinate. The ivermectin controlled-release capsule has the advantage of outstanding acid resistance, enables drug to reach intestinal tracts to be disintegrated and released, remarkably improves bioavailability and has remarkable treatment effect.
Owner:SOUTH CHINA AGRI UNIV
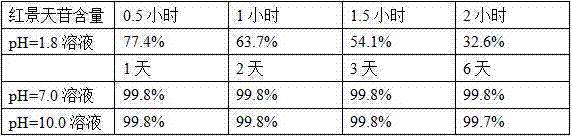
Salidroside enteric-coated tablets and preparation method thereof
ActiveCN104490802AImprove long-term stabilityPromote absorptionOrganic active ingredientsPharmaceutical non-active ingredientsCellulose acetate phthalateSalidroside
The invention discloses salidroside enteric-coated tablets and a preparation method thereof. The tablets comprise salidroside tablet cores and an enteric layer, wherein the salidroside tablet cores are composed of a salidroside main medicine and auxiliary materials, and the auxiliary materials comprise a diluent, a disintegrant, an adhesive, a lubricant, an intestinal absorption accelerant, a pH regulator and the like; an enteric coating comprises hydroxypropyl methylcellulose phthalate or cellulose acetate phthalate and the like. According to the salidroside enteric-coated tablets and the preparation method thereof disclosed by the invention, the problem of instability of salidroside in gastric juice is solved, and the problem of inadequate absorption of salidroside in intestinal tracts is solved simultaneously.
Owner:WUHAN SYNCHALLENGE UNIPHARM INC
Tilmicosin sustained release enteric-coated powder, preparation method and application thereof
ActiveCN106924189AEasy to passGuaranteed releaseAntibacterial agentsPowder deliveryGLYCERYL PALMITATECellulose acetate
The invention discloses a tilmicosin sustained release enteric-coated powder, a preparation method and application thereof. The tilmicosin sustained release enteric-coated powder comprises the following components by mass percentage: 5-40% of tilmicosin; 10-50% of a hydrophobic carrier; and 20-60% of an enteric material. Specifically, the hydrophobic carrier is one or more of cholesterol, palmitin or castor oil wax; the enteric material includes one or more of cellulose acetate phthalate, hydroxypropyl methyl cellulose phthalate or polyacrylic resin II. According to the tilmicosin sustained release enteric-coated powder provided by the invention, on the one hand, drugs are included in the hydrophobic carrier, and the palatability of enteric-coated powder is improved; and on the other hand, the acid resistance of the drugs is improved, so that drugs can be disintegrated and released after reaching the intestinal environment, the plasma concentration in livestock is relatively stable, the cardiotoxicity of tilmicosin is greatly reduced, at the same time, the effective plasma concentration maintenance time is significantly prolonged, the times of administration is reduced, and man power is saved.
Owner:SOUTH CHINA AGRI UNIV
Edible medicinal ink
Owner:武汉科亿华科技有限公司
Enteric capsule
InactiveUS20190000768A1Avoid actionReduce efficacyOrganic active ingredientsAntipyreticCellulose acetate phthalateOrganic acid
There are manufactured an enteric capsule comprising: a seamless capsule consisting of a capsule fill and a shell layer; and an enteric coating layer on the shell layer, the enteric coating layer comprising one selected from a methacrylic acid-based polymer, polyvinyl acetate phthalate, organic acid ester of hydroxypropylmethylcellulose, carboxymethylethylcellulose, and cellulose acetate phthalate; and an enteric seamless capsule further comprising a subcoating layer between the shell layer of the seamless capsule and the enteric coating layer, the subcoating layer consisting of hydroxypropylcellulose and the like. The enteric seamless capsule has acid resistance and also enables controlled dissolution for each application site in the body.
Owner:FUJI CAPSULE
Preparation method and application of chloroprene-rubber-adding building material
The invention discloses a preparation method and application of a chloroprene-rubber-adding building material. The method comprises the following steps of processing Portland cement, perlite powder and sepiolite into powder through ball milling; performing calcination; then, adding modified carboxyl polyester resin and a mixture of polyacrylamide, cellulose acetate-phthalate and polyphenyl thioether subjected to ultrasonic processing for simultaneous heating reaction; then, performing a series of specific processes such as mixing, vulcanization, crushing, stirring, injection molding and heat insulation aging to obtain a finished product of the building material. The prepared building material has the advantages that the toughness is sufficient; the intensity is high; good application prospects are realized. Meanwhile, the invention also discloses application of the building material prepared by the preparation method to building wall body materials.
Owner:SUZHOU XUANLANG PLASTIC PROD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com