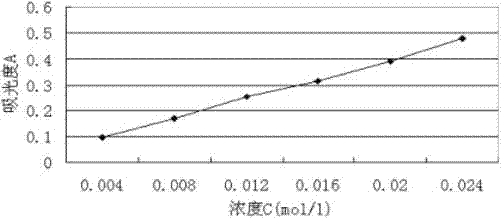
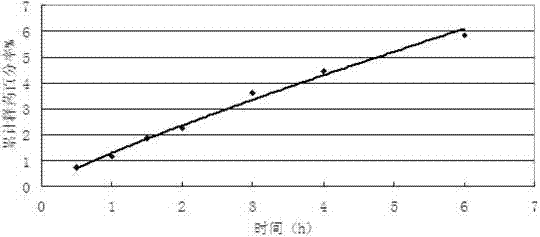
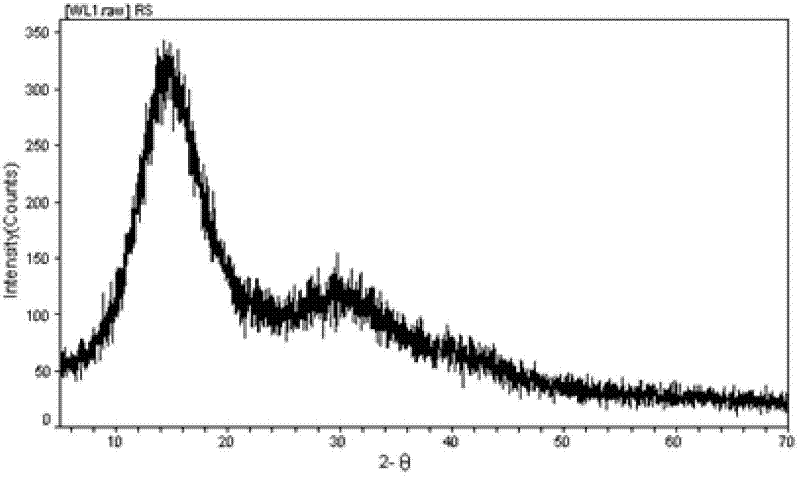
Method for preparing aceclofenac enteric microcapsules
An aceclofenac, enteric-coated technology, applied in the field of preparation of pharmaceutical preparations, can solve the problems of difficulty in optimal process investigation, difficulty in achieving the effect of capsule shells, poor reproducibility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Take 3.8g of acrylic resin II and dissolve it in acetone to obtain solution A;
[0027] 2) Then add 2.6g of aceclofenac powder to solution A to fully dissolve to obtain solution B, put solution B in a flask and stir at 200r / min at 8-10°C to obtain colostrum;
[0028] 3) Take 5ml of Span 80 and add it to 100ml of liquid paraffin and stir fully at the bottom of the temperature at 15°C;
[0029] 4) Add the colostrum prepared in step 2) into the stirred liquid paraffin in step 3), gradually heat up to 40°C while stirring at 200r / min, keep stirring for 3 hours, then gradually heat up to 75°C, keep stirring for 1h, Prepare microcapsules;
[0030] 5) After filtration, wash with n-hexane for 3 times and dry to obtain the finished microcapsules.
Embodiment 2
[0032] 1) Take 4.5g of hydroxypropyl methylcellulose phthalate and disperse it in acetone to obtain solution A;
[0033] 2) Then add 2.8g of aceclofenac powder to solution A to fully dissolve to obtain solution B, put solution B in a flask and stir at 200r / min at 8-10°C to obtain colostrum;
[0034] 3) Take 8ml of Span 80 and add it to 70ml of liquid paraffin and stir fully at the bottom of the temperature at 15°C;
[0035] 4) Add the colostrum prepared in step 2) into the stirred liquid paraffin in step 3), gradually heat up to 40°C while stirring at 200r / min, keep stirring for 3 hours, then gradually heat up to 75°C, keep stirring for 1h, Prepare microcapsules;
[0036] 5) After filtration, wash with n-hexane for 3 times and dry to obtain the finished microcapsules.
Embodiment 3
[0038] 1) Take 5.1g of cellulose acetate phthalate and disperse it in acetone to obtain solution A;
[0039] 2) Then add 2.5g of aceclofenac powder to solution A to fully dissolve to obtain solution B, put solution B in a flask and stir at 200r / min at 8-10°C to obtain colostrum;
[0040] 3) Take 10ml of Span 80 and add it to 200ml of liquid paraffin and stir fully at the bottom of the temperature at 15°C;
[0041] 4) Add the colostrum prepared in step 2) into the stirred liquid paraffin in step 3), gradually heat up to 40°C while stirring at 200r / min, keep stirring for 3 hours, then gradually heat up to 75°C, keep stirring for 1h, Prepare microcapsules;
[0042] 5) After filtration, wash with n-hexane for 3 times and dry to obtain the finished microcapsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com