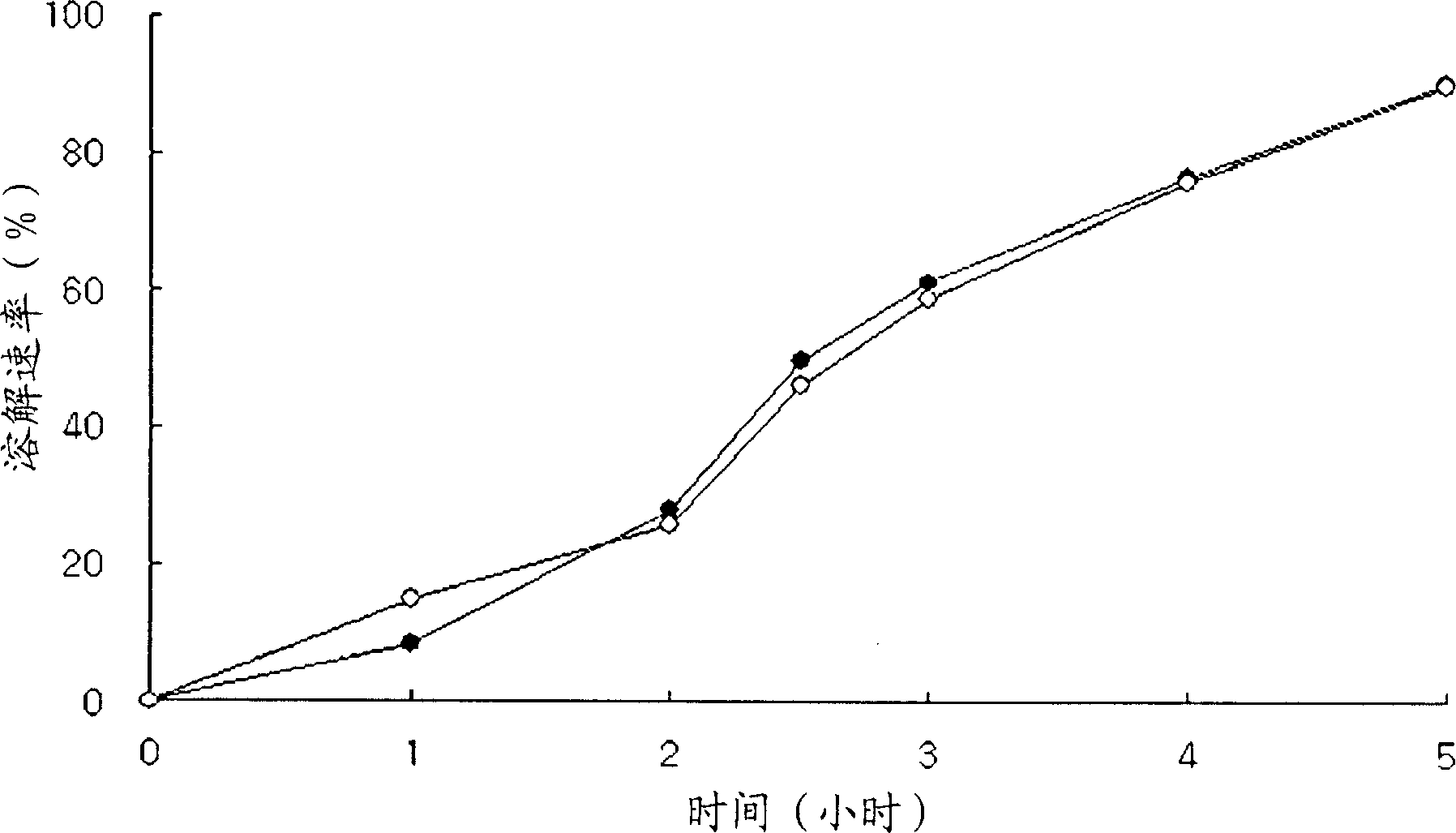
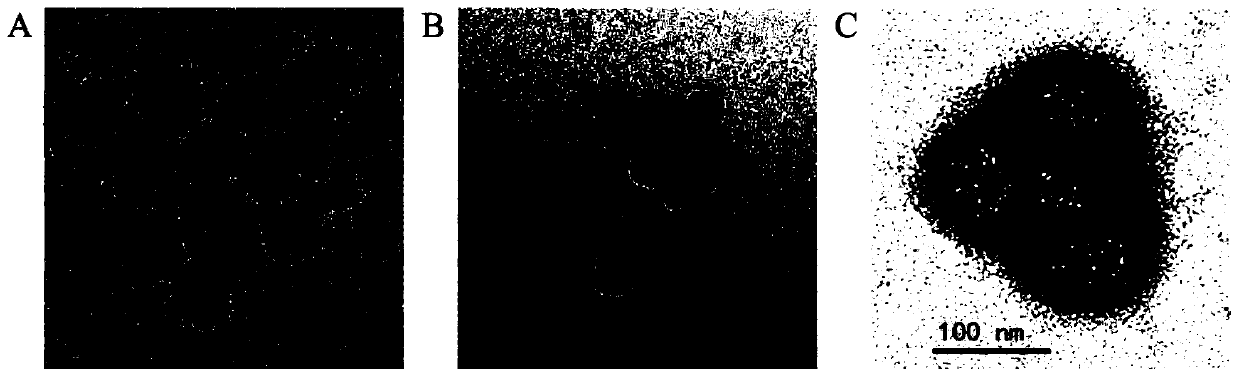
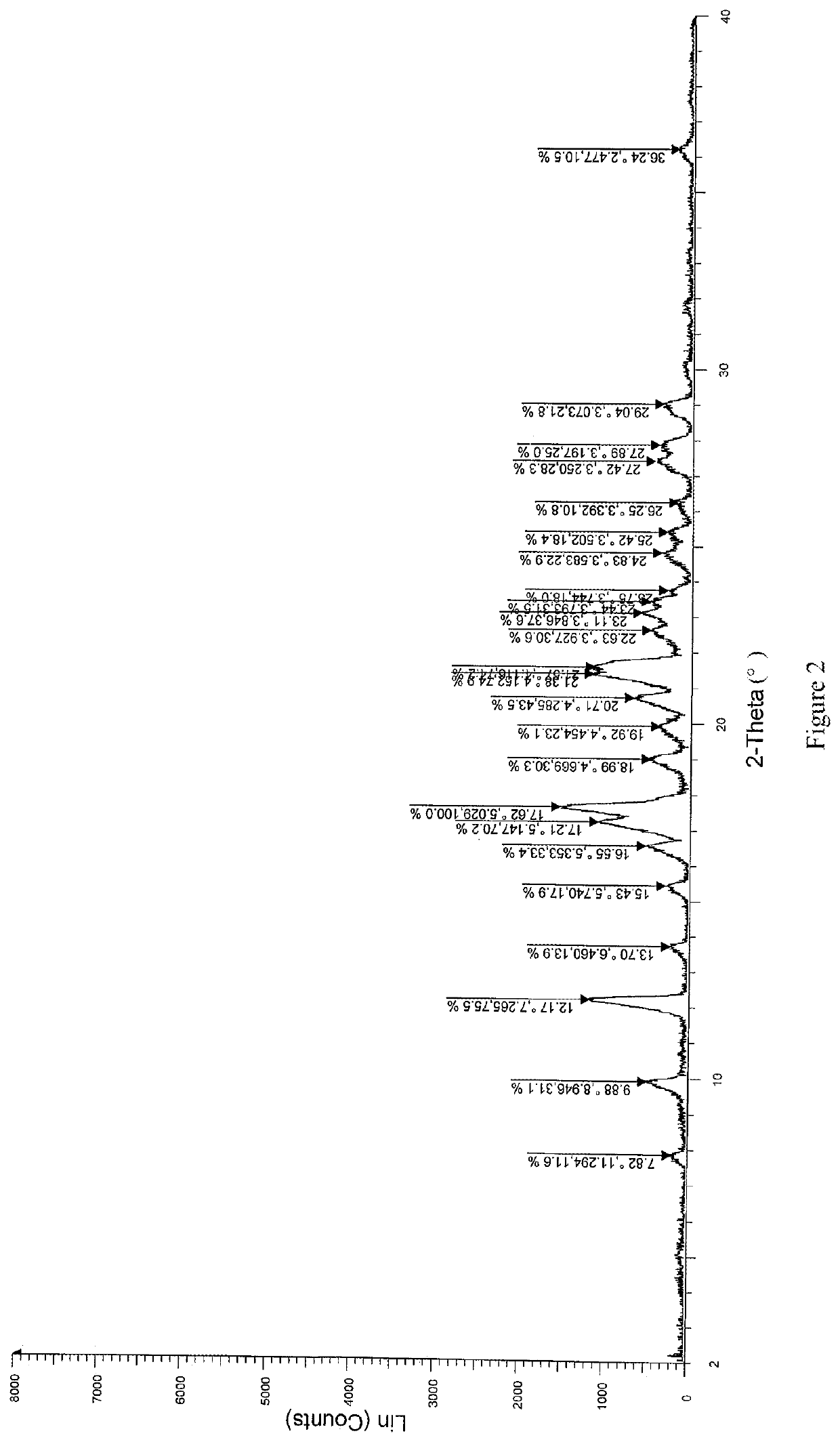
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Hydroxypropylmethylcellulose phthalate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tea polyphenol liposoluble microcapsules and preparation method thereof
InactiveCN103330213ASmall droplet sizeSmall particle sizeTripeptide ingredientsAntinoxious agentsPhenolic content in teaPolyphenol
The invention relates to microcapsules and a preparation method thereof, particularly to tea polyphenol liposoluble microcapsules and a preparation method thereof, wherein a core material of the tea polyphenol liposoluble microcapsules comprises tea polyphenol and reduced glutathione, a mass ratio of the tea polyphenol to the reduced glutathione is 95-99:1-5, a wall material of the tea polyphenol liposoluble microcapsules is one or a plurality of materials selected from arabic gum, dextrin, corn syrup, ethyl cellulose, methylcellulose, and hydroxypropyl methylcellulose phthalate, and a mass weight ratio of the core material to the wall material is 1:1-4. The tea polyphenol liposoluble microcapsule preparation method comprises the following steps: respectively preparing a core material emulsion liquid and a wall material solution, adding the core material emulsion liquid to the wall material solution, carrying out high speed stirring, homogenizing, and carrying out spray drying to obtain the tea polyphenol liposoluble microcapsules. The tea polyphenol liposoluble microcapsules have characteristics of fine average particle size and slow tea polyphenol release, such that anti-oxidation and absorption utilization efficiency of the tea polyphenol are substantially improved.
Owner:ZHEJIANG MINGHUANG NATURAL PRODS DEV
Precipitation modifying method for cellulose type solid enteric coatings
The invention relates to an improved precipitation method for the cellulose-based solid enteric-coating material; wherein, the solid enteric-coating material comprises a series of cellulose ether esters such as hydroxypropyl methylcellulose of phthalate ester (HPMCP), hydroxypropyl methylcellulose of acetic acid succinate (HPMCAS) and hydroxypropyl methylcellulose of trimellitic ester. The improved precipitation method is characterized in that the mixed solution of diluent and bleaching agent with a certain quantity is added in the reaction system at the later reaction phase, then the product is enabled to form even powdery particles in the viscous system via cooling and adding purified water for continuous precipitation. The improved precipitation method for the cellulose-based solid enteric-coating material has the advantages that the content of free acid in the product is greatly reduced; the dense and powdery particles do not need to be grinded; and dissolution rate of the product is improved to a great extent.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Biodegradable microbicidal vaginal barrier device
An intravaginal bio-erodible microbicidal barrier device. The device comprises (a) at least one micronized compound selected from the group consisting of cellulose acetate phthalate and hydroxypropylmethylcellulos- e phthalate, and (b) at least one water soluble or water dispersible cellulose compound selected from the group consisting of hydroxypropylmethylcellulose, methylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, hydroxyethylmethylcellulose, hydroxyethylethylcellulose and hydroxypropylethylcellulose; or a pectin, such as an apple pectin. The device is prepared by a combination of foaming, freezing and freeze-drying processes.
Owner:NEW YORK BLOOD CENT
Slow releasing integument composition of cysteamine or its salts, processing technique and application thereof
InactiveCN1666631AIncrease contentGood coating effectClimate change adaptationAnimal feeding stuffCysteaminePolyethylene glycol
The invention relates to a new composition by slowing-releasing envelope with cysteamine or its saline compound. Said composition comprises: A, cysteamine or its saline compound in 1-95 wt %; main materials used for covering in 1-80 wt % which is mainly sodium alginate, polyacrylic resin II, polylactic acid, polyamino acid, corn zein plasm, poly(D.L-Lactide)-poly(ethylene glycol) block copolymer, crosslinked polyvinyl pyrrolidone, hydroxypropylmethylcellulose phtha late, polyvinyl acetate phthalic acid esters, cellulose ethyl ether, and succinic acetate methylcellulose. This invention also relates to process of slowing-releasing envelope on new compound with cysteamine or its saline, and its use as animal feedstuff additive. The strongpoint of said invention is the excellent effect on envelope; the available utilization by animals of envelope materials degraded in the inner body of animals; further, the reached slowing-releasing technique on the combined use of envelope materials which can express the biological value more effectively in inner body of animals.
Owner:李浙烽
Hemostatic device
ActiveUS20090156711A1Impression capsSurgical adhesivesCellulose acetate phthalateHydroxypropylmethylcellulose phthalate
A hemostatic device comprising (i) a carrier comprising at least one component selected from the group consisting of hydroxypropyl methylcellulose phthalate, hydroxypropyl methylcellulose acetate succinate, cellulose acetate phthalate; polyvinylacetate phthalate, cellulose acetate phthalate, acetaldehyde dimethylcellulose acetate, polymethacrylate-based polymers, and derivatives, salts, copolymers or combinations thereof; and (ii) thrombin
Owner:ETHICON INC
Camellia nitidissima tea polyphenols slow release microsphere particle and production method thereof
InactiveCN101822750AHigh recovery rateHigh activityAntinoxious agentsPharmaceutical non-active ingredientsMicrosphereAdditive ingredient
The invention relates to a Camellia nitidissima tea polyphenols slow release microsphere particle made of Camellia nitidissima, wherein the main ingredients comprise Camellia nitidissima tea polyphenols extract, hydroxypropyl methyl cellulose phthalate and dodecyl sodium sulfate; the production method comprises the following steps: cleaning and cutting up the fresh blades of Camellia nitidissima, ultrasonic digestion, separating and concentrating sodium filter membrane, purifying macroporous absorption resin and concentrating at low temperature in vacuum so as to obtain Camellia nitidissima tea polyphenols; adding the Camellia nitidissima tea polyphenols extract to the ethanol aqueous solution of hydroxypropyl methyl cellulose phthalate according to the mass ratio of Camellia nitidissima tea polyphenols extract to hydroxypropyl methyl cellulose phthalate of 2: 1, and adding dodecyl sodium sulfate which is 1 / 16 of the mass of Camellia nitidissima tea polyphenols extract as the emulsifying agent and mixing uniformly, spraying and drying so as to obtain the Camellia nitidissima tea polyphenols slow release microsphere product.
Owner:GUANGXI FUXIN SCI & TECH
Solid preparation comprising npyy5 receptor antagonist
A preparation which can improve solubility of a NPYY5 receptor antagonist in water, even when the NPYY5 receptor antagonist is contained in the preparation at a high content is provided. A solid preparation containing a NPYY5 receptor antagonist, an amorphous stabilizer, and optionally an amorphousization inducing agent. Particularly, when the amorphous stabilizer is hydroxypropylmethylcellulose phthalate and / or hydroxypropylmethylcellulose acetate succinate, and the amorphousization inducing agent is urea and / or saccharine sodium at an addition amount of less than 8% by weight, dissolution out property of a water-hardly soluble NPYY5 receptor antagonist could be improved.
Owner:SHIONOGI & CO LTD
Hemostatic device
A hemostatic device comprising (i) a carrier comprising at least one component selected from the group consisting of hydroxypropyl methylcellulose phthalate, hydroxypropyl methylcellulose acetate succinate, cellulose acetate phthalate; polyvinylacetate phthalate, cellulose acetate phthalate, acetaldehyde dimethylcellulose acetate, polymethacrylate-based polymers, and derivatives, salts, copolymers or combinations thereof; and (ii) thrombin.
Owner:ETHICON INC
Ranolazine oral sustained-release preparation and preparation method thereof
The invention provides a Ranolazine oral sustained-release preparation and a preparation method. The Ranolazine oral sustained-release preparation comprises Ranolazine, a sustained-release skeleton material, a filling agent, an adhesive and a lubricating agent, and is characterized in that the weight of the Ranolazine is between 35 and 85 percent in the sustained-release preparation; a prescription composition is preferably selected according to a large number of experiments; hydroxypropyl methyl cellulose phthalate and methyl cellulose are served as the sustained-release skeleton material ofthe sustained-release preparation in the preferable weight ratio of 2:1-1.5; and microcrystalline cellulose is served as the filling agent; the 29 / 39 ethanol solution of polyvinyl pyrrolidone K is served as the adhesive, and magnesium stearate is served as the lubricating agent.
Owner:FUREN PHARMA GROUP
Cellulosic films incorporating a pharmaceutically acceptable plasticizer with enhanced wettability
An enteric coating for a solid pharmaceutical carrier or substrate wherein the enteric coating includes a cellulosic polymeric material selected from selected from the group consisting of hydroxypropyl cellulose, hydroxyethyl cellulose, hydroxypropyl methyl cellulose, methyl cellulose, ethyl cellulose, cellulose acetate, cellulose acetate butyrate, cellulose acetate phthalate, cellulose acetate succinate, cellulose acetate propionate, cellulose acetate trimellitate, hydroxypropylmethyl cellulose phthalate, hydroxypropylmethyl cellulose succinate, hydroxypropylmethyl cellulose acetate succinate, cellulose acetate succinate butyrate, cellulose acetate succinate propionate, carboxymethylcellulose sodium, cellulose butyrate, and mixtures thereof and a plasticizer selected from a water-soluble preparation of a fat-soluble vitamin. A preferred plasticizer is Vitamin E polyethylene glycol 1000 succinate.
Owner:EASTMAN CHEM CO
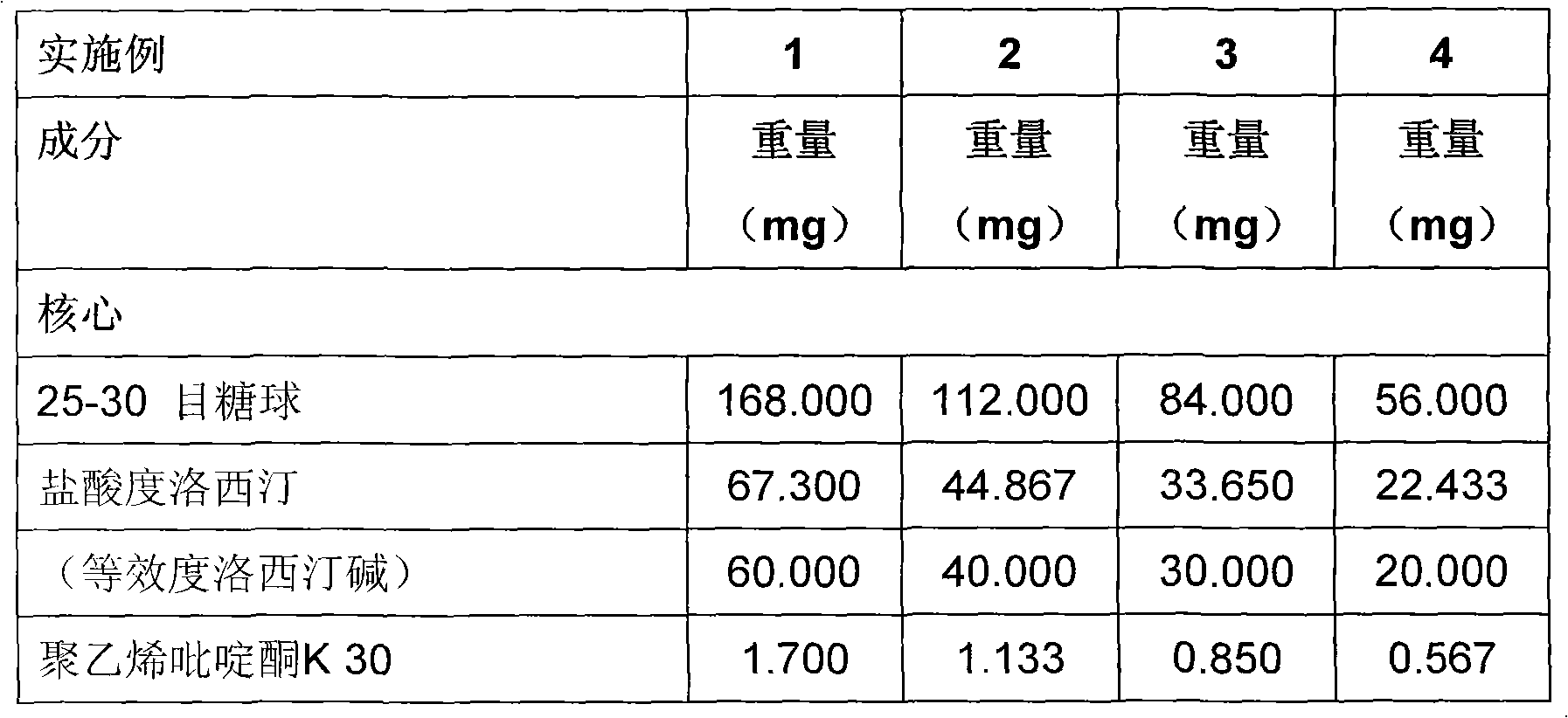
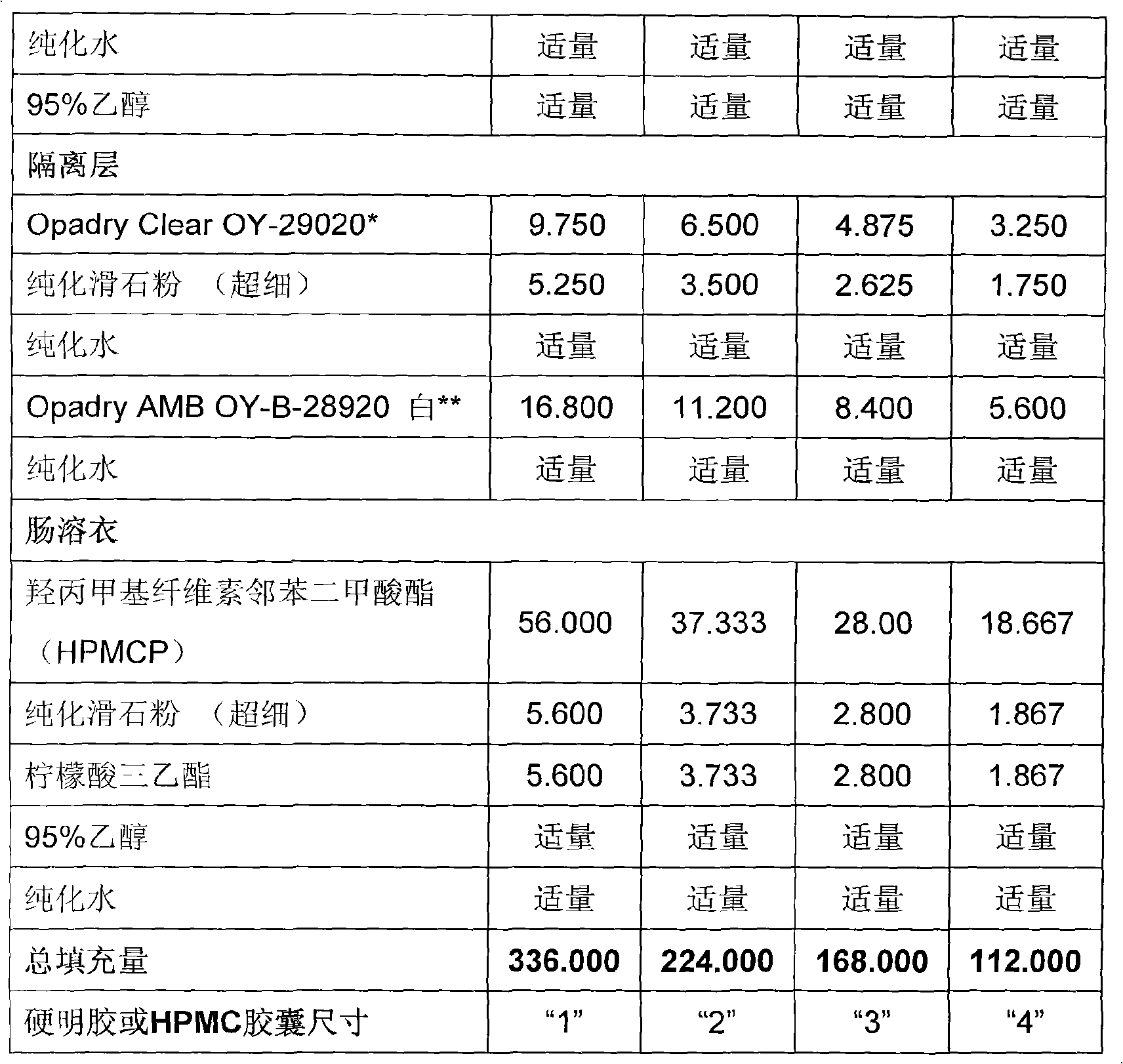
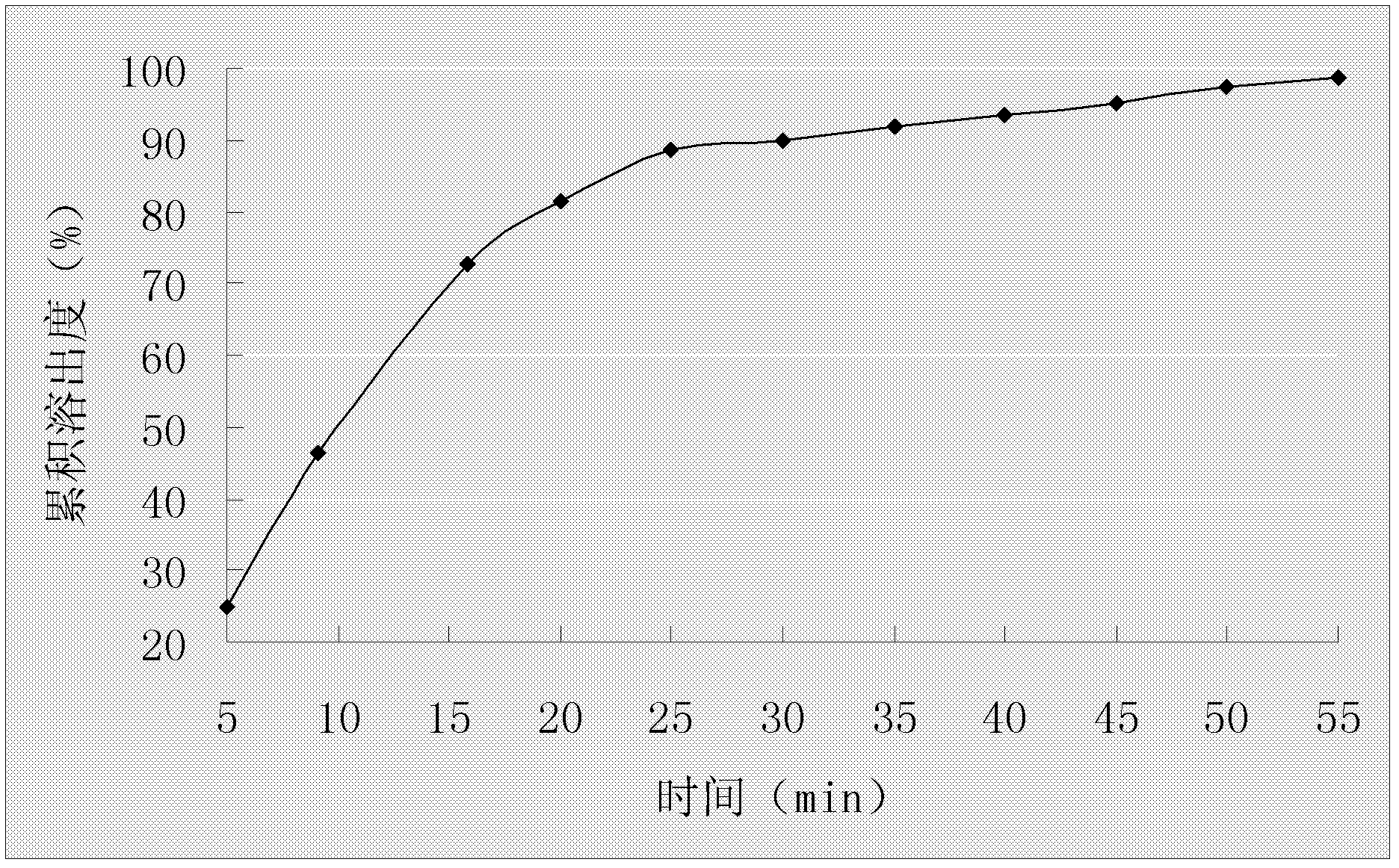
Duloxetine formulation
A duloxetine pellet formulation comprises: (i) a core including a desired amount of duloxetine; (ii) an enteric coating comprising hydroxypropylmethylcellulose phthalate (HPMCP) as an enteric polymer;and, optionally, (iii) a separating layer located between the core and the enteric coating, the separating layer including polyvinyl alcohol and a low molecular weight hydroxypropylmethylcellulose (HPMC).
Owner:箭锋国际有限公司
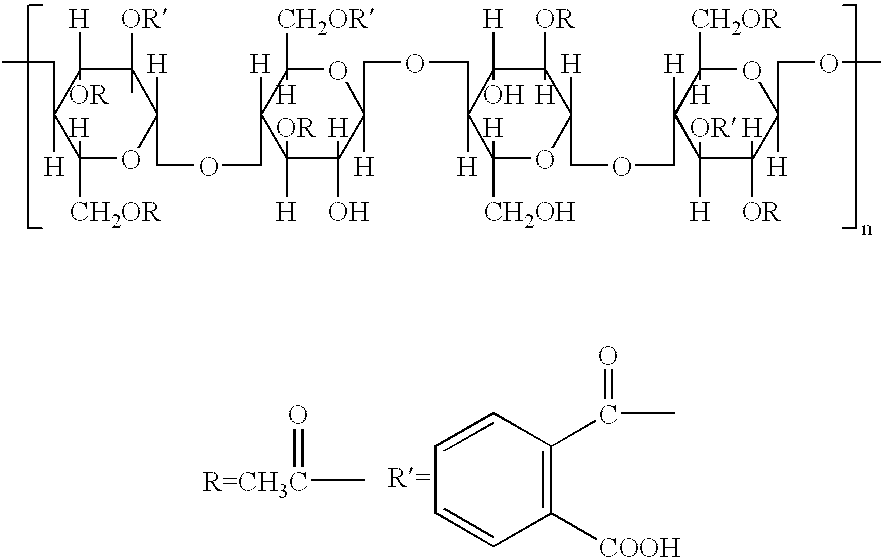
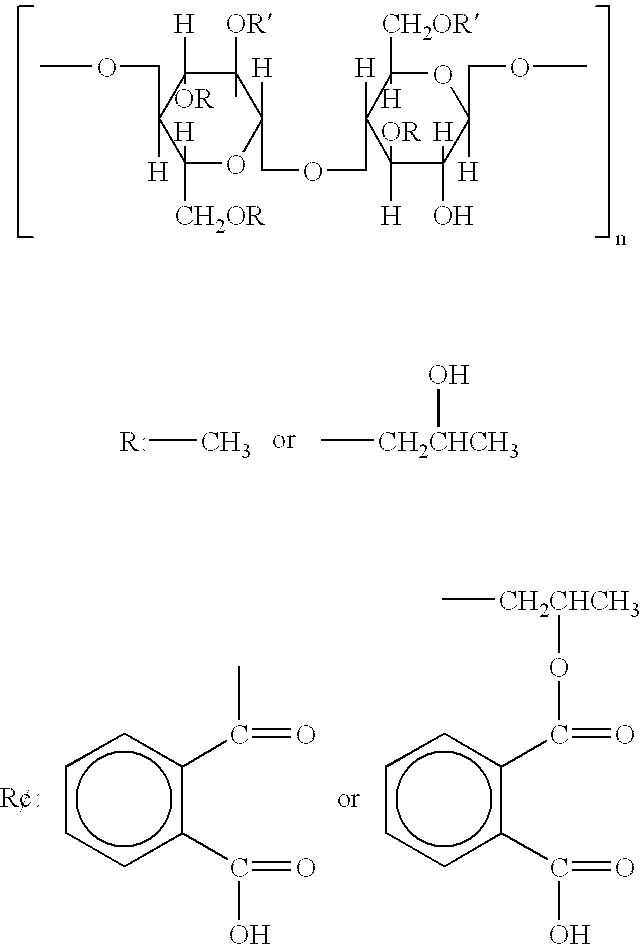
Preparation method of hydroxypropyl methyl cellulose phthalate
ActiveCN104231091ASimple methodReaction conditions are easy to controlPharmaceutical non-active ingredientsInternal pressureHydroxypropylmethylcellulose phthalate
The invention belongs to the field of auxiliary materials of medicines, particularly relates to a preparation method of hydroxypropyl methyl cellulose phthalate, and aims to provide the preparation method for the hydroxypropyl methyl cellulose phthalate, wherein the hydroxypropyl methyl cellulose phthalate prepared according to preparation method is higher in phthalic acyl content. The preparation method disclosed by the invention is characterized in that the reaction temperature is raised to 85-100 DEG C, the internal pressure of a reaction container is maintained to 0.3-0.9MPa, and the reacting time is 3.5-6 h. For the preparation method disclosed by the invention, the aim of promoting a positive reaction is achieved by adjusting the reaction temperature and reaction pressure, and the phthalic acyl content of a product can be increased to 25% from 21%. The preparation method disclosed by the invention has the advantages that the synthetic technology is simple, and the reaction condition is easy to control, so that the preparation method is suitable for expanding the production.
Owner:HERCULES TIANPU CHEM
Omeprazole enteric-coated capsules and preparation method thereof
ActiveCN102274204AImprove acid resistanceNo significant change in contentOrganic active ingredientsDigestive systemHydroxypropylmethylcellulose phthalateHypromellose
The invention discloses an omeprazole enteric-coated capsule, containing omeprazole, hydroxypropyl-beta-cyclodextrin, hydroxypropyl methylcellulose phthalate, mannitol and hydroxypropyl methylcellulose, wherein the mole ratio of omeprazole to hydroxypropyl-beta-cyclodextrin is 1: (0.8-2); and the weight ratio of omeprazole to hydroxypropyl methylcellulose phthalate to mannitol is 1: (10-18): (12-20). The omeprazole enteric-coated capsule disclosed by the invention has good acid resistance, dissolution and stability; and the preparation method has simple process, easiness in operation and goodreproducibility and is applicable for industrialization production.
Owner:YOUCARE PHARMA GROUP
Modified Release Pharmaceutical Composition of Bupropion Hydrochloride
A delayed extended release pharmaceutical composition includes a compressed core containing an effective amount of bupropion or its pharmaceutically acceptable salt, a water-attractant polymer. The core is preferably devoid of a stabilizer. The core is surrounded by an extended release layer, which is free of plasticizer and pore-forming agent. The extended release layer is surrounded by a delayed release layer. Alternating coats of extended release layer and delayed release layer may follow. A preferred extended release layer includes ethylcellulose and hydroxypropyl cellulose or hydroxypropyl methylcellulose and a preferred delayed release layer includes methacrylic acid copolymer and hydroxypropyl methylcellulose phthalate, lactose and a combination of triethyl citrate and polyethylene glycol and talc. A method of preparing the delayed extended release bupropion hydrochloride containing pharmaceutical composition is also disclosed.
Owner:JUBILANT ORGANOSYS LTD
Method for preparing aceclofenac enteric microcapsules
InactiveCN102688221AThe preparation process is stableEase of industrial productionOrganic active ingredientsAntipyreticCellulose acetateAceclofenac
Disclosed is a method for preparing aceclofenac enteric microcapsules, comprising dissolving eudragit II, hydroxypropylmethylcellulose phthalate (HPMCP) or cellulose acetate phthalate in acetone to acquire a solution A; then adding aceclofenac powder into the solution A and fully dissolving the aceclofenac powder to obtain a solution B, and acquiring a primary emulsion by placing the solution B in a flask and stirring; adding span 80 into liquid paraffin and fully stirring; adding the primary emulsion into the well-stirred liquid paraffin, heating up to 75 DEG C gradually while stirring, and acquiring microcapsules by stirring continuously while preserving the temperature; and washing the microcapsules by using n-hexane three times after filtering and drying to acquire the finished microcapsules. According to the invention, the aceclofenac slow-release enteric microcapsules are prepared by using a property that decomposition of a capsule wall material is influenced by pH values. Through controlling the capsule wall material, the following effects can be achieved: drugs are sent directionally to the small intestine and released slowly to gentle the plasma concentration, prolong the action time, and improve the curative effect; the dosing frequency can be reduced and the plasma concentration is maintained in the body; and an adverse reaction in the gastrointestinal tract is reduced effectively and patient compliance is also improved effectively.
Owner:SHAANXI UNIV OF SCI & TECH
Preparation method of solvent-free water-dispersible hydroxypropyl methyl cellulose phthalate nanoparticle
InactiveUS6893493B2Transportation and packagingUltrafiltrationWater dispersibleHydroxypropylmethylcellulose phthalate
The present invention relates to a preparation method of solvent-free water-dispersible hydroxypropyl methyl cellulose phthalate nanoparticle, and more particularly, to a preparation method of solvent-free water-dispersible hydroxypropyl methyl cellulose phthalate nanoparticle, which is environment-friendly and advantageous in disintegration and dissolution when used as an enteric coating material, which is prepared by obtaining suitable hydroxypropyl methyl cellulose phthalate (HPMCP) particle through aqueous emulsification process and regulating content of remaining electrolyte through ion exchange process.
Owner:LOTTE FINE CHEM CO LTD
Premix for piglets, as well as preparation method and application method thereof
InactiveCN106509436AHigh content of small peptidesLow in free amino acidsAnimal feeding stuffAccessory food factorsAnti stressPhytase
The invention discloses a premix for piglets. The premix for the piglets comprises the following raw materials in parts by weight: 15-20 parts of soybean peptide protein, 10-15 parts of a cortex eucommiae extract, 20-25 parts of an acidifying agent, 60-65 parts of compound trace elements, 8-10 parts of compound vitamins, 20-25 parts of choline chloride, 70-80 parts of lysine, 20-30 parts of phytase, 60-80 parts of calcium hydrogen phosphate, 5-10 parts of an antioxidant, 100-120 parts of zeolite powder, and 62-95 parts of HPMCP, namely hydroxypropylmethyl cellulose phthalate. The premix for the piglets is a safe and efficient strengthened feed which is rational in formula. The premix is capable of increasing food intake of the piglets, improving anti-stress ability and immunity of the piglets, and effectively raising growth rates of the piglets. The premix is a real safe and efficient feed.
Owner:无锡华诺威动物保健品有限公司
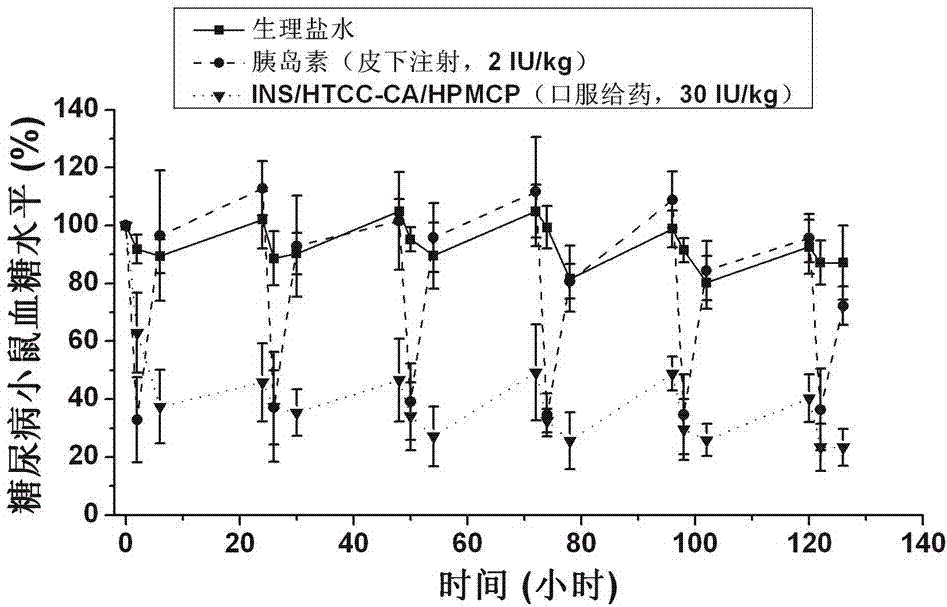
Insulin oral nano-preparation and preparation method thereof
The invention belongs to the technical field of medicines, and particularly relates to an insulin oral nano-preparation and a preparation method thereof. The insulin oral nano-preparation is an insulin loaded nano-particle solution prepared by taking cholic acid modified quaternized chitosan and hydroxypropyl methylcellulose phthalate as an insulin composite carrier. A quaternary amine group and a cholic acid group are irregularly connected to chitosan to obtain the cholic acid modified quaternized chitosan, the cholic acid modified quaternized chitosan and insulin interact in an electrostatic and hydrophobic manner to form nano-particles, and the nano-particles can deliver the insulin to the liver to play a role by a bile acid liver and intestine circulation channel in ilea and the liver through intestinal wall cells; and the hydroxypropyl methylcellulose phthalate is added in the nano-particles, the nano-particles can be protected from dissociating in the stomach, therefore, the insulin is protected from being degraded in the digestive tract, the staying time of the nano-particles in a small intestine part is prolonged, and then the nano-particles can be assisted to pass through an intestinal mucosa and the intestinal wall cells.
Owner:FUDAN UNIV
Omeprazole-containing enteric coated tablets
ActiveCN102379856AImprove acid resistanceNo significant change in contentOrganic active ingredientsDigestive systemHydroxypropylmethylcellulose phthalateCellulose acetate
Owner:贝克诺顿(浙江)制药有限公司
Preparing method for controlled released type tablet tamsulosin hcl and the tablet thereof
InactiveUS20060204570A1Sharp increase in production costProduct yield is lowBiocideWood working apparatusTamsulosin hclDrug release rate
The present invention relates to a simple and effective method for preparing a tamsulosin HCl sustained-release tablet and a tamsulosin HCl sustained-release tablet produced thereby. The method comprises the steps of: dissolving tamsulosin HCl as an active ingredient in an organic solvent; dissolving the tamsulosin HCl solution in hydroxypropylmethylcellulose phthalate to prepare a binder solution; and kneading the binder solution with a hydroxypropylmethylcellulose phthalate / glyceryl dibehenate mixture as an excipient and allows tamsulosin HCl to be released at uniformly controlled amounts in a subtained-release manner in vivo by controlling drug release rate according to different pH environments in vivo, so that it shows improved bioavailability and minimized side effects.
Owner:KYUNG DONG PHARM
PH-sensitive insulin sustained-release oral preparation and preparation method thereof
InactiveCN105920587AEfficient releaseRelease effective controlPeptide/protein ingredientsMetabolism disorderHalloysiteOral medication
The invention discloses a pH-sensitive insulin sustained-release oral preparation and a preparation method thereof, and relates to the technical field of biological medicine. The pH-sensitive insulin sustained-release oral preparation is prepared by taking halloysite (HNTs) as an insulin carrier material, assisted by hydroxypropylmethyl cellulose phthalate (HPMCP). The preparation method comprises the following steps: firstly, loading insulin in a halloysite tube, and then coating the HPMCP in a form of magnetic stirring, so that an insulin-loaded halloysite / hydroxypropylmethyl cellulose phthalate complex (INS / HNTs-HPMCP) is obtained. The preparation method disclosed by the invention is simple and is mild in condition. The obtained complex can release in simulated gastric liquid with a relatively low amount and can slowly and continuously release in simulated intestinal fluid; and with the natural tubular structure of the halloysite, the bio-activity of the medicine (the oral preparation) is guaranteed, and the oral administration of the insulin is expected to be achieved.
Owner:YANGZHOU UNIV
Duloxetine Formulation
A duloxetine pellet formulation comprises: (i) a core including a desired amount of duloxetine; (ii) an enteric coating comprising hydroxypropylmethylcellulose phthalate (HPMCP) as an enteric polymer; and, optionally, (iii) a separating layer located between the core and the enteric coating, the separating layer including polyvinyl alcohol and a low molecular weight hydroxypropylmethylcellulose (HPMC).
Owner:ARROW INT INC
Preparation method of mold release agent durable in storage
ActiveCN106955968AImprove heat resistanceImprove stabilityFoundry mouldsFoundry coresHydroxypropylmethylcellulose phthalateTert-Butyloxycarbonyl protecting group
The invention provides a preparation method of a mold release agent durable in storage. A mold release agent product is obtained by adding (heptadecafluoro-1,1,2,2-tetradecyl)trimethoxysilane, poly(ethylene glycol) methyl ether meth-acrylate, poly-4-methyl-1-amylene, methyl isobutyl carbinol, hydroxypropyl methylcellulose phthalate, 5,7-difluoro-2,3-dihydrobenzofuran and N-(tert-butoxycarbonyl)-1-[(4-methylphenyl)sulfonyl]-L-histidine in an agitated vessel to be stirred and diffused.
Owner:江苏康姆罗拉特种陶瓷有限公司
Preparation method of probiotics and dunaliella salina tablets
InactiveCN105963326AReduce lossesEasy to compressDigestive systemAlgae medical ingredientsMagnesium stearateSilicon dioxide
The invention discloses a preparation method of probiotics and dunaliella salina tablets. The method comprises the following steps that 1, probiotics for health-care foods are inoculated into a liquid fermentation medium, anaerobic culture, centrifugal separation and supernatant removing are conducted, and probiotics thalli are collected; 2, the collected probiotics thalli are subjected to resuspension with sterile purified water, and a probiotics suspension is obtained; 3, dunaliella salina powder, xylitol, mannitol, hydroxypropylmethyl cellulose phthalate, polyvinylpyrrolidone and aspartame are fully mixed, smashed and sieved, and dunaliella salina tablet coarse powder is obtained; 4, the dunaliella salina tablet coarse powder is added to the probiotics suspension, the dunaliella salina tablet coarse powder and the probiotics suspension are mixed, granulated and dried, and intermediate granules are obtained; 5, magnesium stearate, silicon dioxide and the intermediate granules are mixed to be uniform, tabletting is conducted, and the probiotics and dunaliella salina tablets are obtained.
Owner:合肥赛为智慧医疗有限公司
Preparing method for controlled released type tablet tamsulosin hcl and the tablet thereof
InactiveCN1835742AEvenly distributedSmooth releasePharmaceutical non-active ingredientsPill deliveryTamsulosin hclDrug release rate
The present invention relates to a simple and effective method for preparing a tamsulosin HCl sustained-release tablet and a tamsulosin HCl sustained-release tablet produced thereby. The method comprises the steps of: dissolving tamsulosin HCl as an active ingredient in an organic solvent; dissolving the tamsulosin HCl solution in hydroxypropylmethylcellulose phthalate to prepare a binder solution; and kneading the binder solution with a hydroxypropylmethylcellulose phthalate / glyceryl dibehenate mixture as an excipient and allows tamsulosin HCl to be released at uniformly controlled amounts in a subtained-release manner in vivo by controlling drug release rate according to different pH environments in vivo, so that it shows improved bioavailability and minimized side effects.
Owner:KYUNG DONG PHARM
Oral nano-vaccine of large yellow croaker and immune type large yellow croaker feed prepared by the same
ActiveCN110935016ALow costSimple preparation processAntibacterial agentsPharmaceutical non-active ingredientsCelluloseMesoporous silica
The invention relates to an oral nano-vaccine of large yellow croaker and an immune type large yellow croaker feed prepared by the same. The oral nano-vaccine comprises MSN-DLDH nano-particles and enteric coatings coating the surfaces of the MSN-DLDH nano-particles. The MSN-DLDH nano particles are nano particles formed by assembling DLDH protein and a mesoporous silica micro-nano carrying system;and the enteric coatings are hydroxypropyl methylcellulose phthalate. The nano-vaccine disclosed by the invention has the advantages of low cost, simple preparation process, good capability of tolerating a strongly acidic stomach environment of fishes and the like. Besides, the vaccine has no toxicity to kidney cells of the large yellow croaker and is good in biocompatibility; and thus a technicalsupport is provided for research and development of oral nano-vaccines and clinical application in the future.
Owner:FUJIAN NORMAL UNIV
Pharmaceutical composition and method for preparing same
ActiveUS11304945B2Rapid onsetImprove bioavailabilityPowder deliveryOrganic active ingredientsCellulosePolymer science
A solid dispersion, a method for preparing same, and a solid preparation including the solid dispersion. The solid dispersion contains (R)-4-amino-1-(1-(but-2-ynylacyl)pyrrolidin-3-yl)-3-(4-(2,6-difluorophenoxy)phenyl)-1,6-dihydro-7H-pyrrolo[2,3-d]pyridazine-7-one or a pharmaceutically acceptable salt thereof, and a carrier material. The carrier material is selected from hydroxypropyl methylcellulose acetate succinate and hydroxypropyl methylcellulose phthalate.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Preparation method of composite microencapsulated anti-ultraviolet finishing agent
InactiveCN110063926AStrong absorption capacityImprove bindingCosmetic preparationsToilet preparationsFreeze-dryingPolyethylene glycol
The invention provides a preparation method of a composite microencapsulated anti-ultraviolet finishing agent. The method includes the following steps that walnut shells are dried, crushed and subjected to ethanol extraction, extraction liquid is concentrated, refrigerated, filtered and heated in sequence, and then the extraction liquid is subjected to standing and is filtered; water is added in the extraction liquid, stirring is performed, activated carbon is added, stirring is performed, heat preservation is conducted, ethanol is added, and then stirring, filtering, heat preservation, standing, filtering and washing are conducted sequentially to obtain walnut shell extract; egg white is mixed with water, polyethylene glycol is added, stirring is conducted, the mixture is subjected to standing for layering, supernate is removed to obtain a precipitate, magnesium chloride is added in the precipitate, stirring is performed, and then stirring centrifugation, washing centrifugation and freeze-drying are conducted sequentially to obtain crude mucin; hydroxypropyl methyl cellulose phthalate, a silane coupling agent, ethyl orthosilicate and an ethanol aqueous solution are taken and stirred, and then the walnut shell extract and the crude mucin are added and stirred; sodium stearyl lactate and sodium lignosulphonate are added and stirred; homogenization, spray drying and cooling are performed sequentially to obtain the finishing agent. A fabric treated with the finishing agent prepared by the method has a good anti-ultraviolet effect and certain antimicrobial property.
Owner:余晨
Empty capsule for targeted release in intestinal tracts
PendingCN112773773ALess moistureHigh transparencyInorganic non-active ingredientsCapsule deliveryCelluloseCarrageenan
The invention discloses an empty capsule for targeted release in intestinal tracts. The empty capsule is prepared from the following raw materials in parts by weight: 1000 parts of hydroxypropyl methylcellulose, 5.0-9.0 parts of carrageenan, 8.5-12.5 parts of gel, 1.0-5.0 parts of potassium chloride, 4800-5200 parts of purified water, 1.5-2 parts of auxiliary materials, 2400-4400 parts of acetone, 700-900 parts of hydroxypropyl methyl cellulose phthalate, 2400-4400 parts of coating liquid, 50-150 parts of polyethylene glycol, 6.5-8.5 parts of triethyl citrate, 6.5-8.5 parts of glycerol and 50-150 parts of diethyl phthalate. The empty capsule designed and prepared by the invention has the advantages of being natural, free of pollution, long in storage period, consistent in uniformity, free of peculiar smell, low in production cost, not easy to absorb moisture, not easy to break, high in transparency and the like, can meet the requirements of people with various religious and vegetarian diet habits, and is relatively high in acceptability.
Owner:广州玖洲胶囊生物科技集团有限公司
Tea polyphenol liposoluble microcapsules and preparation method thereof
InactiveCN103330213BPreserve antioxidant activityEvenly dispersedTripeptide ingredientsAntinoxious agentsPolyphenolPhthalate
Owner:ZHEJIANG MINGHUANG NATURAL PRODS DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com