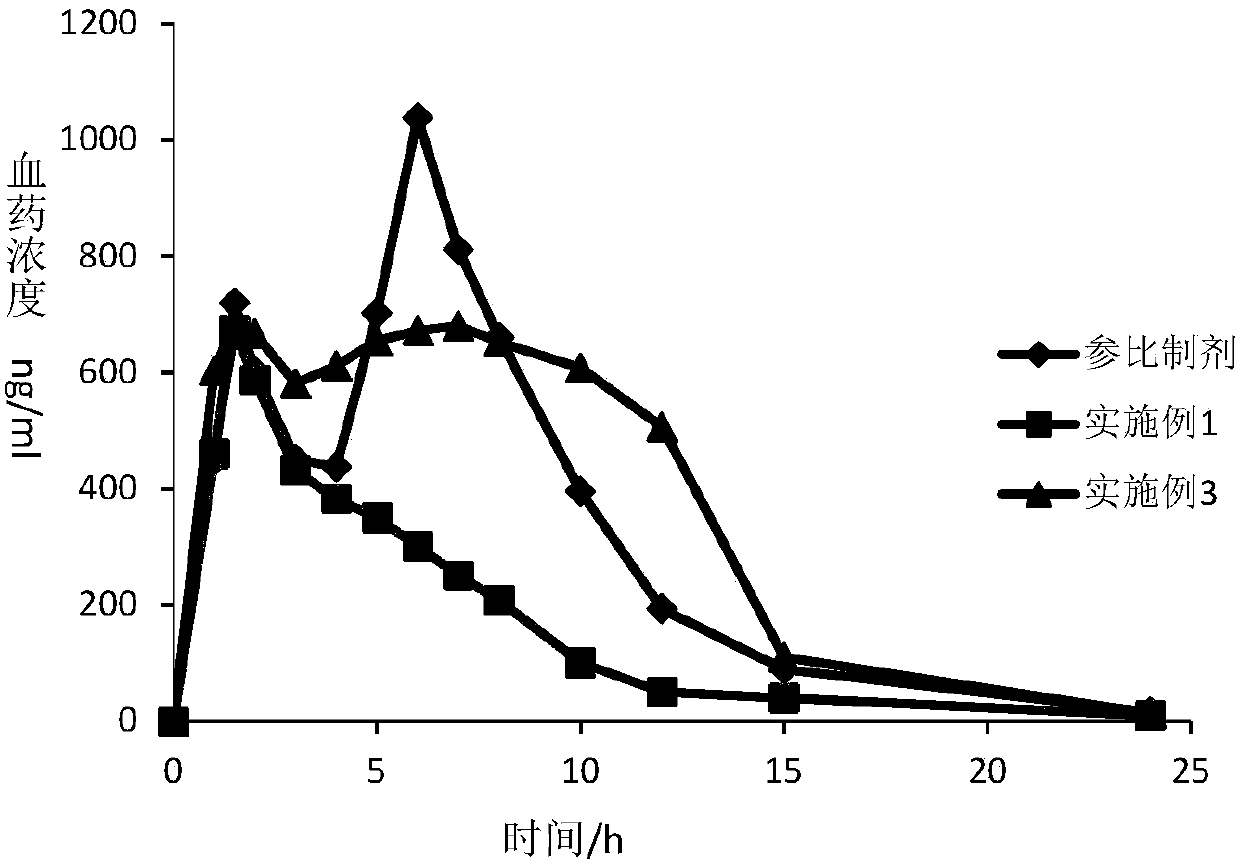
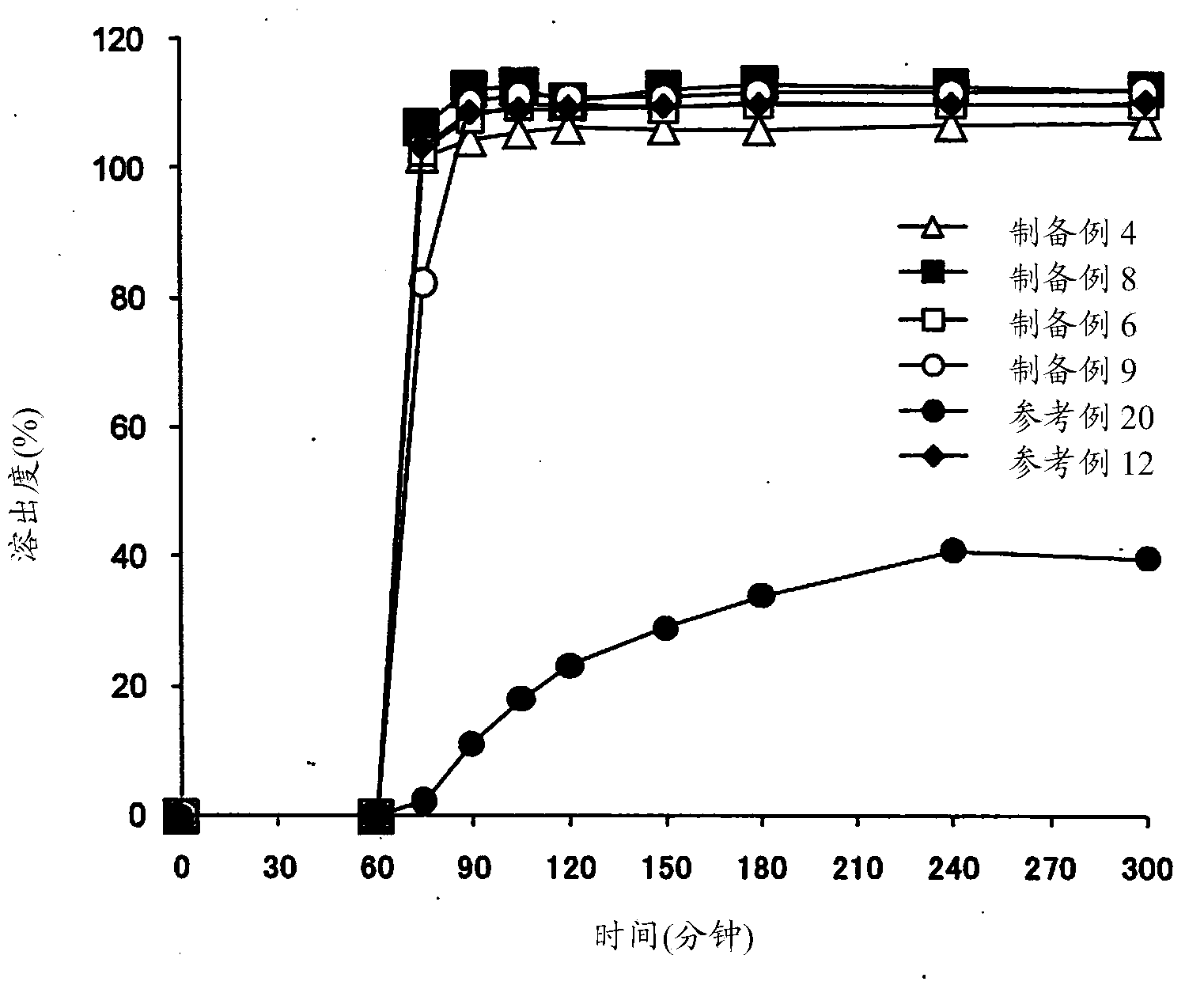
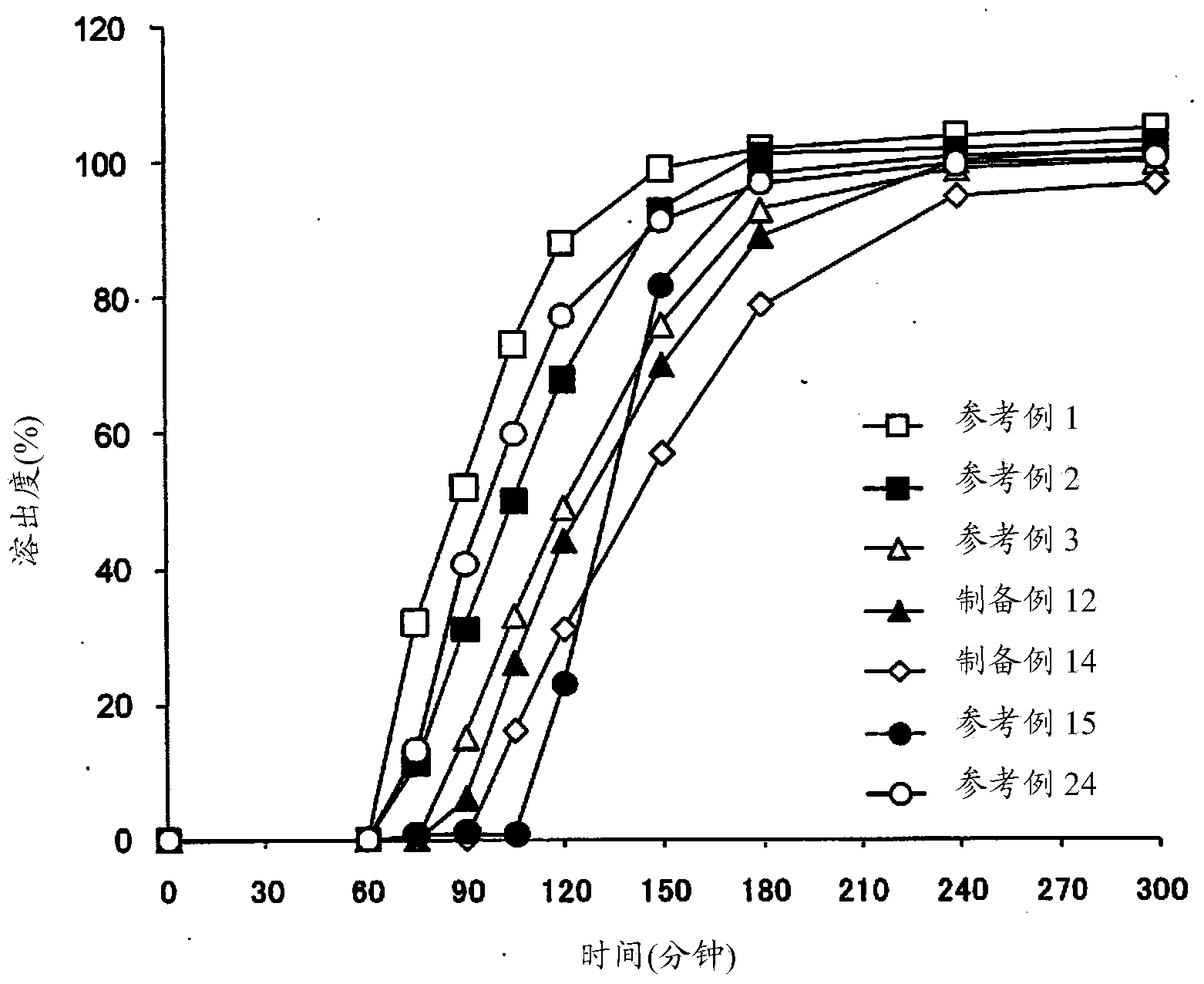
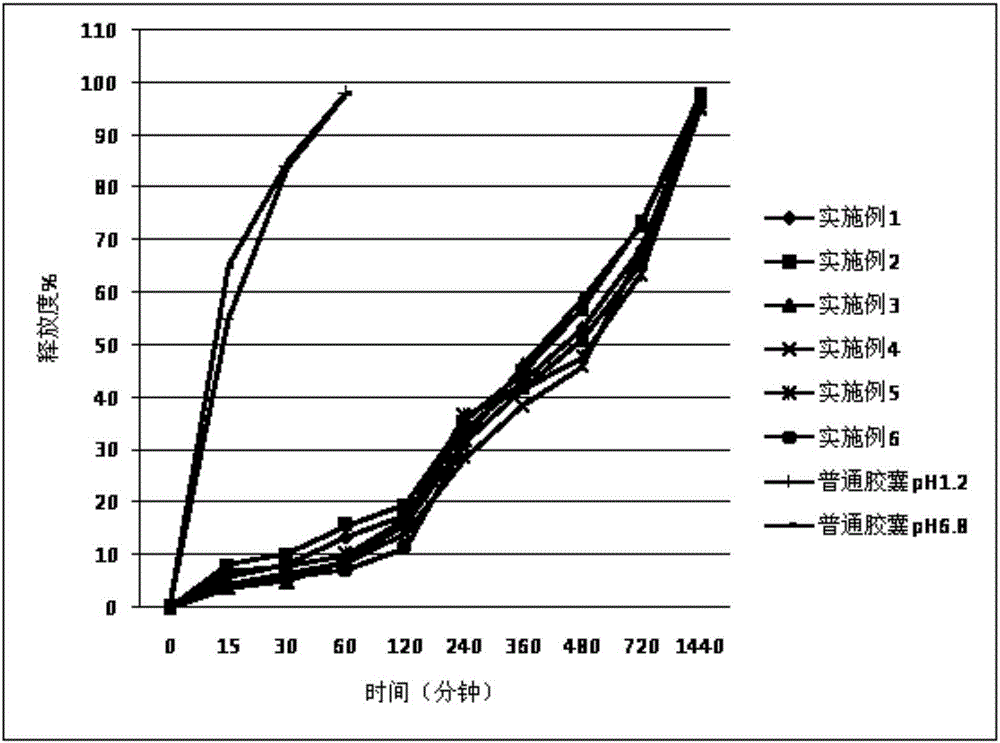
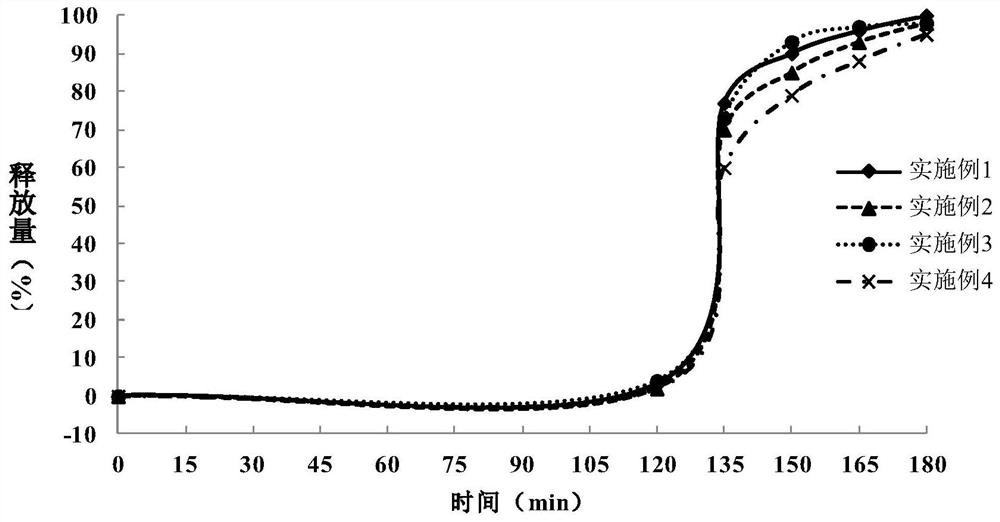
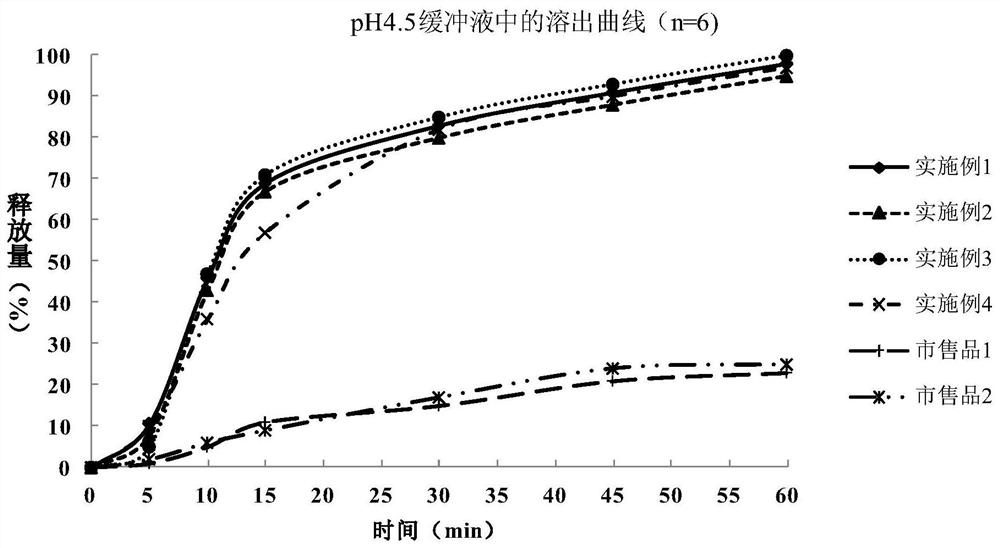
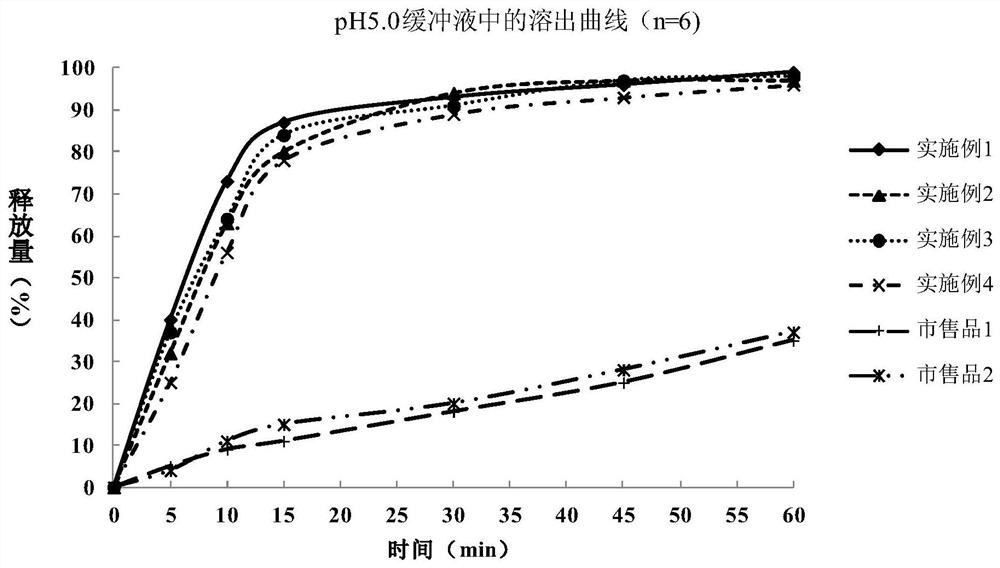
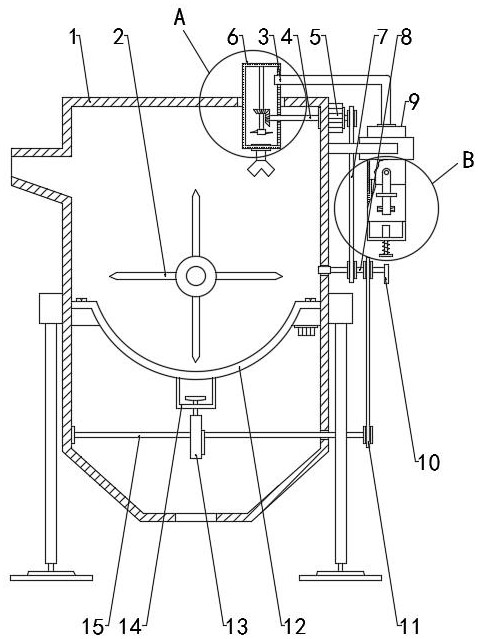
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Hypromellose phthalate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
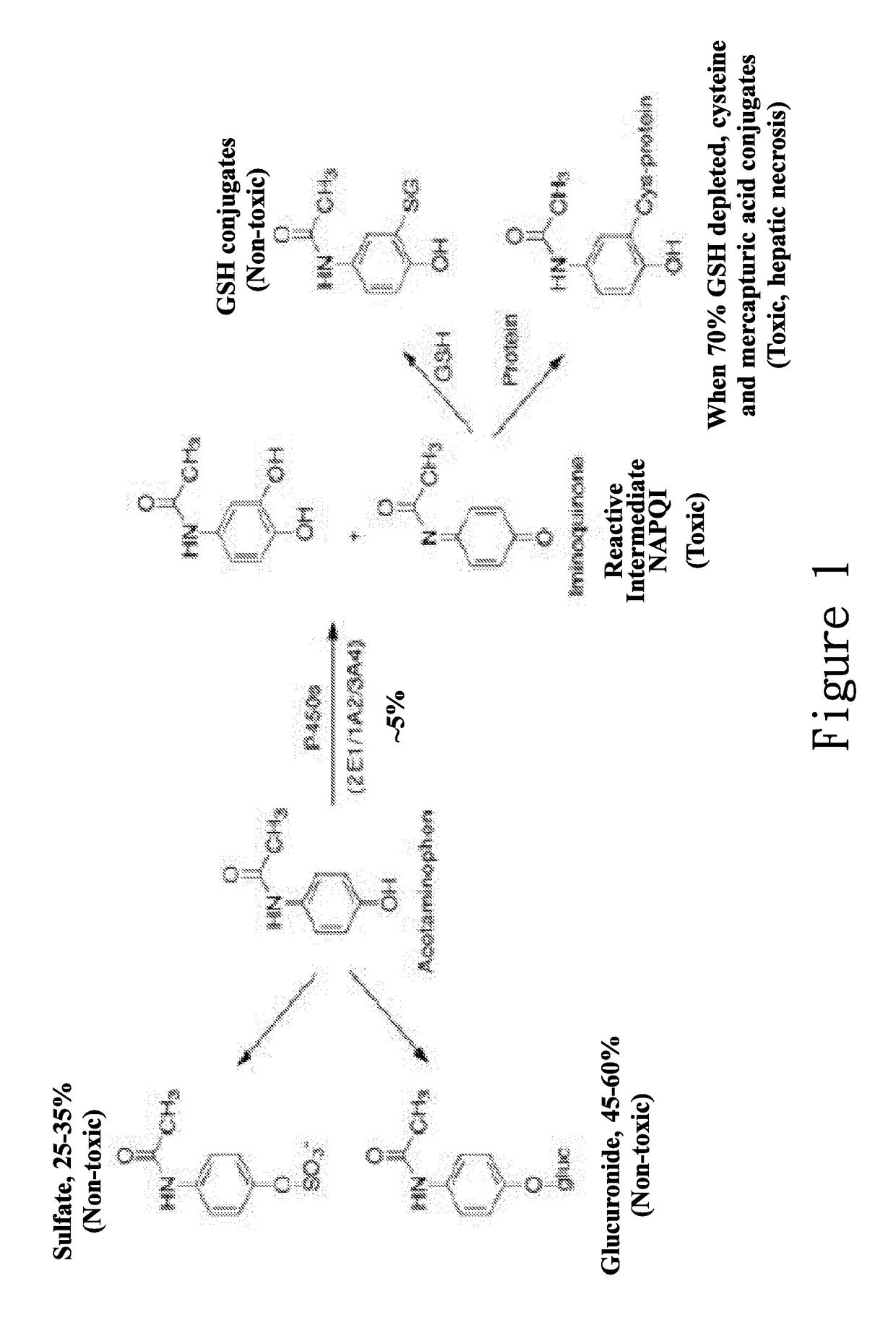
Hypromellose phthalate (hydroxypropyl methylcellulose phthalate, or HPMCP) is a phthalic acid ester of hydroxypropyl methylcellulose. In the pharmaceutical industry, hypromellose phthalate is used as a coating agent for tablets and granules. It is a colorless, odorless white powder.
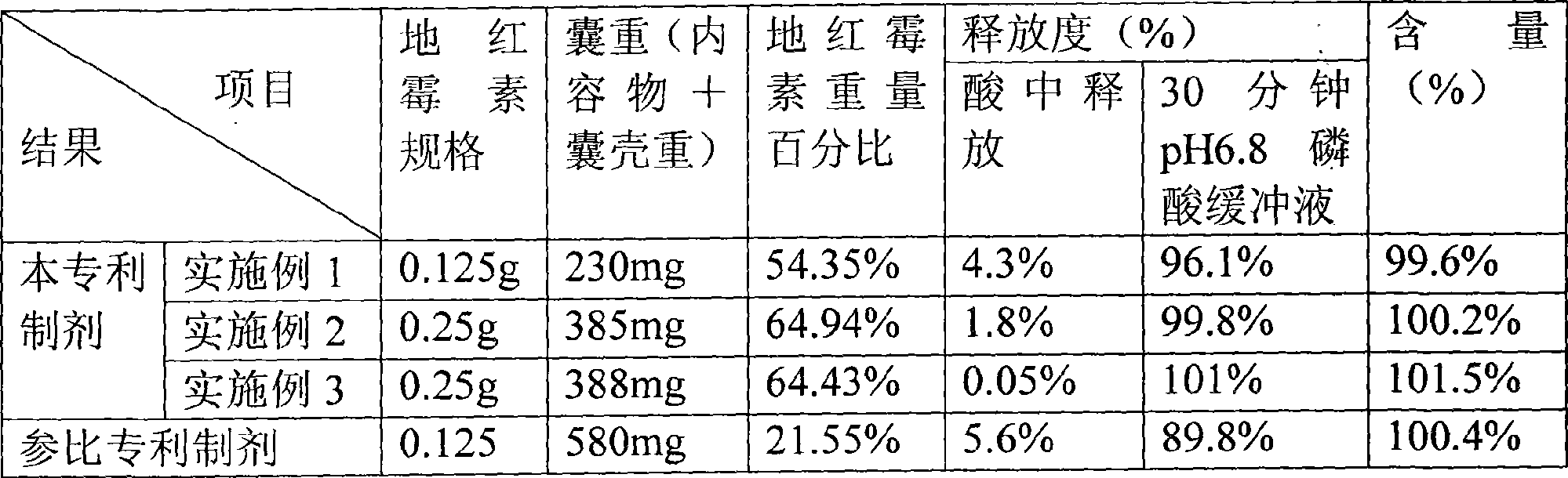
Dirithromycin enteric-coated formulation
The invention relates to a dirithromycin (DRM) enteric preparation with hydroxypropyl methylcellulose phthalate (HPMCP) being the framework material of drug-containing core and the coating material of an enteric coating layer. The enteric preparation has the advantages that the drug content is higher, the drug dissolution rate is less affected by the coating material, the preparation cost is low and the preparation process is simple.
Owner:SHANDONG INST OF PHARMA IND
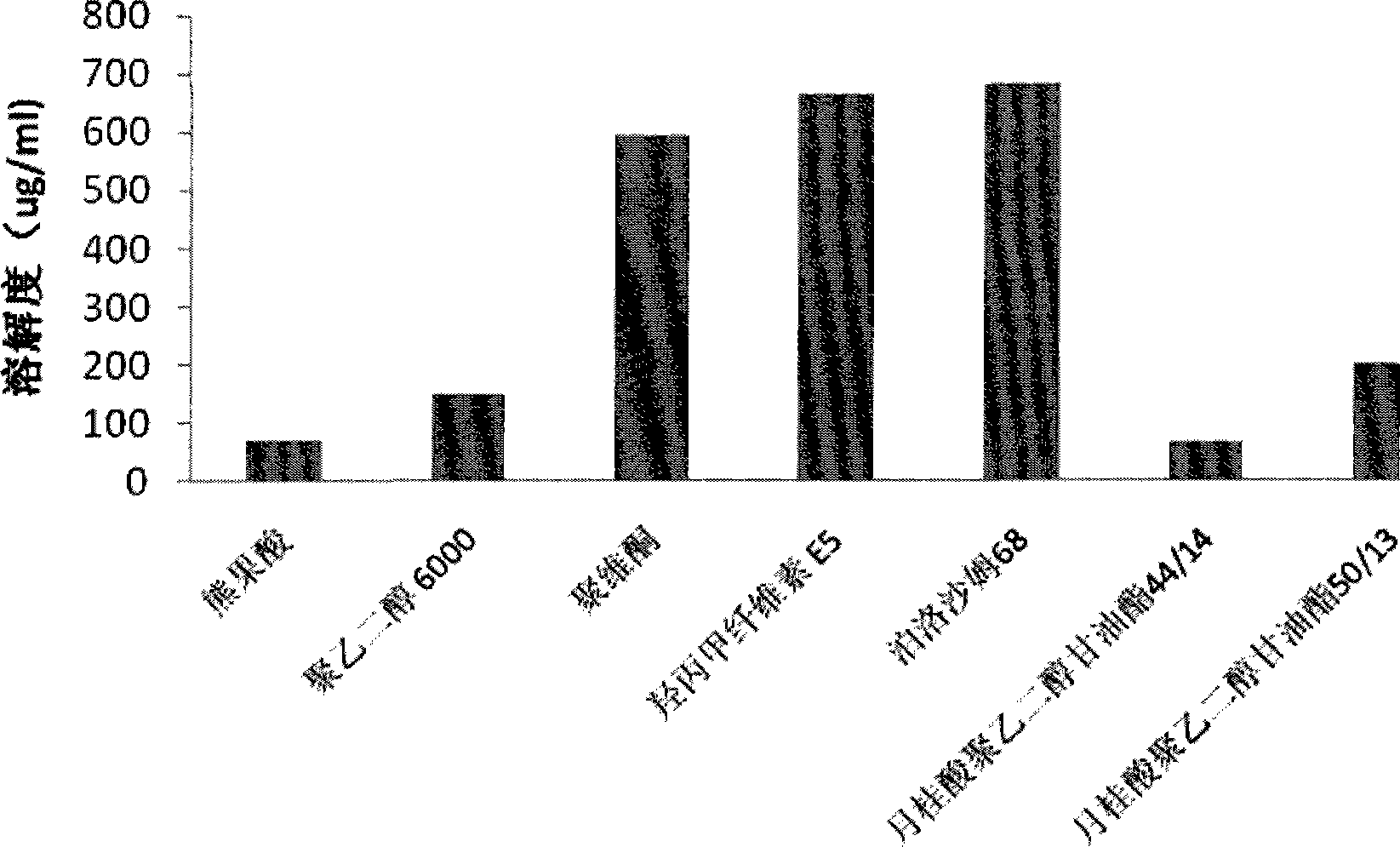
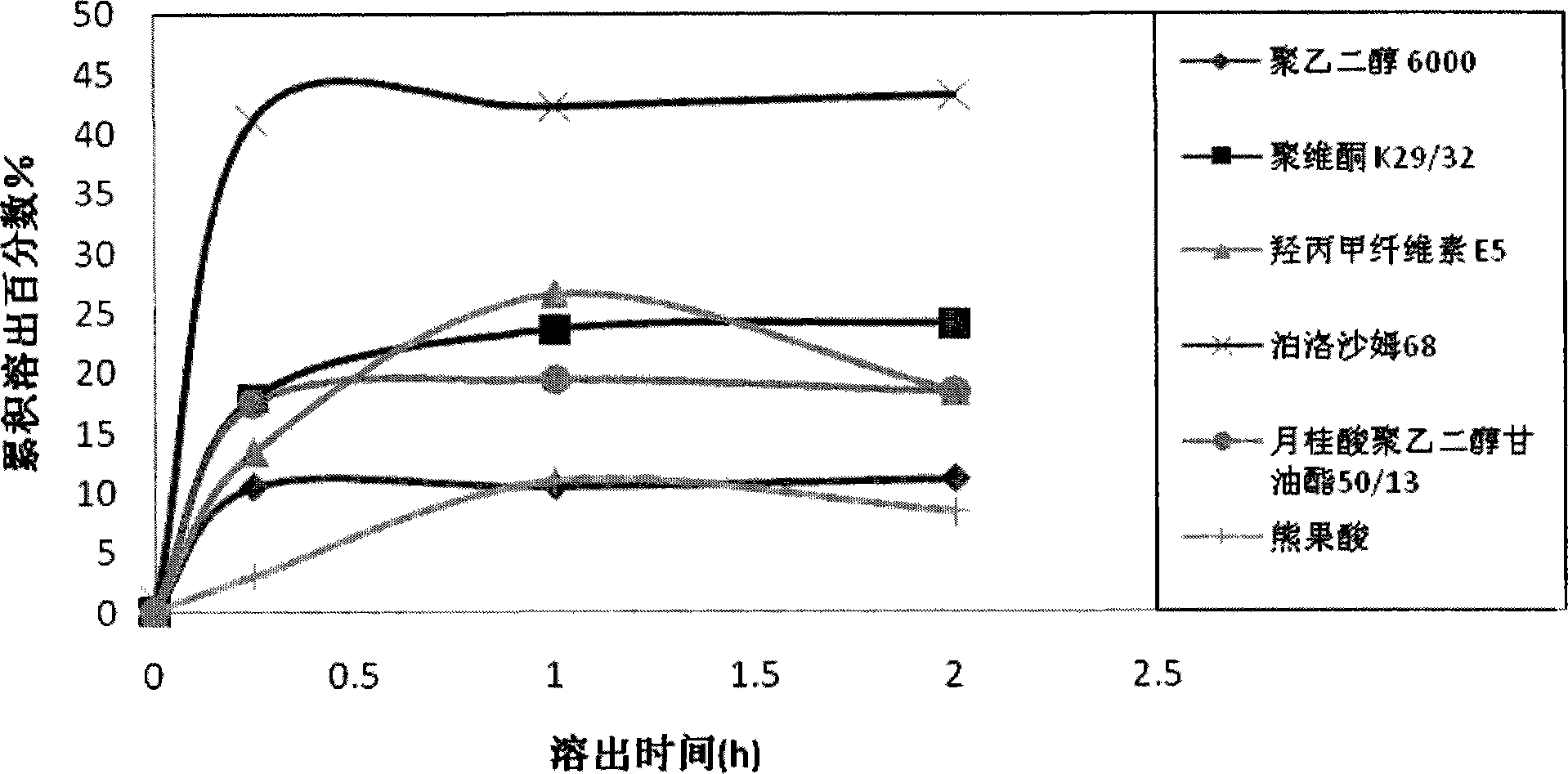
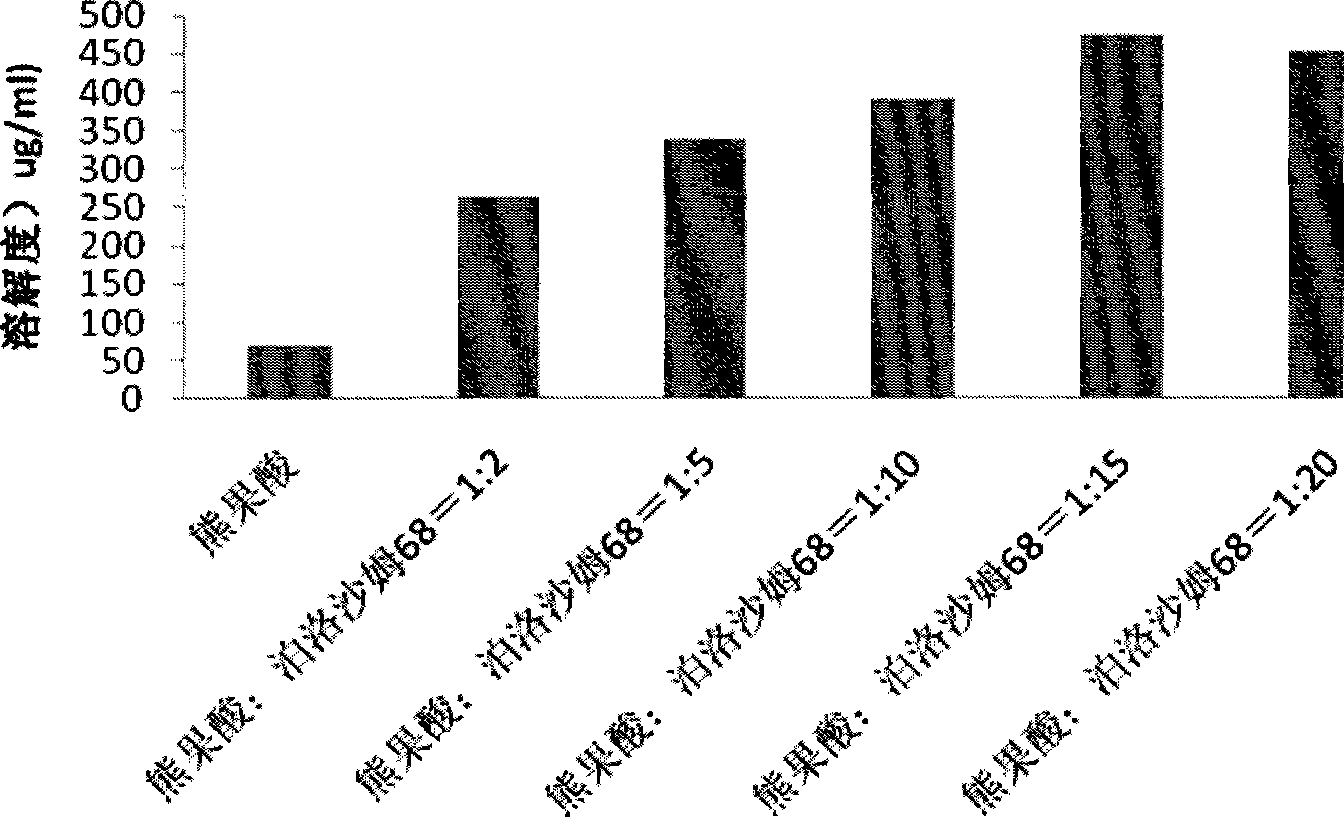
Ursolic acid solid dispersion and preparation method thereof
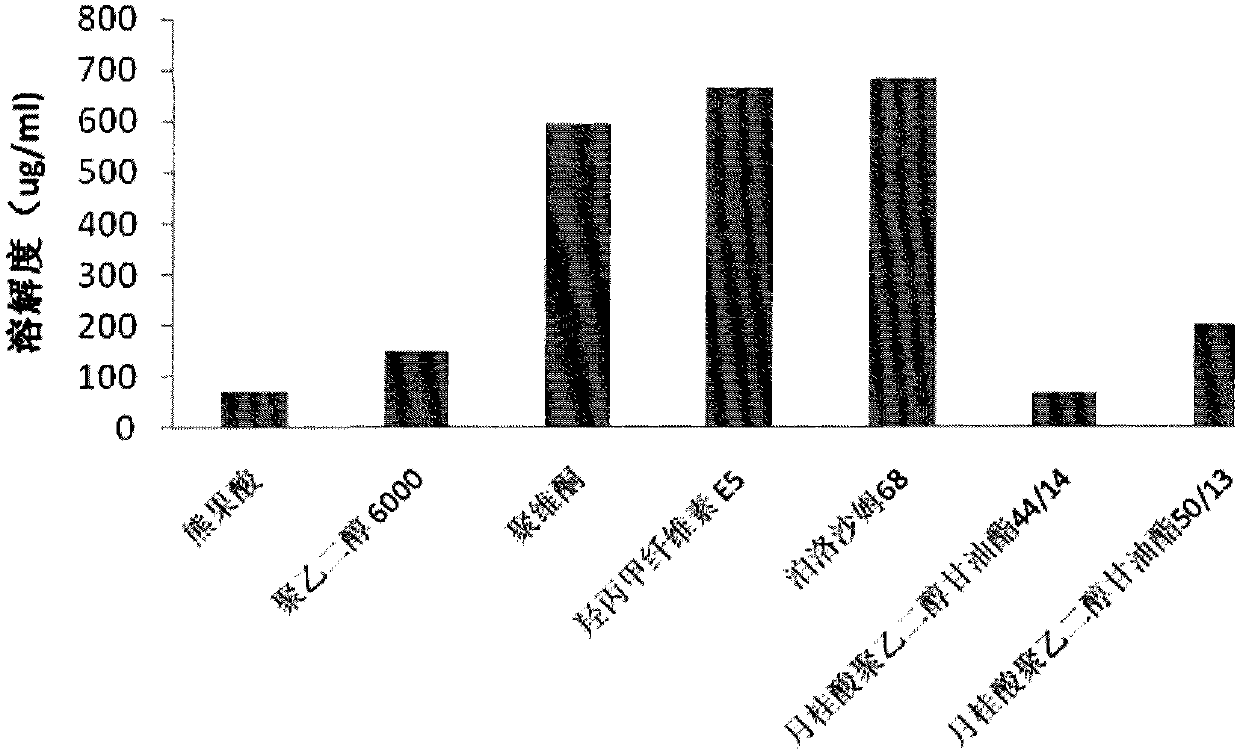
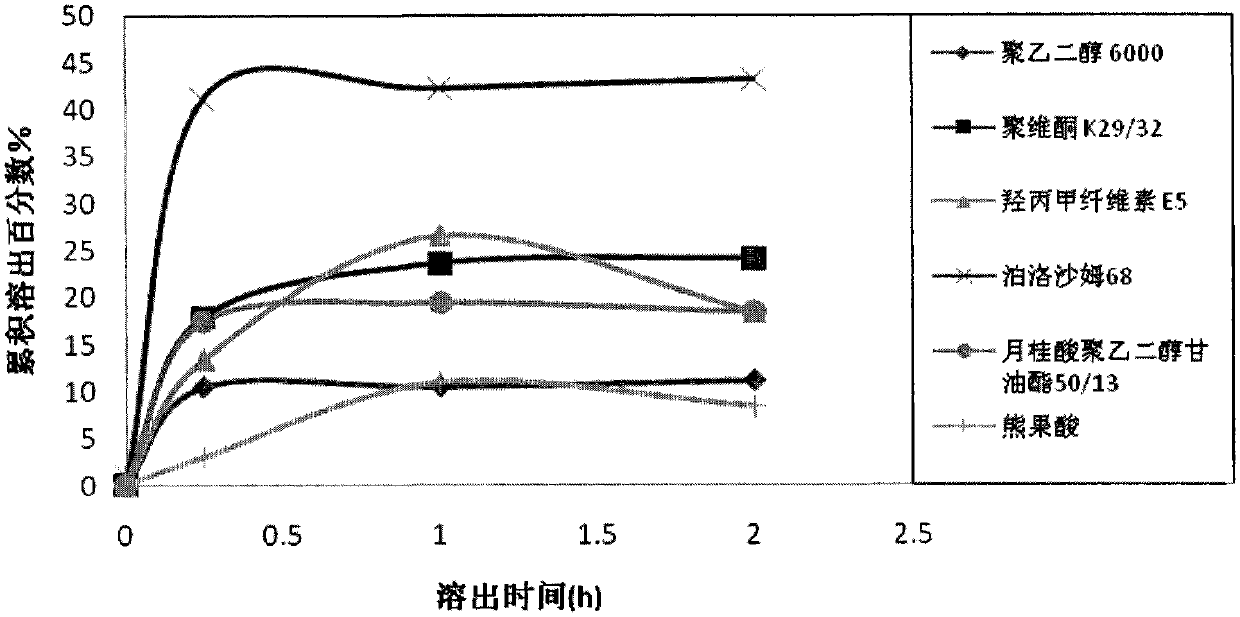
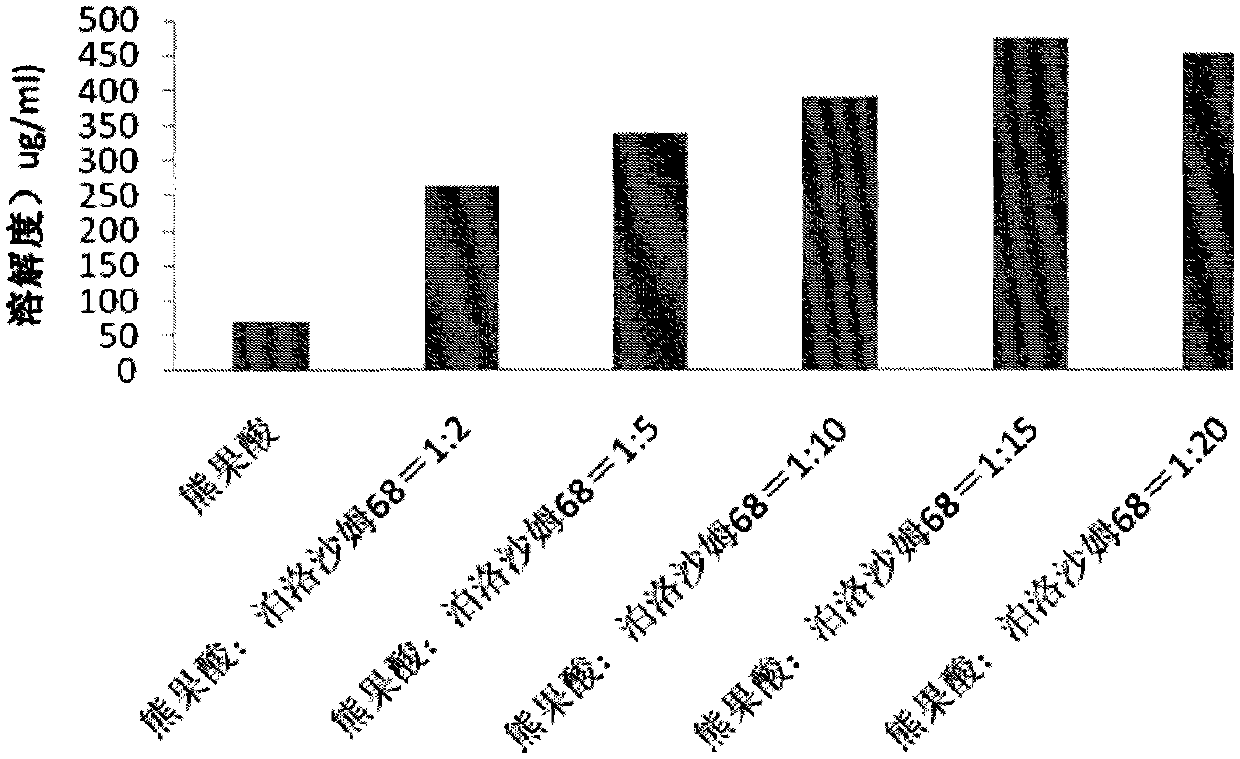
ActiveCN102871950AImprove solubilityIncrease dissolution rateAntibacterial agentsOrganic active ingredientsSolubilityUrsolic acid
The invention discloses an ursolic acid solid dispersion and a preparation method thereof. The formula of the ursolic acid solid dispersion comprises ursolic acid and a carrier material; the mass ratio of the ursolic acid to the carrier material is 1:2 to 1:20; and the carrier material is one or more of PEG6000 (polyethylene glycol 6000), povidone k29 / 33, hypromellose E5, polyving alcohol, poloxamer 68, GELUCIRE 50 / 13, polyacrylic resin EPO, polyacrylic resin L 100-55, povidone-vinyl alcohol, hypromellose AS-HG, hypromellose AS-LG, hypromellose AS-MG, low substituted hypromellose, hypromellose phthalate, cellulose acetate phthalate, glucan, polyoxyethylene, polyoxyethylene stearates and polyvinyl acetate phthalate. The preparation method comprises the following steps: dissolving the ursolic acid and the carrier material into an organic solvent according to the formula; removing the organic solvent; and grinding. The ursolic acid solid dispersion has good solubility and has high bioavailability.
Owner:CHINA GATEWAY PHARMA DEV CO LTD
Febuxostat enteric preparation
ActiveCN101716132ADissolves and absorbs quicklyImprove bioavailabilityOrganic active ingredientsSkeletal disorderPylorusHypromellose phthalate
The invention provides a Febuxostat enteric preparation comprising a medicament inner core, an isolated layer and a casing layer. The Febuxostat enteric preparation adopts hypromellose phthalate ester to wrap an isolated coat and selects cellulose acetate propionate (CAP) and polyvinylacetate phthalate of an enteric-coated material dissolvable in a pH value range of 5-6.8 to wrap an enteric coat. The enteric preparation does not leak after being orally taken into the stomach of a human being, is rapidly dissolved and disintegrated after entering the pylorus with a pH value larger than 5 and releases the Febuxostat medicament for the ntestinal tract to rapidly absorb so as to achieve the aim of improving the bioavailability and the healing effect. Compared with a common Febuxostat tablet or capsule, the invention has the advantages of stability in gastric juice, rapid dissolution and absorption in the ntestinal tract and high bioavailability, is prepared by general process and is easy to realize the industrialized production.
Owner:BEIJING KONRUNS PHARMA TECH CO LTD
Preparation method of taste-masked suspension granules of Gegenqinlian decoction
ActiveCN102309562AGood taste masking effectSimple processAntibacterial agentsAntipyreticAdhesiveHypromellose phthalate
The invention relates to a preparation method of taste-masked suspension granules of Gegenqinlian decoction. The preparation method comprises the following steps of 1, taking appropriate amounts of dispensing granules of three traditional Chinese medicines comprising radix puerariae, coptis chinensis and scutellaria baicalensis and respectively carrying out coating processes in a fluidized bed through adopting one or more polymers as coating materials to obtain coated granules for next use, 2, taking an appropriate amount of at least one suspending agent, mixing uniformly the at least one suspending agent and radix glycyrrhizae preparata dispensing granules, then adding an appropriate amount of an adhesive into the mixture to prepare into granules by a wet method, drying the prepared granules in an oven, and then spraying an appropriate amount of an ethanol solution as an aromatic to obtain suspending agent-containing radix glycyrrhizae preparata dispensing granules after ethanol is volatilized, and 3, weighing appropriate amounts of the suspending agent-containing radix glycyrrhizae preparata dispensing granules and the coated granules containing radix puerariae, coptis chinensisand scutellaria baicalensis, mixing well, and carrying out sub-packaging to obtain the taste-masked suspension granules of Gegenqinlian decoction, wherein the one or more polymers as coating materials are selected from enteric-coated polyacrylic resin, hypromellose acetate succinate and hydroxypropyl methylcellulose phthalate. The invention provides the preparation method of the taste-masked suspension granules of Gegenqinlian decoction. The preparation method is also suitable for taste masking of a traditional Chinese medicine with a bitter taste or a fishy smell.
Owner:安徽天祥药业有限公司
Aprepitant oral pharmaceutical preparation
InactiveCN105534987ADissolution rate is fastImprove bioavailabilityOrganic active ingredientsDigestive systemLow-substituted hydroxypropylcelluloseHypromellose phthalate
The invention discloses an aprepitant oral pharmaceutical preparation. The aprepitant oral pharmaceutical preparation comprises 15wt%-25wt% of aprepitant, 45wt%-75wt% of hydroxypropyl methylcellulose phthalate / hydroxypropyl methylcellulose acetate succinate, 10wt%-25wt% of microcrystalline cellulose, lactose or mannitol, 2wt%-8wt% of low-substituted hydroxypropyl cellulose as well as croscarmellose sodium and / or crospovidone, 0-2wt% of silicon dioxide and / or talc and 0-2wt% of magnesium stearate. The pharmaceutical preparation can be prepared in a form of tablets or capsules andhas high stability and good bioavailability.
Owner:北京颐诺赛医药科技有限公司
Rebeprazole sodium enteric-coated tablet and preparation process thereof
InactiveCN105640915AImprove acid resistanceImprove bioavailabilityOrganic active ingredientsDigestive systemFiller ExcipientHypromellose phthalate
A rebeprazole sodium enteric-coated tablet comprises, by weight, 65-125 parts of tablet core, 10-40 parts of isolation layer and 80-170 parts of enteric-coated layer, wherein filler is one or more of microcrystalline cellulose, lactose and mannitol, stabilizer is one or more of magnesium oxide and anhydrous sodium carbonate, disintegrating agent is one or more of polyvinyl pyrrolidone, cross-linking sodium carboxymethyl cellulose and lauryl sodium sulfate, lubricating agent is one or two of talcum powder and magnesium stearate, binder is the anhydrous ethanol solution of crospovidone or the aqueous solution of hydroxypropyl methylcellulose, the isolation layer is one or two of magnesium oxide and ethyl cellulose, and the enteric-coated layer is one or more of hydroxypropyl methylcellulose phthalic acid ester, enteric-coated acrylic resin, cellulose acetate phthalic ester, talcum powder and 93F19255 type coating agent. A preparation process of the rebeprazole sodium enteric-coated tablet is simple, low in equipment requirement, high in yield, and controllable in product quality. The dissolution rate of the rebeprazole sodium enteric-coated tablet is highly consistent with the release behavior of originally-developed drugs, and the rebeprazole sodium enteric-coated tablet is good in stability.
Owner:吉林修正药业新药开发有限公司
Silymarin compound nanoparticle and preparation method thereof
ActiveCN102614239AImprove stabilityHigh dissolution ratePowder deliveryDigestive systemPrillHypromellose phthalate
The invention discloses a silymarin compound nanoparticle and a preparation method thereof. The silymarin compound nanoparticle is prepared through the following steps of: (1) uniformly mixing silymarin, hypromellose phthalate and water by using a dual-screw extruder to obtain a silymarin solid dispersant; (2) mixing the silymarin solid dispersant with phospholipid, brij 35 and ethanol to obtain an ethanol suspension; and (3) making the ethanol suspension flow through supercritical CO2 fluid equipment, and preparing the silymarin compound nanoparticle by applying a supercritical fluid anti-solvent technology. A nanoparticle system has very high stability and high medicament dissolution rate. The method provided by the invention is simple, refining operation is controlled. The nanoparticle prepared by using the method disclosed by the invention has a small particle diameter standard difference, the dispersant is in a good state, and the defects of easiness in agglomerating, poor refining operation safety and the like existing in the prior art are overcome.
Owner:晨光生物科技集团天津有限公司
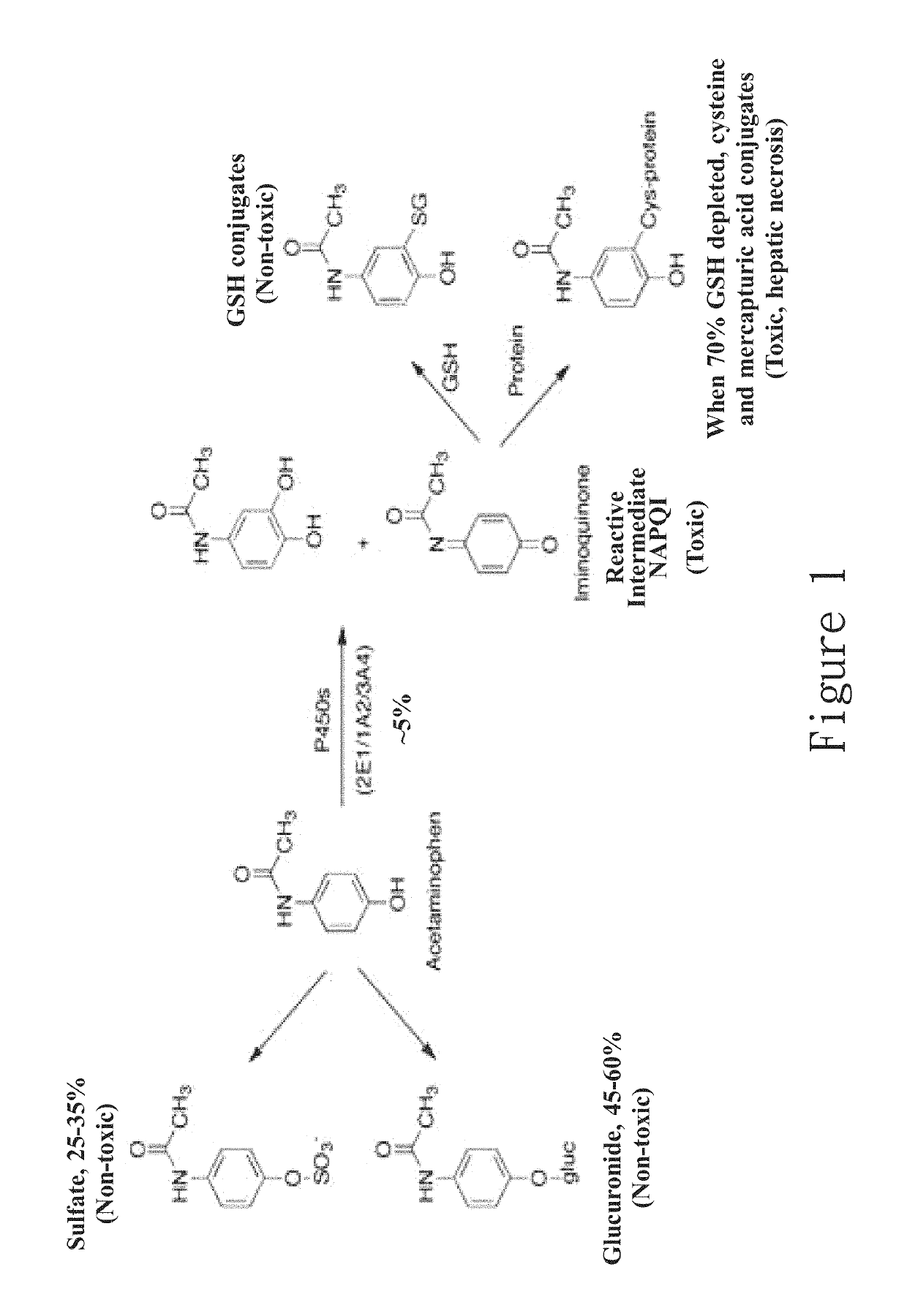
New hepatotoxicity-free pharmaceutical composition containing acetaminophen drugs
ActiveUS20170172950A1Reduce liver toxicityOrganic active ingredientsAntipyreticColloidal silicaPolyoxyethylene castor oil
A new compound composition that is free of a side effect to a liver and used for alleviating the toxicity of an acetaminophen (APAP) medicament to the liver. The compound composition comprises (a) a pharmaceutically effective amount of acetaminophen and (b) a frequently-used safe and pharmaceutically acceptable excipient that can be combined with one or more than two medicaments that can reduce the toxicity of a drug via liver enzyme CYP2E1 metabolism to the liver. The compound is selected from the following group: Tween 20, microcrystalline cellulose, dicalcium phosphate, polyoxyethylene 23 lauryl ether, saccharin, mannitol, polyoxyethylene alkyl ether, sucralose, pyrrolidone, sodium starch glycolate, acrylic resin S100, carboxymethyl cellulose sodium, polyoxyethylene polyoxypropylene, menthol, low-substituted hydrocarbon propyl cellulose, pregelatinized starch, Dextrates NF hydrated, citric acid, polyoxyethylene castor oil, colloidal silica, polyethylene glycol monostearate aliphatic ester, sorbic acid, lemon oil, hydroxypropyl cellulose, sorbitol, acesulfame potassium, hypromellose phthalate, lactose monohydrate, maltodextrin, Brij 58, Brij 76, Tween 80, Tween 40, PEG 400, PEG 4000, PEG 2000, and the like, so as to reduce the side effect of the toxicity caused by acetaminophen to the liver.
Owner:INT EDUCATION FOUND
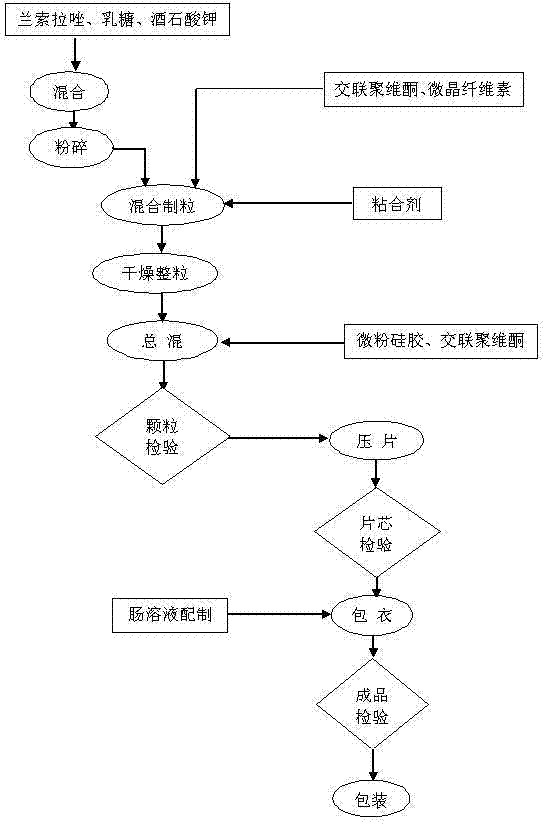
Dexlansoprazole medicinal preparation
ActiveCN107648231AGuaranteed stabilityShort preparation cycleOrganic active ingredientsDigestive systemMedicineDexlansoprazole
The invention discloses a dexlansoprazole medicinal preparation, and in particular relates to a dexlansoprazole medicinal composition having a bi-phase drug release behavior and a preparation method of the medicinal composition. The medicinal composition consists of enteric quick release pellets and enteric delay pellets, wherein delay layers of the enteric delay pellets are prepared by mixing hydroxypropyl methylcellulose phthalate (HPMCP) and methacrylic acid copolymers S100 according to specific proportions. Effects of a good acid control effect, stable drug release and long holding time are achieved.
Owner:JIANGSU HANSOH PHARMA CO LTD
Orally disintegrating tablet
InactiveCN103402500AStay acid resistantControl releaseOrganic active ingredientsDigestive systemLansoprazoleControlled release
A orally disintegrating tablet is obtained by tableting fine granules showing controlled release of lansoprazole and an additive, which is capable of suppressing breakage of the fine granules during tableting, and can control the release of lansoprazole for a long time, and can maintain a therapeutically effective concentration for a prolonged time, and shows superior disintegration property in the oral cavity.
Owner:TAKEDA PHARMA CO LTD
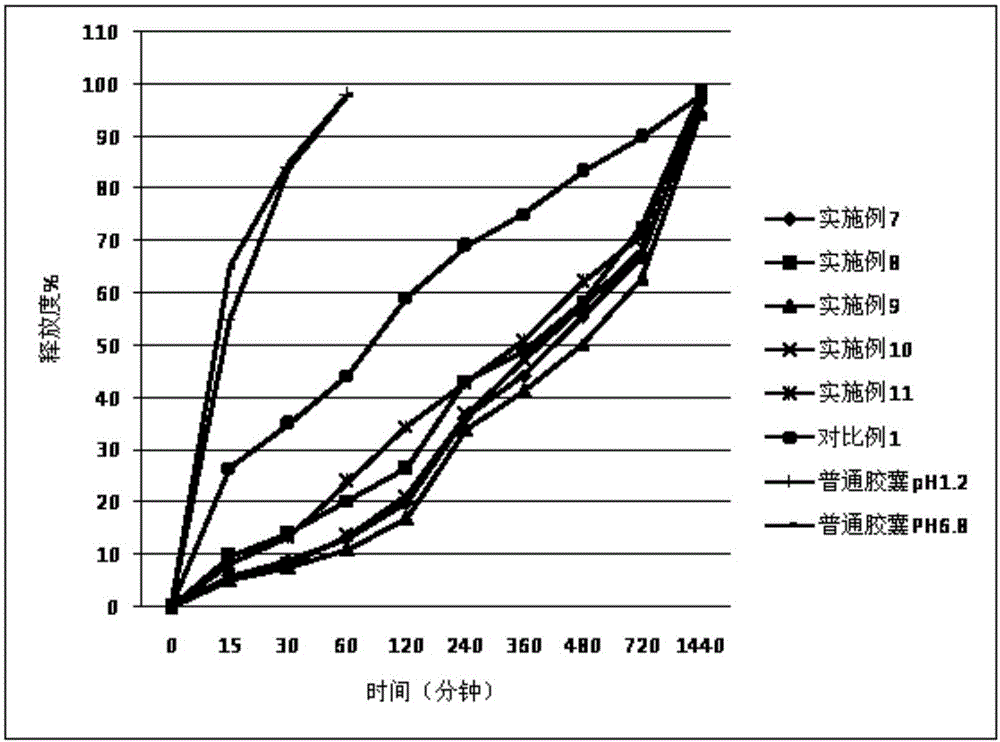
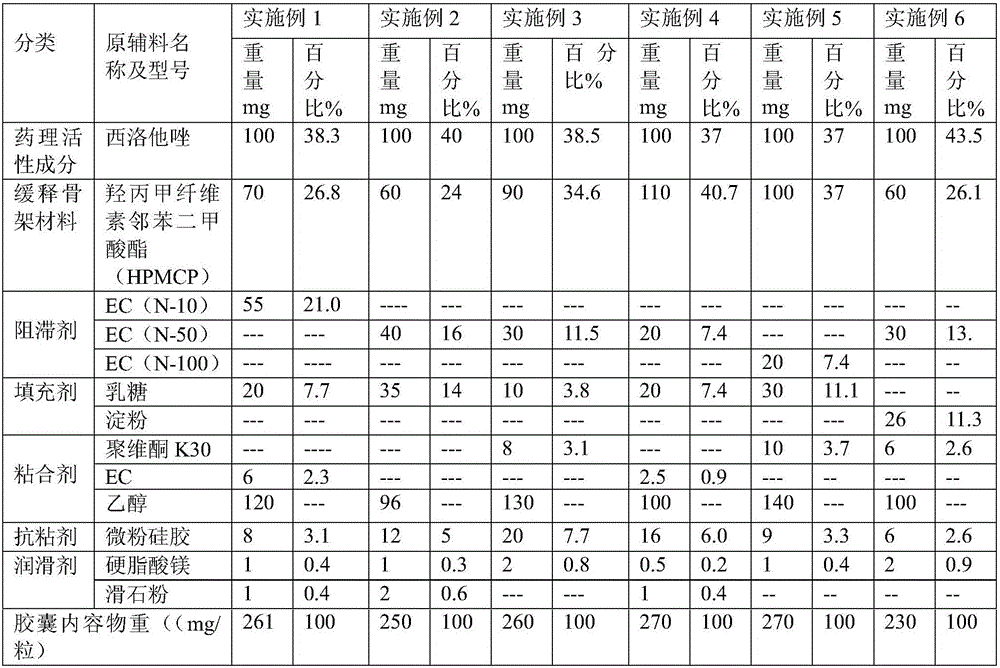
Cilostazol sustained-release capsule compound and preparation method thereof
ActiveCN106511313AImprove controllabilityGood storage stabilityOrganic active ingredientsAntipyreticSolubilitySodium acetate
The invention relates to the pharmaceutical field and discloses a cilostazol sustained-release capsule compound and a preparation method thereof. The sustained-release capsule compound comprises a medicinal hollow capsule body and contents arranged in the medicinal hollow capsule body. The contents comprise cilostazol, a hydroxypropyl methylcellulose phthalate sustained-release framework material, a retardant, an adhesive, a filler, an antisticking agent and a lubricating agent. Hydroxypropyl methylcellulose phthalate is used as the framework sustained-release material and is a product obtained by performing an esterification reaction between hydroxypropyl methylcellulose and phthalic anhydride in acetic acid with sodium acetate serving as a catalyst. Molecules have carboxyl negative charge intermolecular repulsion under a carboxyl alkaline environment, so that twinned macromolecules are stretched to be dissolved, and enteric solubility is achieved. The absorption of the cilostazol by the upper gastrointestinal tract can be reduced while the absorption of the cilostazol by the lower gastrointestinal tract is improved. Adverse drug reactions such as headaches, head heaviness and tachycardia caused by sudden increase of the drug concentration are overcome. In addition, the cilostazol sustained-release capsule compound is taken only one time per day, so that the adaptability of patients is improved.
Owner:浙江为康制药有限公司
Sodium bicarbonate enteric capsule and preparation method thereof
ActiveCN112472715AAvoid adverse reactionsReduce adverse effectsInorganic active ingredientsSkeletal disorderSodium bicarbonateCellulose
The invention belongs to the technical field of medicinal preparation processing, and particularly relates to a sodium bicarbonate enteric capsule and a preparation method thereof. The preparation method of the sodium bicarbonate enteric capsule comprises the following steps: adding a capsule shell material suitable for alkaline contents into water, heating and stirring to obtain a solution, and dipping with glue to obtain a hollow capsule shell; dipping the hollow capsule in an enteric coating solution, and drying to obtain an enteric hollow capsule shell; filling the content containing sodium bicarbonate, coating a sealing material solution at the sleeved part of the capsule, and drying to obtain the sodium bicarbonate enteric capsule. The capsule shell material suitable for the alkalinecontents is prepared from the following raw materials in parts by weight: 10-20 parts of hydroxypropyl methyl cellulose phthalate, 1-2 parts of curdlan and 0.5-1.5 parts of xanthan gum. According tothe invention, the enteric hollow capsule is filled with the alkaline contents, so adverse reactions of the stomach can be avoided, and the effects of neutralizing uric acid and protecting the intestinal tract can be more effectively realized; and compared with the coating by a spraying technology, the enteric hollow capsule has less influence on the contents.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
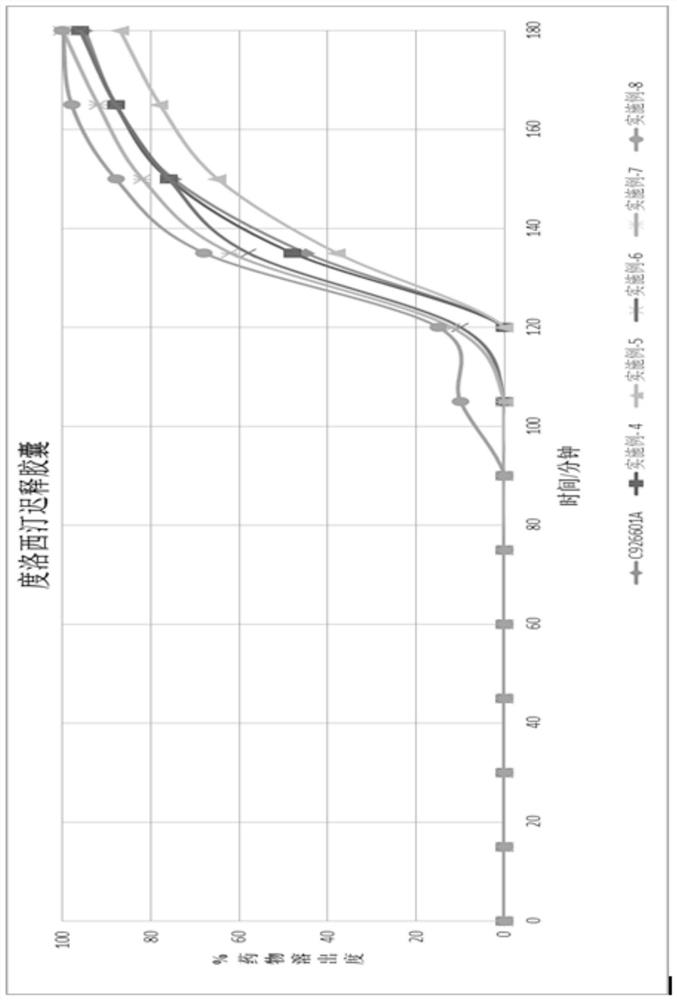
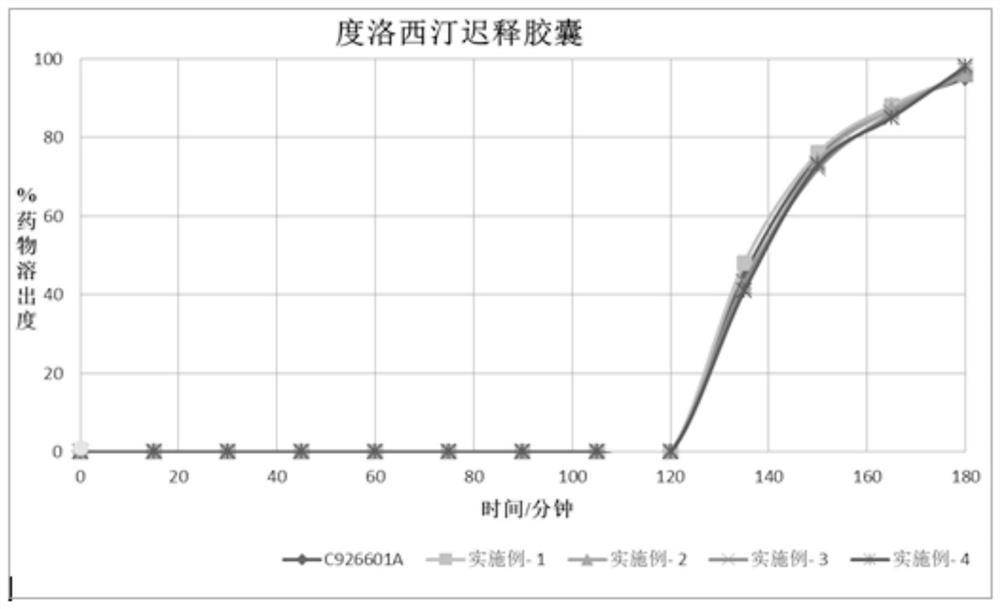
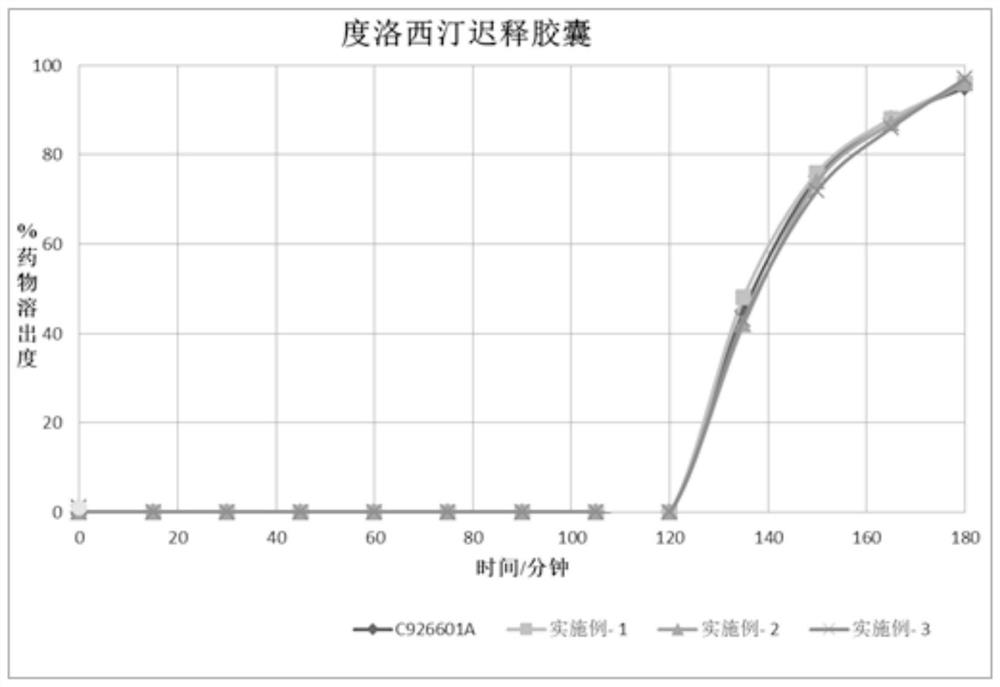
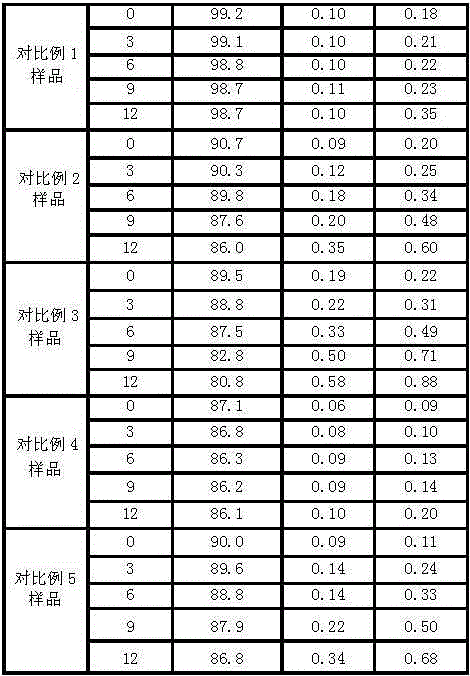
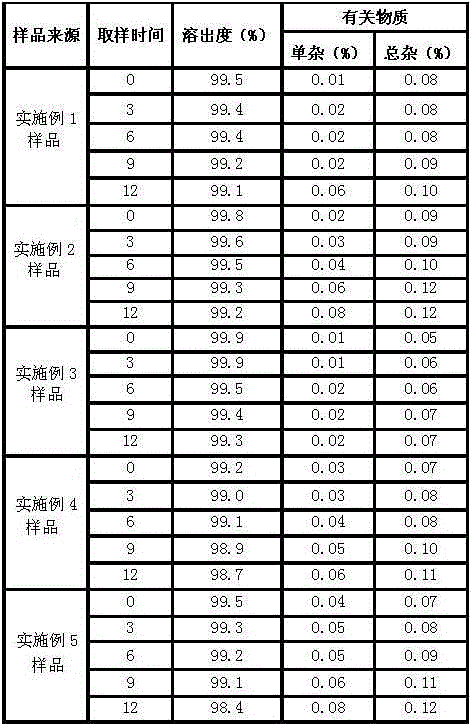
Duloxetine pharmaceutical composition
PendingCN112168797APrecision releaseReduced risk of spillsOrganic active ingredientsNervous disorderDuloxetineCellulose
The invention discloses a duloxetine pharmaceutical composition. The duloxetine pharmaceutical composition comprises (1) a pellet loaded with a certain amount of duloxetine; and (2) an enteric layer consisting of two types of hydroxypropylmethyl cellulose phthalate different in pH solubility, wherein the enteric layer coats the pellet. According to the pharmaceutical composition, the polymer hydroxypropylmethyl cellulose phthalate (HPMCP) with different levels of pH solubility is used, and adding ammonia for neutralization is not needed, so that the process is shortened, the reaction between the enteric layer and active substances of the duloxetine on the pellet is greatly reduced, and stability is increased; in addition, different HPMCP forms the enteric layer, so that the duloxetine is prevented from being damaged by the acidic condition of a stomach, and the duloxetine is released in a gastrointestinal tract (GIT) with relatively high pH, for example, in a small intestine, and thus,accurate release of the drug at a target part is facilitated, and the risk of dose dumping of the drug within an initial time interval is greatly lowered.
Owner:宁波高新区美诺华医药创新研究院有限公司
Omeprazole hydrotalcite composite tablet and preparation process thereof
PendingCN113384547ARelieve painReduce stomach acid concentrationOrganic active ingredientsDigestive systemHypromellose phthalateOmeprazole
The invention discloses an omeprazole hydrotalcite composite tablet and a preparation process thereof. The omeprazole hydrotalcite composite tablet is of a three-layer composite structure of an omeprazole tablet core layer, an enteric layer and a hydrotalcite layer which are sequentially pressed from inside to outside. The preparation process comprises the following steps: firstly, pressing omeprazole and auxiliary materials into a tablet core; then, directly carrying out secondary pressing by using hydroxypropyl methylcellulose phthalate and the tablet core, and pressing the enteric layer; and finally, carrying out third-time pressing on hydrotalcite, the auxiliary materials and the tablet core subjected to secondary pressing to obtain a pressed tablet of an omeprazole, enteric layer and hydrotalcite quick-acting layer. After a patient takes the composite tablet, the hydrotalcite layer in the medicine is rapidly disintegrated, the concentration of gastric acid is rapidly reduced, the pain of the patient is reduced, then the omeprazole wrapping the enteric layer enters the intestinal tract to be released, the gastric acid release capacity of gastric cells is reduced, and the symptoms are treated firstly and then the cost is reduced.
Owner:SHANGHAI SINE WANXIANG PHARMA +1
Drug composition for treating digestive system diseases
InactiveCN104606168AEasy to prepareIncrease productionOrganic active ingredientsDigestive systemDiseaseCellulose
The invention relates to the technical field of medicines, in particular to drug composition for treating digestive system diseases. The drug composition comprises an active pill core and an enteric coating layer, wherein the active pill core is prepared from lansoprazole, starch, sucrose, hydroxypropylcellulose, sodium potassium tartrate, sodium carboxymethylcellulose, sodium dodecyl sulfate, povidone K30 and 95% ethanol; the enteric coating layer is prepared from hydroxypropyl methylcellulose phthalate, n-butyl stearate, talcum powder and 95% ethanol. A preparation process is simplified, the production cost is reduced, acceleration test and long-term test results show that a lansoprazole enteric capsule prepared from the composition has the advantages of good stability, high dissolution rate and low impurity content in comparison with the prior art, health and safety of a human body are relatively facilitated, and large-scale industrialized production is facilitated.
Owner:崔银方
Preparation method of pharmaceutical composition for treating digestive system disease
InactiveCN104739802AEasy to prepareSuitable for mechanized productionOrganic active ingredientsDigestive systemDiseaseHypromellose phthalate
The invention discloses a preparation method of a pharmaceutical composition for treating a digestive system disease. The preparation method comprises the following steps: preparing a tablet core from the medical components, such as lansoprazole, lactose, microcrystalline cellulose, sodium potassium tartrate, polyvinylpolypyrrolidone, povidone K30, 95% ethyl alcohol, polysorbate 80 and superfine silica powder by wet granulation; and then coating the tablet core with hydroxypropyl methylcellulose phthalate, n-butyl stearate, talcum powder and 95% ethanol to prepare an enteric coated tablet. Through screening by a lot of experiments, coating materials hydroxypropyl methylcellulose phthalate and n-butyl stearate are combined; and the sodium potassium tartrate in the tablet core is added, so that an isolating layer does not need to coat between an enteric coat layer and the tablet core; the preparation technology is simplified; the production cost is reduced; and acceleration test and long-term test results show that compared with the prior art, the lansoprazole enteric coated tablet prepared by the preparation method has the advantages of good stability, high dissolution degree and low impurity content.
Owner:崔银方
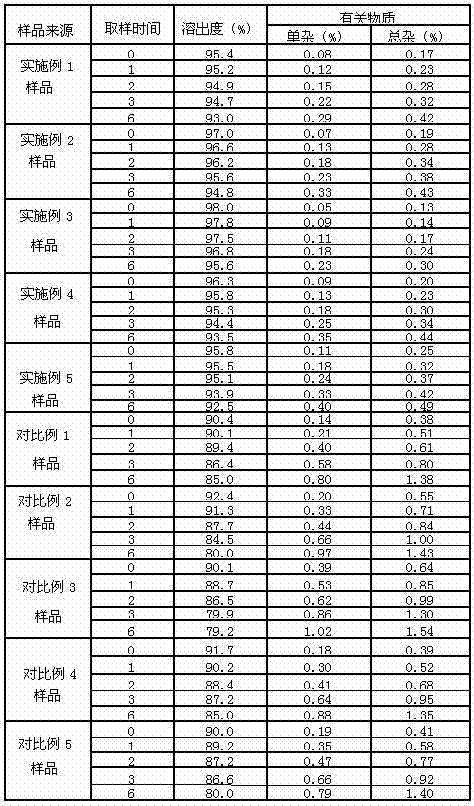
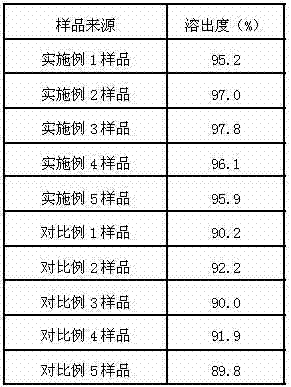
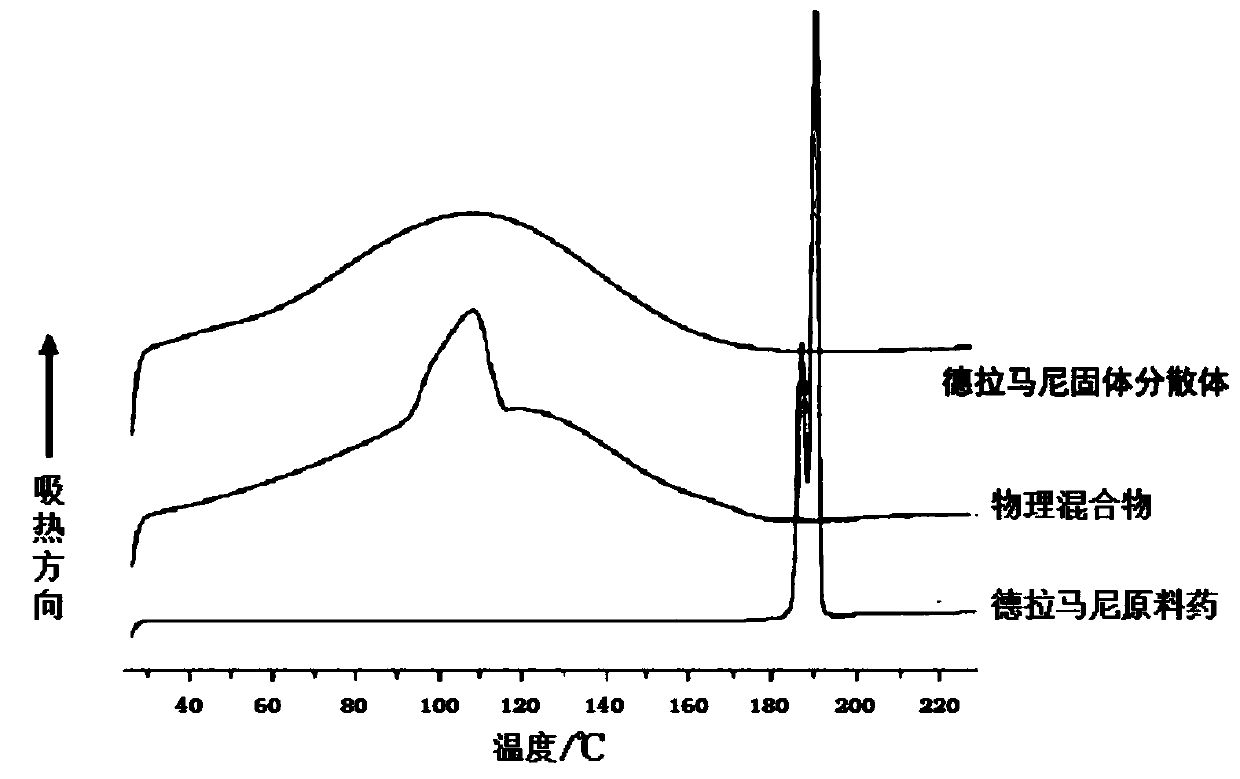
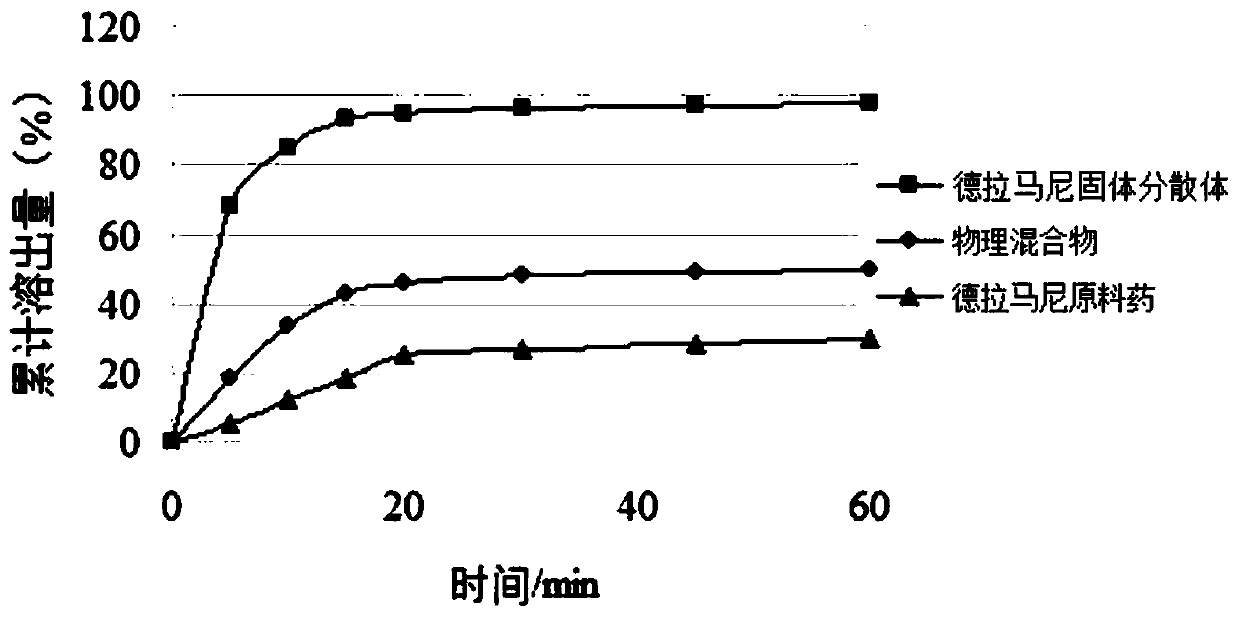
Delamanid quick-release preparation and preparation method thereof
InactiveCN109730970ARapid dissolutionImprove stabilityAntibacterial agentsOrganic active ingredientsAdditive ingredientAlcohol sugars
The invention belongs to the field of pharmacy, in particular to a Delamanid quick-release preparation and a preparation method thereof. The Delamanid quick-release preparation takes hydroxypropyl methylcellulose acetate phthalate or hypromellose phthalate as a dispersed carrier material, functional auxiliary materials such as sugar alcohols are added as plasticizers, the materials and Delamanid active pharmaceutical ingredients are subjected to high-temperature extrusion through a hot melt extruder, then cooled and crushed into powder, then the powder is fully mixed with a filler, a disintegrating agent and a lubricant, tabletting is conducted directly, and the preparation is obtained. The problem that Delamanid is poor in in vitro dissolution is solved, the in vivo bioavailability is improved, and the therapeutic effect is ultimately improved; the preparation method is simple, energy consumption is small, no residual solvents exist, other impurities cannot be introduced in the wholeprocess, and continuous production is easy to achieve.
Owner:REYOUNG PHARMA
Hypromellose phthalate and method for producing hypromellose phthalate
Provided is a method for producing HPMCP capable of reducing the average particle size of HPMCP particles to an intended range without a pulverization step. Specifically provided is a method for producing hypromellose phthalate, comprising an esterification step of reacting hypromellose with an esterification agent in the presence of a catalyst to obtain a reaction solution containing crude hypromellose phthalate, a precipitation step of mixing the reaction solution with water to precipitate the crude hypromellose phthalate, thereby obtaining a hypromellose phthalate suspension, a liquid removal step of removing a liquid from the hypromellose phthalate suspension with a centrifugal decanter to obtain liquid-removed hypromellose phthalate, and a drying step of drying the liquid-removed hypromellose phthalate.
Owner:SHIN ETSU CHEM CO LTD
Febuxostat enteric preparation
ActiveCN101716132BAvoid destructionImprove solubilityOrganic active ingredientsSkeletal disorderPylorusHypromellose phthalate
Owner:BEIJING KONRUNS PHARMA TECH CO LTD
Preparation of solid dispersion of paclitaxel and its homologues and oral preparations thereof
InactiveCN103083240BImprove bioavailabilitySimple production processPowder deliveryOrganic active ingredientsCelluloseDrug content
A solid dispersion and solid dispersion microsphere of paclitaxel or a homologue thereof and a preparation method therefor. By means of preparing a solid dispersion and solid dispersion microsphere to improve the rapid release in vivo and in vitro of paclitaxel or a homologue thereof, and by maintaining a supersaturated drug concentration or, in the case of high molecularity, increasing the apparent solubility of the drug, the bioavailability of the drug is increased. The solid dispersion carrier of paclitaxel or a homologue thereof is either hydroxypropyl methylcellulose acetate succinate or hypromellose phthalate or a mixture thereof or a silica gel-based micropowder mixture. The prepared solid dispersion microsphere has a particle size of between 100 and 600 mum and a yield rate of over 80%; the appropriate drug content therefore is between 5% and 40%.
Owner:SHENYANG PHARMA UNIVERSITY
Hepatotoxicity-free pharmaceutical composition containing acetaminophen drugs
A new compound composition that is free of a side effect to a liver and used for alleviating the toxicity of an acetaminophen (APAP) medicament to the liver. The compound composition comprises (a) a pharmaceutically effective amount of acetaminophen and (b) a frequently-used safe and pharmaceutically acceptable excipient that can be combined with one or more than two medicaments that can reduce the toxicity of a drug via liver enzyme CYP2E1 metabolism to the liver. The compound is selected from the following group: Tween 20, microcrystalline cellulose, dicalcium phosphate, polyoxyethylene 23 lauryl ether, saccharin, mannitol, polyoxyethylene alkyl ether, sucralose, pyrrolidone, sodium starch glycolate, acrylic resin S100, carboxymethyl cellulose sodium, polyoxyethylene polyoxypropylene, menthol, low-substituted hydrocarbon propyl cellulose, pregelatinized starch, Dextrates NF hydrated, citric acid, polyoxyethylene castor oil, colloidal silica, polyethylene glycol monostearate aliphatic ester, sorbic acid, lemon oil, hydroxypropyl cellulose, sorbitol, acesulfame potassium, hypromellose phthalate, lactose monohydrate, maltodextrin, Brij 58, Brij 76, Tween 80, Tween 40, PEG 400, PEG 4000, PEG 2000, and the like, so as to reduce the side effect of the toxicity caused by acetaminophen to the liver.
Owner:INT EDUCATION FOUND
A kind of ursolic acid solid dispersion and preparation method thereof
ActiveCN102871950BImprove solubilityIncrease dissolution rateAntibacterial agentsOrganic active ingredientsSolubilityHypromellose phthalate
The invention discloses an ursolic acid solid dispersion and a preparation method thereof. The formula of the ursolic acid solid dispersion comprises ursolic acid and a carrier material; the mass ratio of the ursolic acid to the carrier material is 1:2 to 1:20; and the carrier material is one or more of PEG6000 (polyethylene glycol 6000), povidone k29 / 33, hypromellose E5, polyving alcohol, poloxamer 68, GELUCIRE 50 / 13, polyacrylic resin EPO, polyacrylic resin L 100-55, povidone-vinyl alcohol, hypromellose AS-HG, hypromellose AS-LG, hypromellose AS-MG, low substituted hypromellose, hypromellose phthalate, cellulose acetate phthalate, glucan, polyoxyethylene, polyoxyethylene stearates and polyvinyl acetate phthalate. The preparation method comprises the following steps: dissolving the ursolic acid and the carrier material into an organic solvent according to the formula; removing the organic solvent; and grinding. The ursolic acid solid dispersion has good solubility and has high bioavailability.
Owner:CHINA GATEWAY PHARMA DEV CO LTD
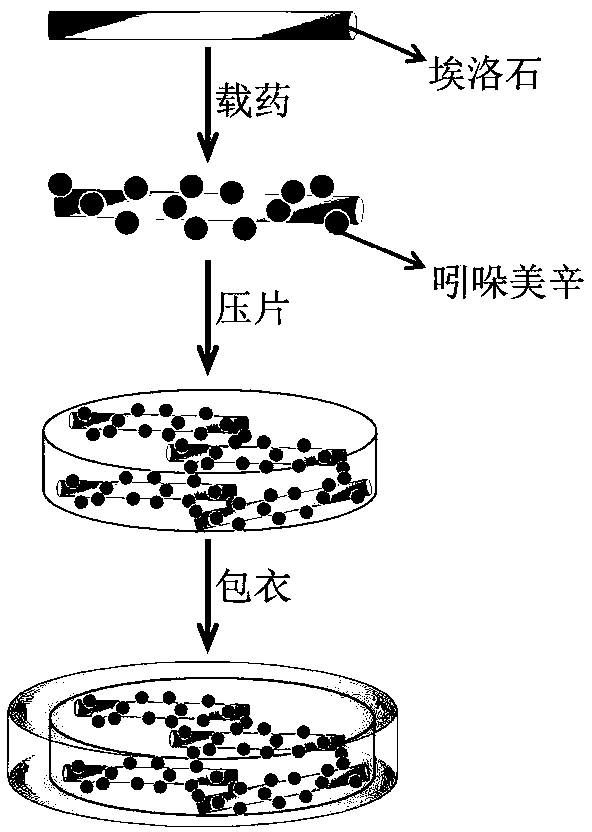
Enteric composite drug-loading system with halloysite nanotube as skeleton and preparation method thereof
The invention provides an enteric complex drug-loaded system with a halloysite nanotube being a skeleton. A preparation method includes the steps that indomethacin is dissolved in acetone, the obtained solution is dropwise added into the halloysite nanotube and stirred evenly, then powder is obtained through vacuum drying and put into a tabletting machine, and tablets are obtained through pressing; hypromellose phthalate, soluble starch, polyvinylpyrrolidone, absolute ethyl alcohol and deionized water are mixed and stirred evenly to obtain a mixed solution; the obtained tablets are coated with the obtained mixed solution, namely a coating solution, cooled to minus 5-0 DEG C in step2 and pulled and taken out after being subjected to complex crosslinking for 3s-2min, coated tablets are obtained, a coating-pulling-freeze drying process is completed once after freeze drying, the process is repeated 1-10 times, and the finished product is obtained. The drug-loaded ratio of the halloysite nanotube for indometacin is greatly increased, slow-release enteric solubility is greatly improved, and the releasing rate of indometacin in artificially simulated gastric fluid is smaller than 20%.
Owner:ZHEJIANG UNIV OF TECH
Method for producing hypromellose phthalate
There is provided a method for producing hypromellose phthalate (HPMCP), the method not requiring any special device, and facilitating removal of impurities. More specifically, there is provided a method for producing HPMCP, including an esterification step of esterifying hypromellose with a carboxybenzoylating agent in the presence of an aliphatic carboxylic acid to obtain a reaction product solution containing HPMCP; a precipitation step of precipitating the HPMCP by mixing the reaction product solution with water to obtain a suspension of the precipitated HPMCP; a neutralization step of neutralizing the suspension with a basic substance to obtain a neutralized suspension; and a washing step of washing the HPMCP contained in the neutralized suspension to obtain the washed HPMCP.
Owner:SHIN ETSU CHEM IND CO LTD
Cilostazol sustained-release capsule composition and preparation method thereof
ActiveCN106511313BProlonged drug release timeSimple processOrganic active ingredientsAntipyreticSolubilitySodium acetate
The invention relates to the pharmaceutical field and discloses a cilostazol sustained-release capsule compound and a preparation method thereof. The sustained-release capsule compound comprises a medicinal hollow capsule body and contents arranged in the medicinal hollow capsule body. The contents comprise cilostazol, a hydroxypropyl methylcellulose phthalate sustained-release framework material, a retardant, an adhesive, a filler, an antisticking agent and a lubricating agent. Hydroxypropyl methylcellulose phthalate is used as the framework sustained-release material and is a product obtained by performing an esterification reaction between hydroxypropyl methylcellulose and phthalic anhydride in acetic acid with sodium acetate serving as a catalyst. Molecules have carboxyl negative charge intermolecular repulsion under a carboxyl alkaline environment, so that twinned macromolecules are stretched to be dissolved, and enteric solubility is achieved. The absorption of the cilostazol by the upper gastrointestinal tract can be reduced while the absorption of the cilostazol by the lower gastrointestinal tract is improved. Adverse drug reactions such as headaches, head heaviness and tachycardia caused by sudden increase of the drug concentration are overcome. In addition, the cilostazol sustained-release capsule compound is taken only one time per day, so that the adaptability of patients is improved.
Owner:浙江为康制药有限公司
A kind of enteric-coated hollow cellulose capsule and preparation method thereof
ActiveCN112546016BCapsule mechanical properties are goodImprove toleranceInorganic non-active ingredientsCapsule deliveryCelluloseIntestinal fluid
The enteric-coated hollow cellulose capsules disclosed in the present invention are composed in weight percent: hypromellose phthalate: 45-60%, hypromellose: 25-40%, agar: 5.5-10%, chlorinated Potassium: 0.5-1%, Tween-80: 0.5-3%, the balance is water; among them, hypromellose phthalate is Hp55S or Hp55, and hypromellose is HPMC-E4. The preparation method of the enteric-coated hollow cellulose capsule of the present invention does not use any organic solvent, and the general-purpose hollow capsule production line can be used for one-time molding to prepare the enteric-coated hollow cellulose capsule. The surface of the hollow capsule of the present invention is smooth and elastic, and has the stability of not swelling, disintegrating and leaking drugs in the simulated gastric juice environment for 2 hours, and has the characteristics of completely disintegrating and releasing drugs within 1 hour or even 30 minutes in the simulated intestinal juice environment, and The tightness and friability of the product are all qualified, the mechanical properties of the capsule are good, and it has good tolerance to the environment.
Owner:宁波市江南胶囊有限公司
Preparation method of taste-masked suspension granules of Gegenqinlian decoction
ActiveCN102309562BDoes not affect bioavailabilityGood taste masking effectAntibacterial agentsAntipyreticAdhesiveHypromellose phthalate
The invention relates to a preparation method of taste-masked suspension granules of Gegenqinlian decoction. The preparation method comprises the following steps of 1, taking appropriate amounts of dispensing granules of three traditional Chinese medicines comprising radix puerariae, coptis chinensis and scutellaria baicalensis and respectively carrying out coating processes in a fluidized bed through adopting one or more polymers as coating materials to obtain coated granules for next use, 2, taking an appropriate amount of at least one suspending agent, mixing uniformly the at least one suspending agent and radix glycyrrhizae preparata dispensing granules, then adding an appropriate amount of an adhesive into the mixture to prepare into granules by a wet method, drying the prepared granules in an oven, and then spraying an appropriate amount of an ethanol solution as an aromatic to obtain suspending agent-containing radix glycyrrhizae preparata dispensing granules after ethanol is volatilized, and 3, weighing appropriate amounts of the suspending agent-containing radix glycyrrhizae preparata dispensing granules and the coated granules containing radix puerariae, coptis chinensisand scutellaria baicalensis, mixing well, and carrying out sub-packaging to obtain the taste-masked suspension granules of Gegenqinlian decoction, wherein the one or more polymers as coating materials are selected from enteric-coated polyacrylic resin, hypromellose acetate succinate and hydroxypropyl methylcellulose phthalate. The invention provides the preparation method of the taste-masked suspension granules of Gegenqinlian decoction. The preparation method is also suitable for taste masking of a traditional Chinese medicine with a bitter taste or a fishy smell.
Owner:安徽天祥药业有限公司
Metformin hydrochloride enteric-coated tablet and preparation method thereof
ActiveCN111888339BFully absorbedGood pH selectivityOrganic active ingredientsMetabolism disorderMethacrylic acid-ethyl acrylate copolymerCellulose
The invention relates to a metformin hydrochloride enteric-coated tablet and a preparation method thereof. The metformin hydrochloride enteric-coated tablet includes a tablet core and an enteric coating layer; the tablet core is prepared from the following raw materials in parts by weight: 430-570 parts of metformin hydrochloride, 5-40 parts of binder, 0 parts of disintegrant ~30 parts and 1~10 parts of lubricant; the enteric coating layer is prepared from the following raw materials by weight: 5~40 parts of enteric material, 0.5~30 parts of plasticizer and 1~30 parts of antisticking agent 10 parts; the binder is at least one of hypromellose, hypromellose and povidone; the enteric material is methacrylic acid-ethyl acrylate copolymer, methacrylic acid copolymer , at least one of hypromellose phthalate and polyvinyl acetate phthalate. The metformin hydrochloride enteric-coated tablet of the present invention can release rapidly under the condition of pH 4.5-5, and has good bioequivalence with the original drug.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Production process of hypromellose phthalate
ActiveCN113372457BReduce overflowEasy dischargeTransportation and packagingDirt cleaningSodium acetateCellulose
The invention discloses a production process of hypromellose phthalate, comprising the following steps: a. heating and homogenizing raw materials cellulose ether, acetic anhydride, glacial acetic acid, phthalic anhydride, and succinic anhydride ; b. In the process of adding an appropriate amount of catalyst, add the raw material sodium acetate to obtain a mixture; c. After heating the mixture to 60-85°C, perform a constant temperature reaction. In the present invention, the dust generated in the step of crushing can be absorbed to reduce the dust overflow and the influence of the dust on the workers. Under the action of the driving part, the top block continuously beats the filter element and the rubber block continuously beats the sieve plate The bottom of the filter can be prevented from being blocked by particulate matter on the filter element, which will affect the absorption of dust. During the vibration of the sieve plate, it can facilitate the discharge of the crushed material. The crushing equipment involved in this process is more environmentally friendly and more adaptable. In the preparation of hypromellose phthalate.
Owner:TAIAN RUITAI CELLULOSE
Production process of high-purity hypromellose phthalate for photoresist
ActiveCN113372457AReduce overflowEasy dischargeDispersed particle filtrationDirt cleaningCelluloseSodium acetate
The invention discloses a production process of high-purity hypromellose phthalate for photoresist. The production process comprises the following steps of: a, heating and homogenizing raw materials including cellulose ether, acetic anhydride, glacial acetic acid, phthalic anhydride and succinic anhydride; b, in the process of adding a proper amount of catalyst, adding a raw material sodium acetate to obtain a mixture; and c, heating the mixture to 60-85 DEG C, and carrying out a constant temperature reaction. According to the invention, dust generated in the crushing step can be absorbed to reduce dust overflow and the influence of the dust on workers; under the action of a driving piece, a top block continuously beats a filtering piece, a rubber block continuously beats the bottom of a sieve plate, and the situation that particulate matter blocks the filtering piece and influences dust absorption is avoided; andthe crushed materials can be conveniently discharged in the vibration process of the sieve plate.Crushing equipment involved in the process is more environment-friendly and is more suitable for preparation of hypromellose phthalate.
Owner:TAIAN RUITAI CELLULOSE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com