Use of PNAS-4 gene in preparing antineoplastic and antineoplastic auxiliary medicament
An anti-tumor drug and anti-tumor technology, applied in the direction of anti-tumor drugs, drug combinations, recombinant DNA technology, etc., can solve the problem of lack of comprehensive search for homologous genes and functional research, few genes for tumor growth, and damage to the health of the body and other problems, to achieve the effect of overcoming toxic and side effects, good market prospects, and inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0062] The acquisition of embodiment one PNAS-4 gene
[0063] 1. Cloning of human PNAS-4 (hPNAS-4) gene cDNA
[0064] According to the full-length human PNAS-4 gene sequence (NM-016076) submitted in NCBI, primers were designed to amplify the coding sequence of PNAS-4 gene. In order to further clone into the pGEX-6p-1 vector, BamH I and Xho I restriction sites were respectively introduced into the upper and lower primers of the coding sequence.
[0065] Upstream primer (SEQ ID NO.7):
[0066] 5′-GC GGATCC AAGATGGCAGATTTTTTGAAAGG-3′;
[0067] Downstream primer (SEQ ID NO.8):
[0068] 5′-CGC GATATC TTAAGTGTCCTC GTGCATGTCTG-3' (the enzyme cleavage site is underlined).
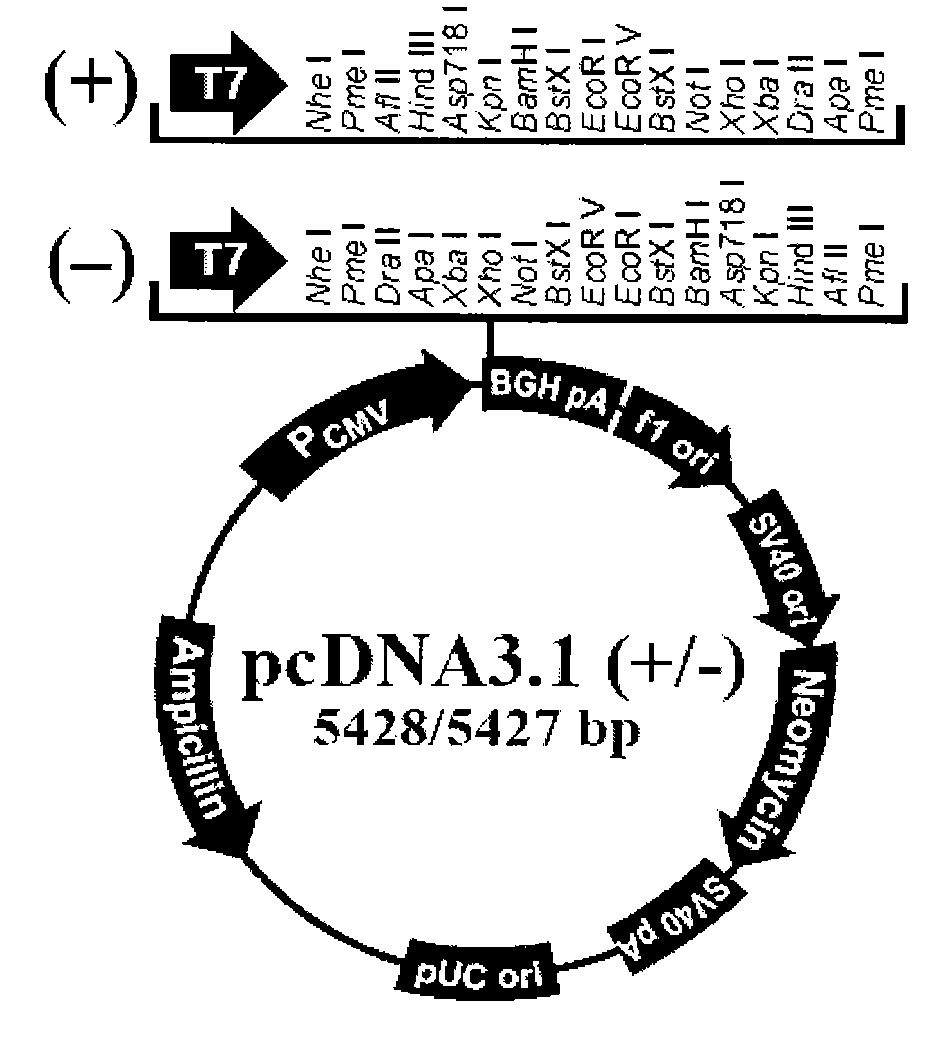
[0069] PCR amplification primers were synthesized by Shanghai Boya Company. To enhance the expression level of the PNAS-4 gene, the following primers were used when constructing the pcDNA3.1-hPNAS-4 recombinant plasmid:
[0070] Upstream primer (SEQ ID NO.9): 5'-GC GGATCC AAGATGGCAGATTTTTTGAAAGG-3' (t...
Embodiment 2
[0160] Embodiment two can be used for the construction of the PNAS-4 recombinant vector of gene therapy
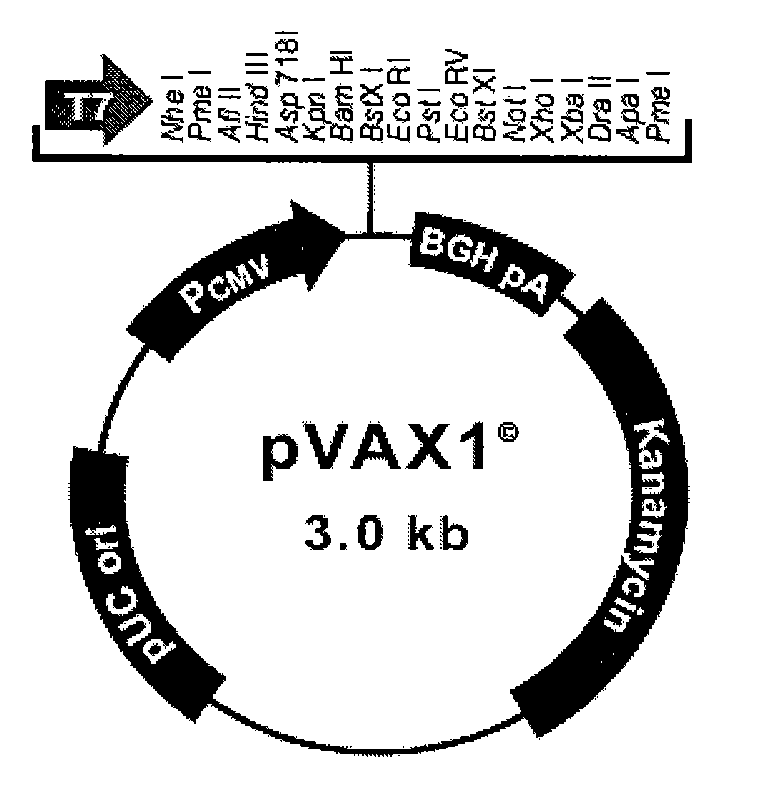
[0161] 1. Construction of recombinant plasmid PNAS-4-pVAX1:
[0162] The PNAS-4 cloned on pcDNA3.1-hPNAS-4 in Example 1 was excised with BamH I and Xho I enzymes according to conventional techniques in the art, and inserted into the BamH I and Xho of the pVAX1 plasmid purchased on the market.
[0163] It was obtained at the I site, and the agarose electrophoresis and sequencing verifications were correct.
[0164] 2. Preparation of liposome complexes of recombinant plasmid PNAS-4-pVAX1
[0165] Lipofectamine (lipofectamine2000) was purchased from Invitrogen.
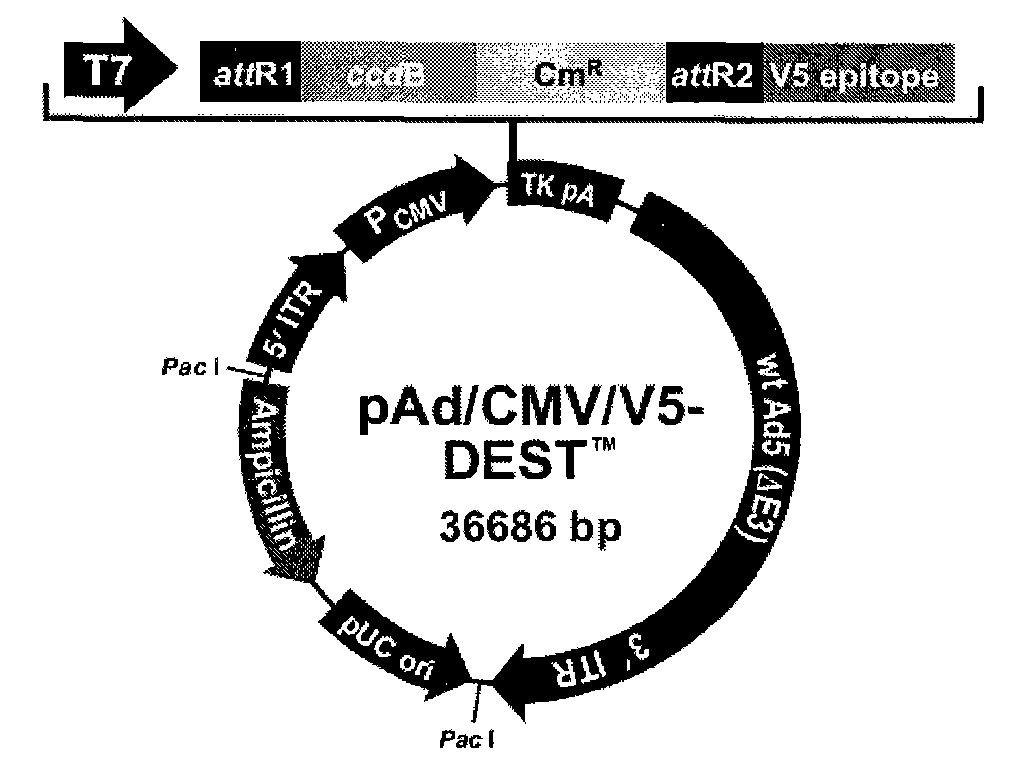
[0166] The liposome complex of the recombinant plasmid PNAS-4-pVAX1 was prepared according to the instructions of lipofectamine2000, the amount of liposome was screened, and liposome:plasmid=1:3 (weight ratio) was finally determined. 3. Construction of PNAS-4 recombinant adenovirus Construction, production and pur...
Embodiment 3
[0192] Example three recombinant adenovirus pAd-DEST-hPNAS-4 and pAd-DEST-mPNAS-4 experimental results
[0193] In vitro experiments
[0194] pAd-DEST-hPNAS-4 was selected for in vitro experiments.
[0195] DNA ladder (DNA ladder strip) test results
[0196] The results of DNA ladder showed that LL / 2 cells were treated with liposome-wrapped pAd-DEST-hPNAS-4, and after 24 hours of treatment, obvious DNA ladder phenomenon was found in this group of cells, while liposome-wrapped pAd-DEST-Null and other control groups did not. The phenomenon of DNA ladder can be observed, which indicates that liposome-wrapped pAd-DEST-hPNAS-4 can obviously induce apoptosis of LL / 2 cells.
[0197] Apoptosis PI staining experiment
[0198] The results of apoptosis PI staining experiment are shown in ( Figure 11) showed that LL / 2 cells were treated with liposome-wrapped pAd-DEST-hPNAS-4 for 24 hours and found that this group of cells had obvious apoptosis, while liposome-wrapped pAd-DEST-Null tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com