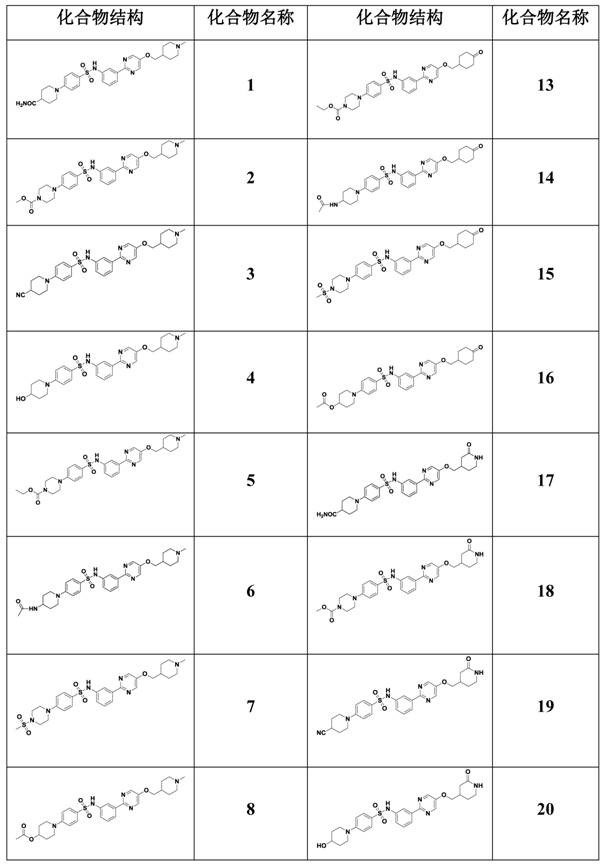
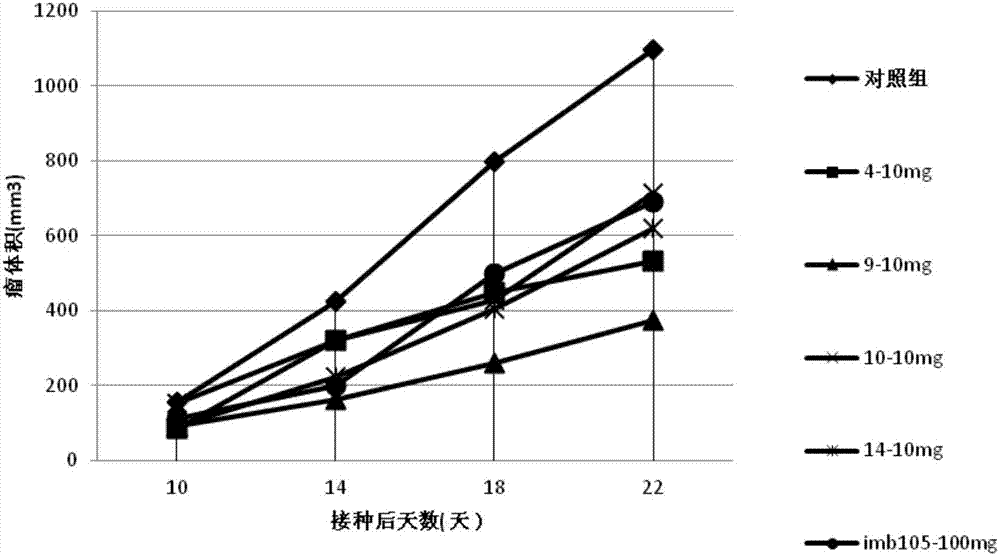
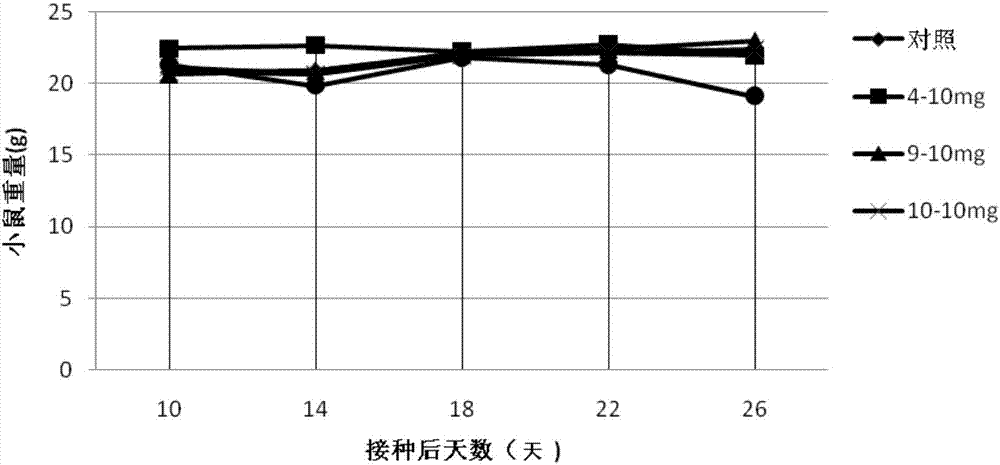
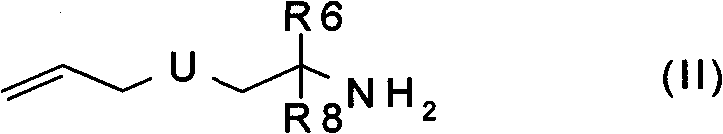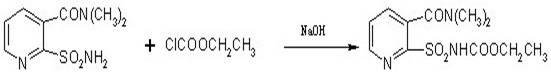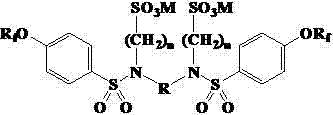Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
160 results about "Sulphonilamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
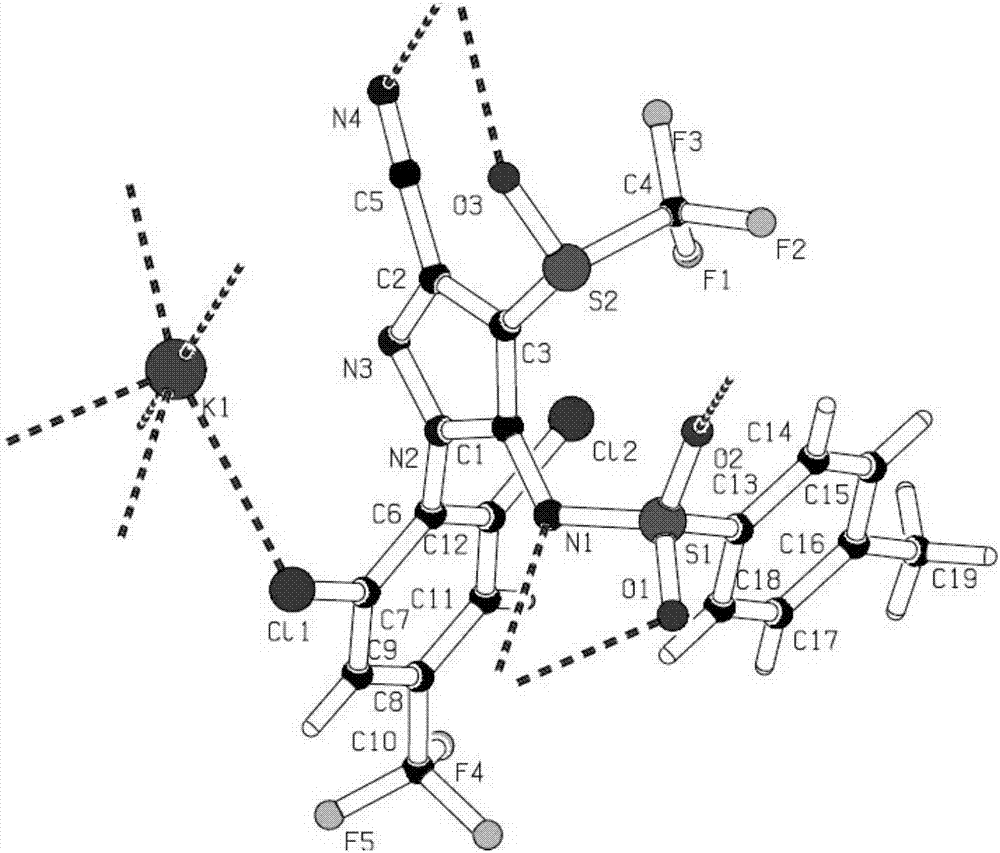
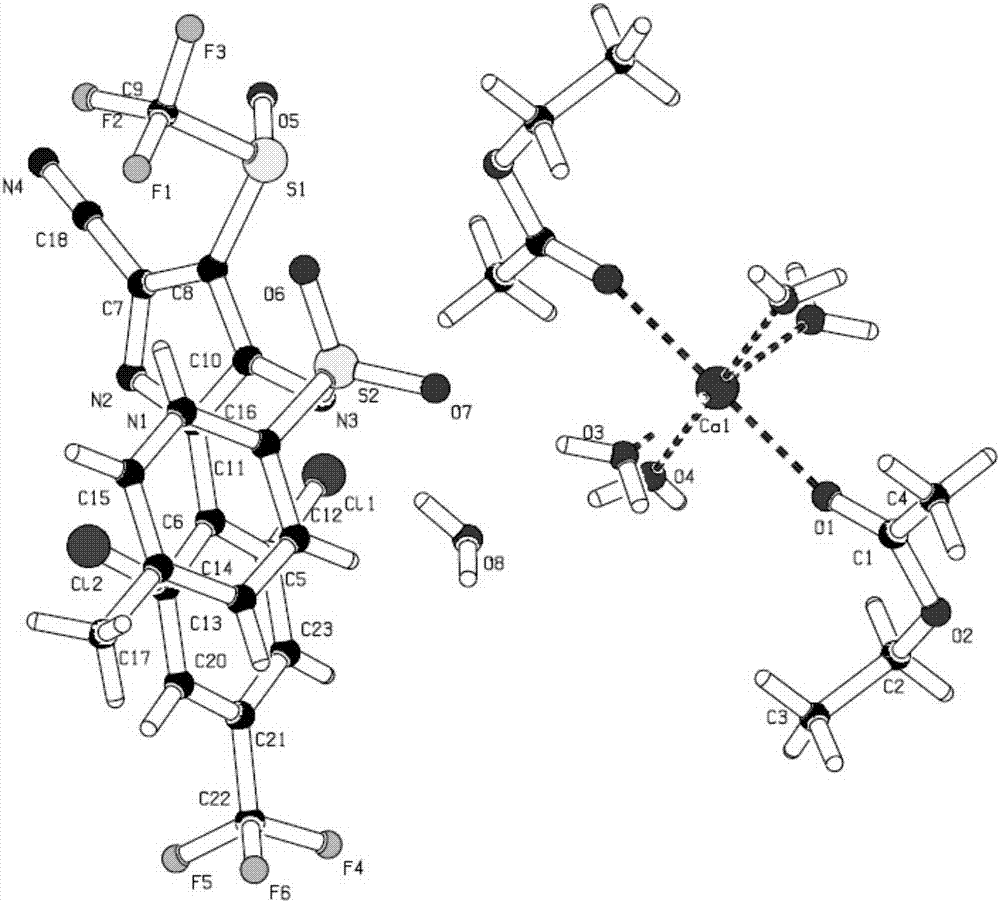
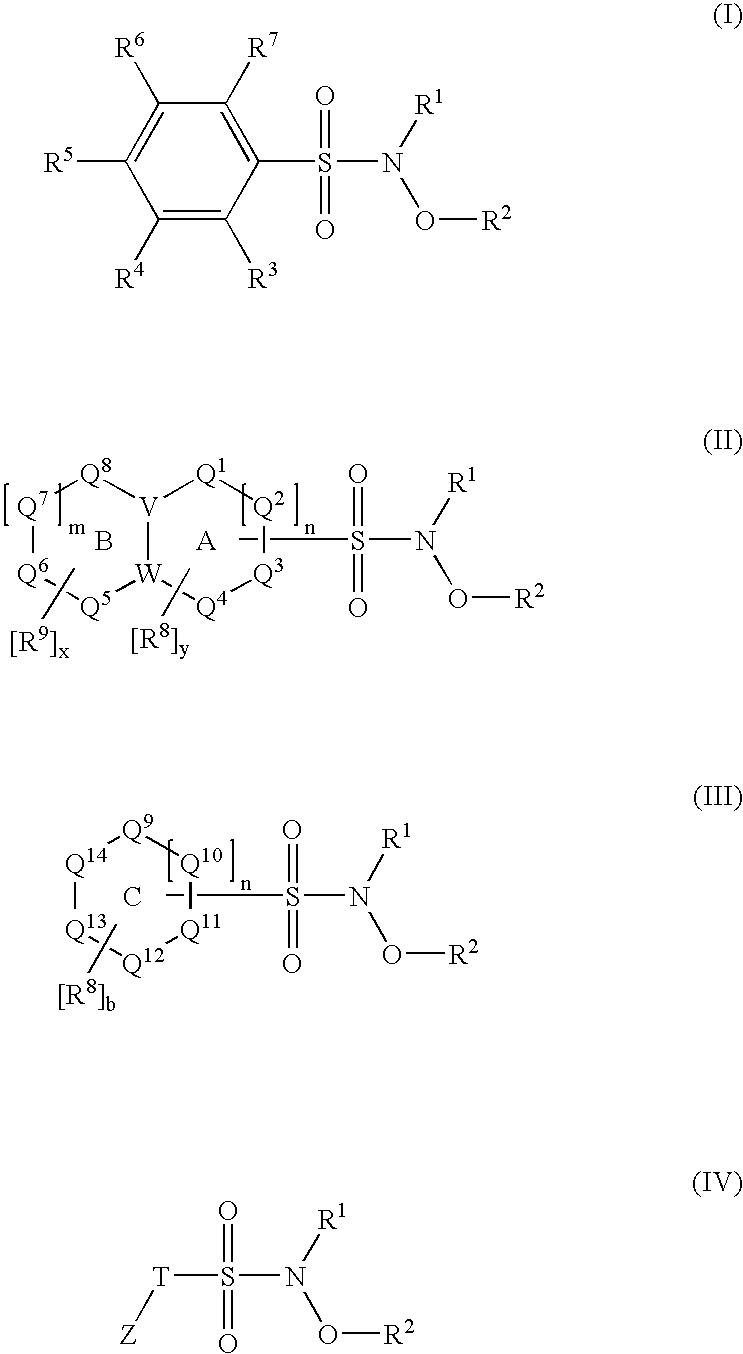
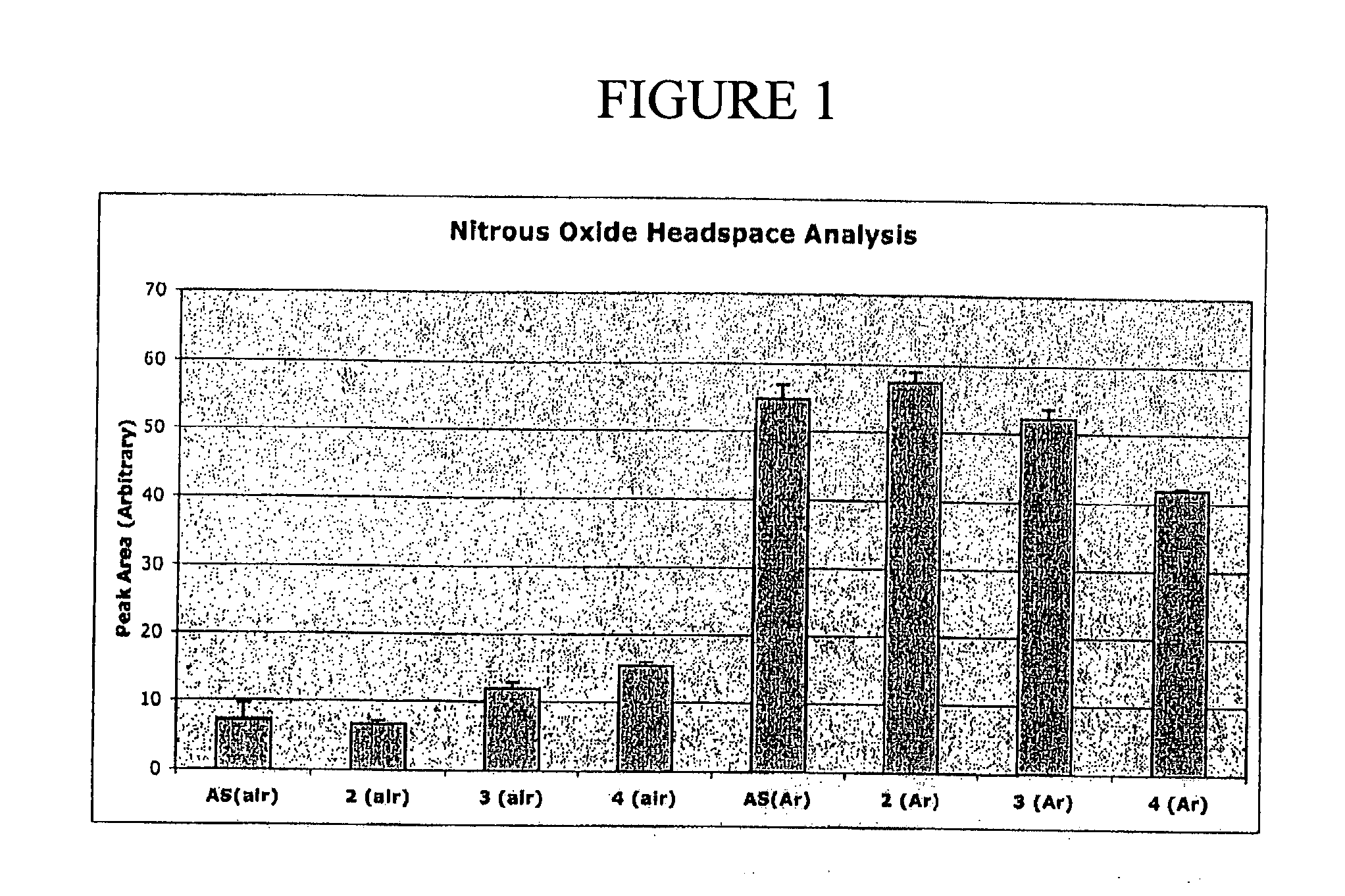
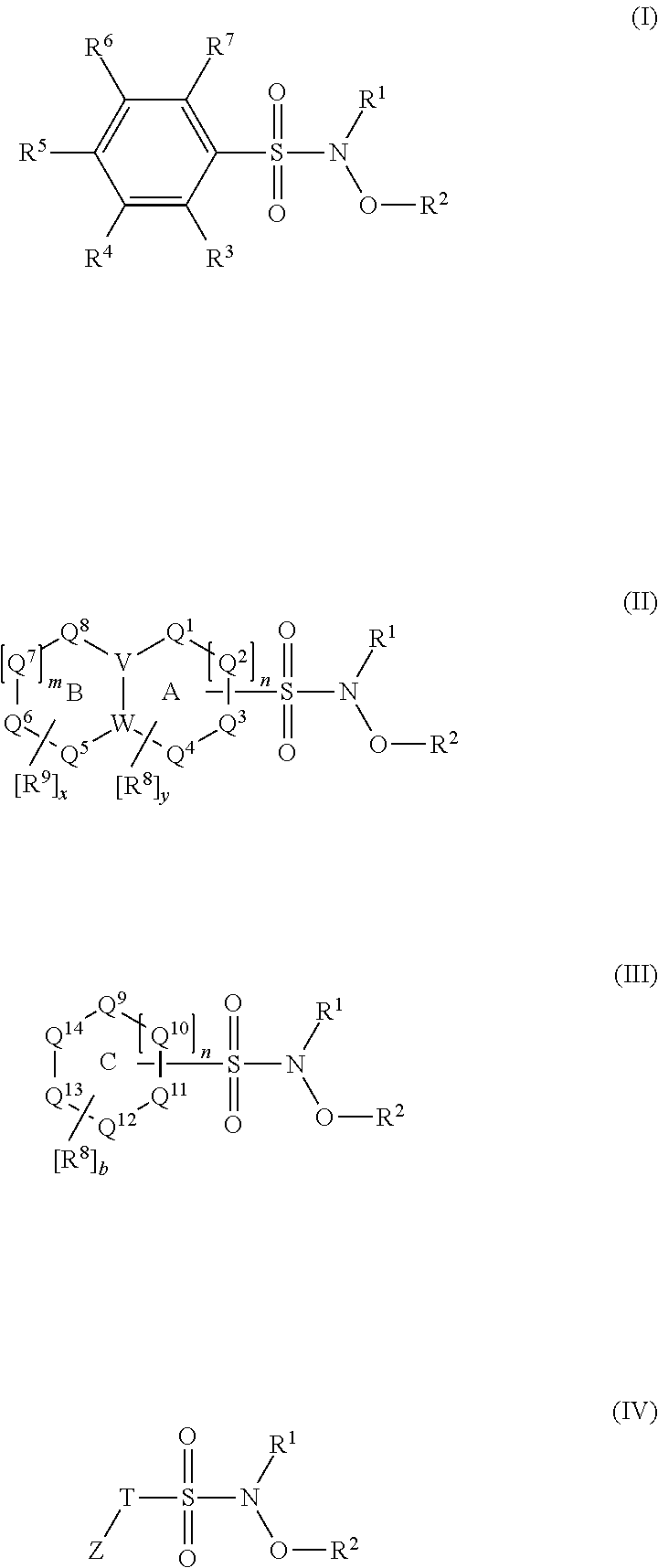
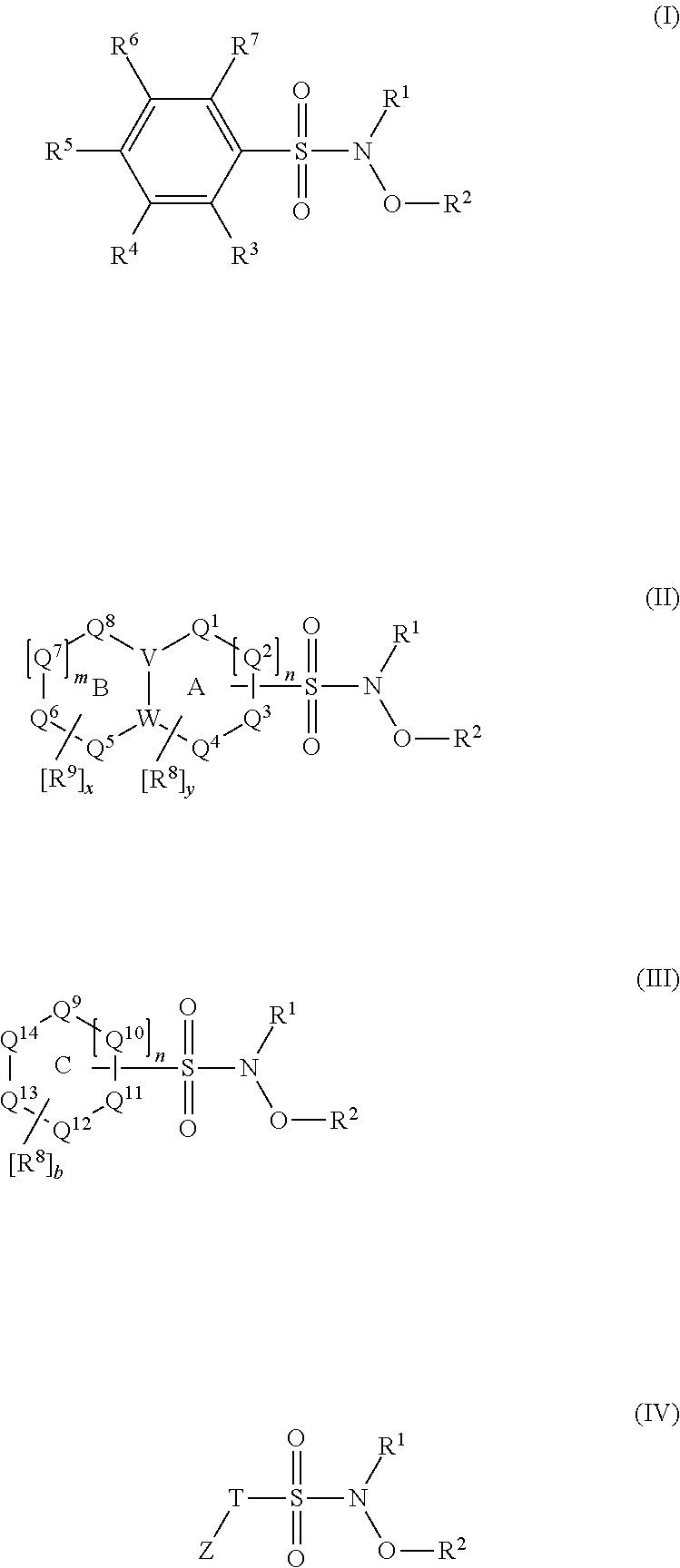
N-hydroxylsulfonamide derivatives as new physiologically useful nitroxyl donors
ActiveUS20070299107A1Increase ischemia/reperfusion injuryIncreased myocardial ischemia/reperfusion injuryBiocideOrganic chemistryDiseaseReperfusion injury
The invention relates to N-hydroxysulfonamide derivatives that donate nitroxyl (HNO) under physiological conditions and are useful in treating and / or preventing the onset and / or development of diseases or conditions that are responsive to nitroxyl therapy, including heart failure and ischemia / reperfusion injury. Novel N-hydroxysulfonamide derivatives release NHO at a controlled rate under physiological conditions, and the rate of HNO release is modulated by varying the nature and location of functional groups on the N-hydroxysulfonamide derivatives.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
N-Hydroxylsulfonamide Derivatives as New Physiologically Useful Nitroxyl Donors
InactiveUS20110306614A1Quality improvementProlong survival timeBiocideOrganic chemistryDiseaseReperfusion injury
The invention relates to N-hydroxysulfonamide derivatives that donate nitroxyl (HNO) under physiological conditions and are useful in treating and / or preventing the onset and / or development of diseases or conditions that are responsive to nitroxyl therapy, including heart failure and ischemia / reperfusion injury. Novel N-hydroxysulfonamide derivatives release NHO at a controlled rate under physiological conditions, and the rate of HNO release is modulated by varying the nature and location of functional groups on the N-hydroxysulfonamide derivatives.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Beta3 agonists and uses thereof
Sulfamide compounds having formula (I) are described as well as their use in the treatment of diseases dependent on the signaling pathways associated with .beta.-adrenergic receptors, such as obesity, diabetes, hypertension, gastrointestinal hypo- or hyper-motility and cardiovascular diseases. 1
Owner:PFIZER INC
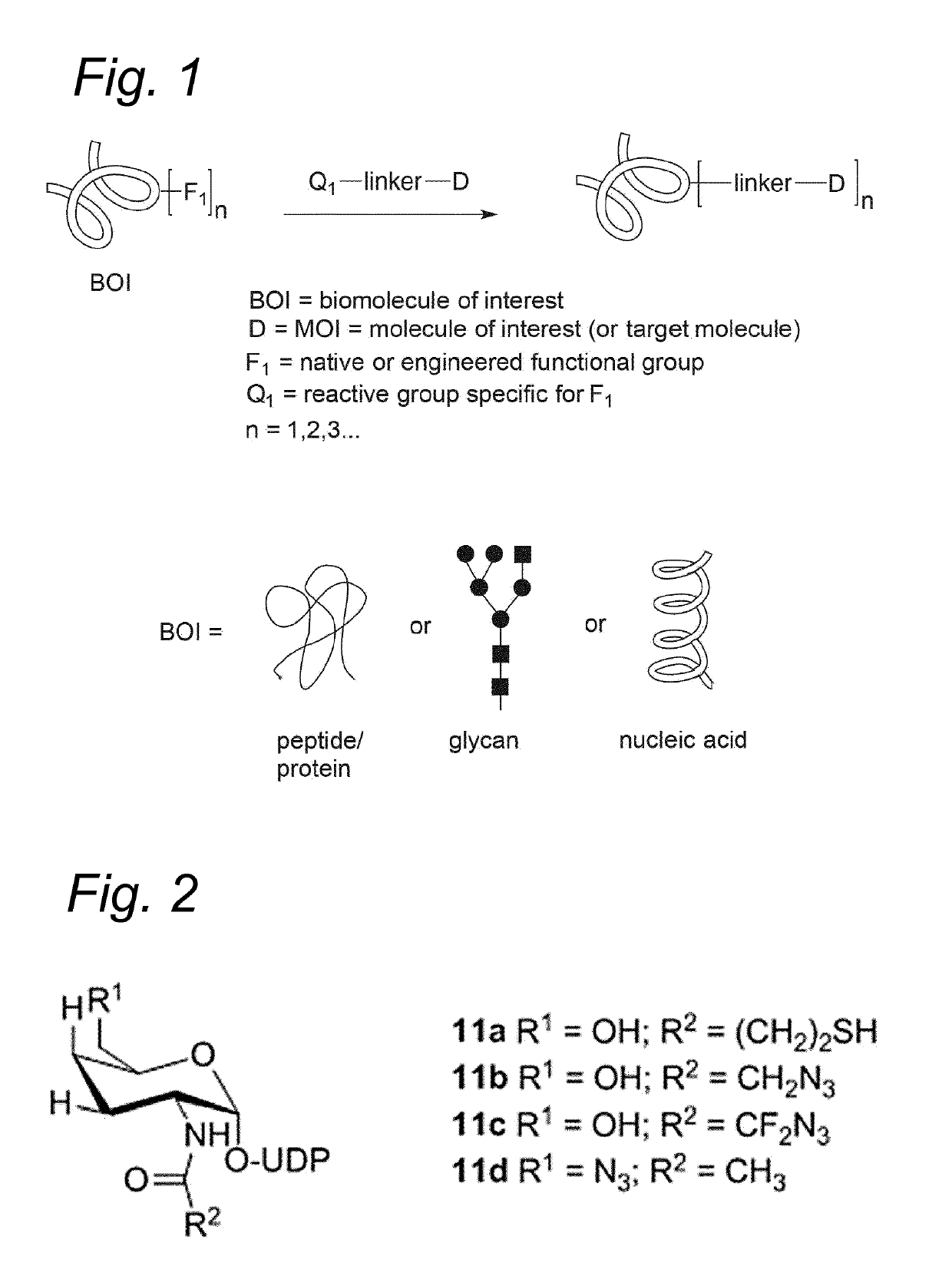
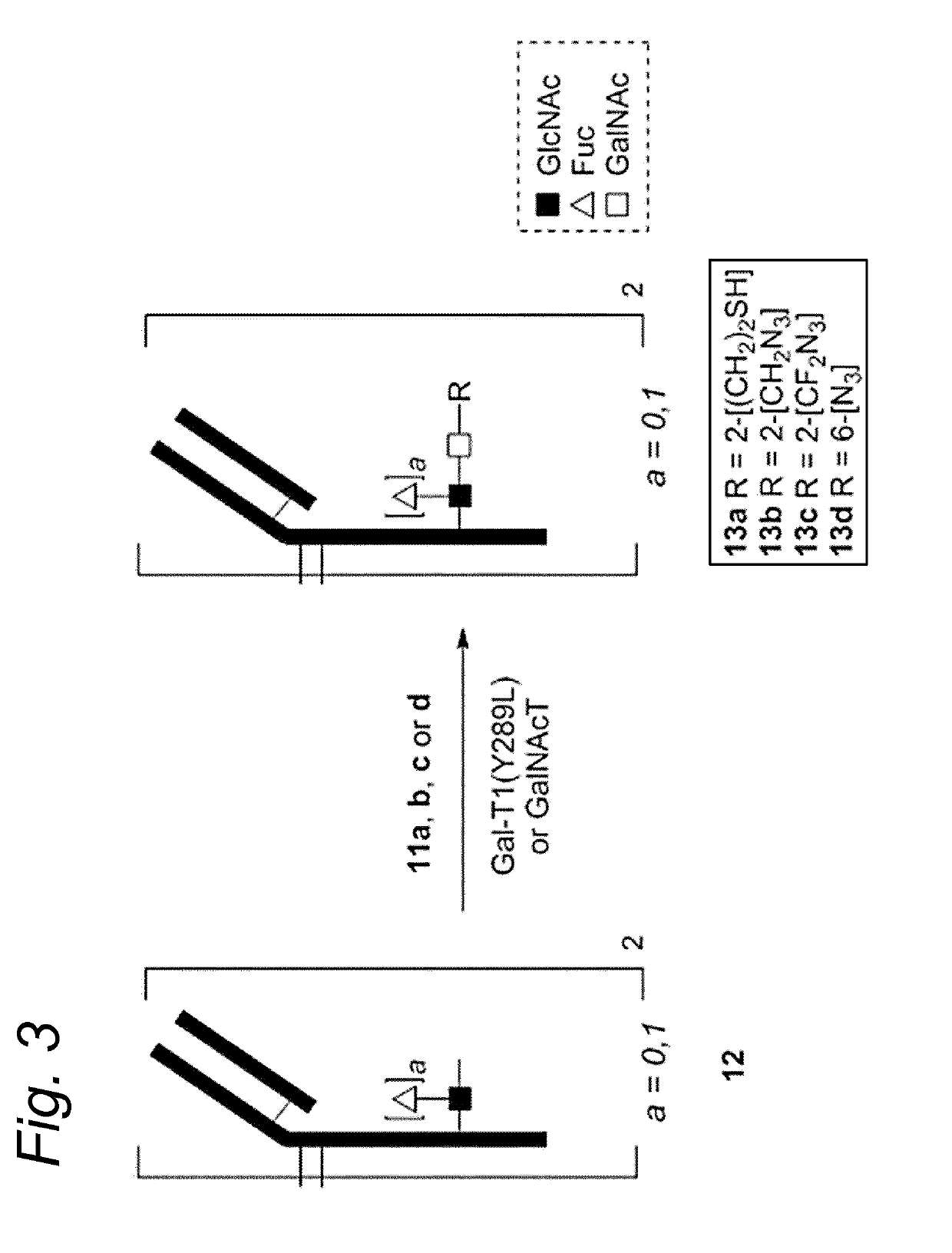
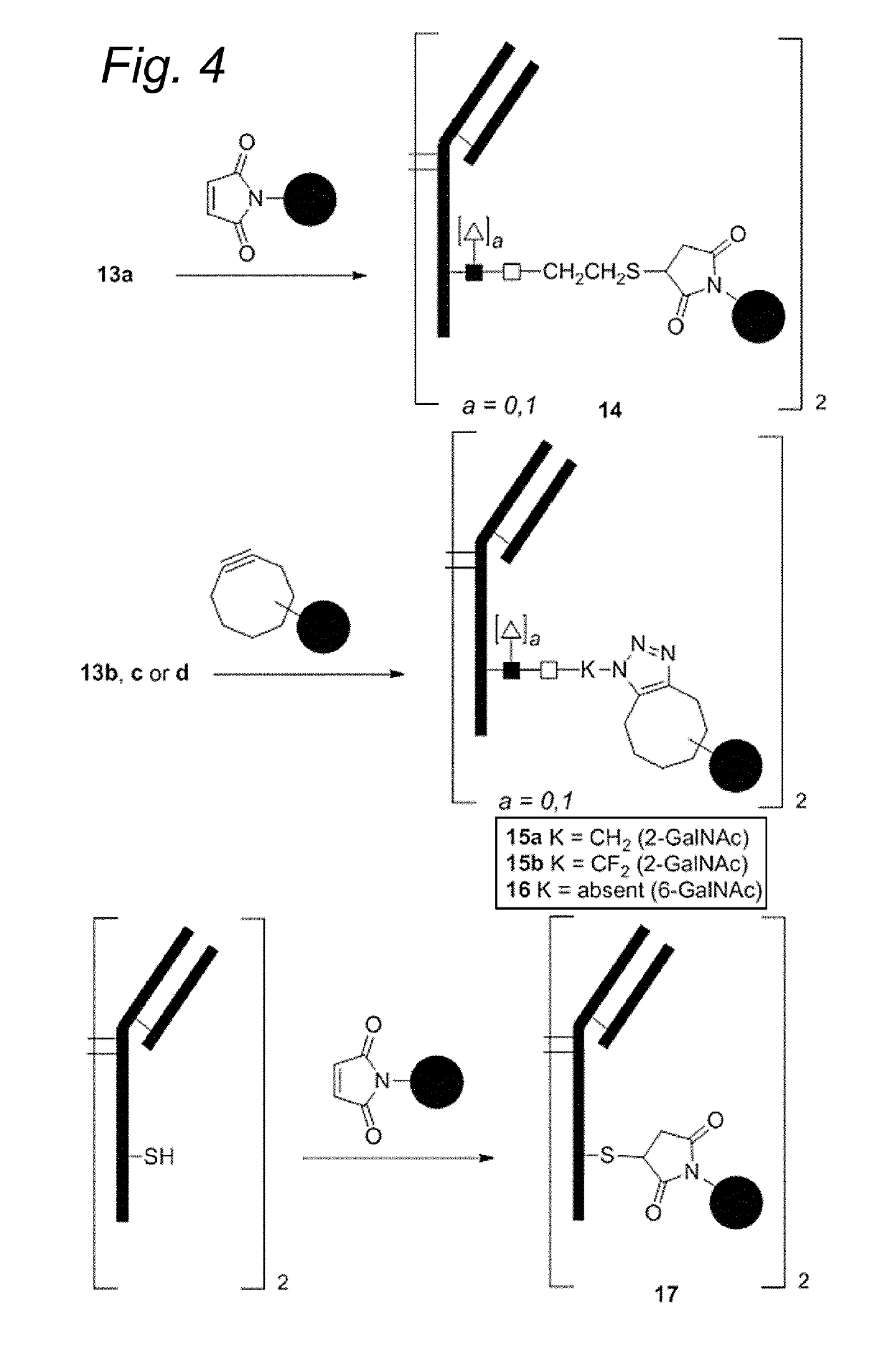
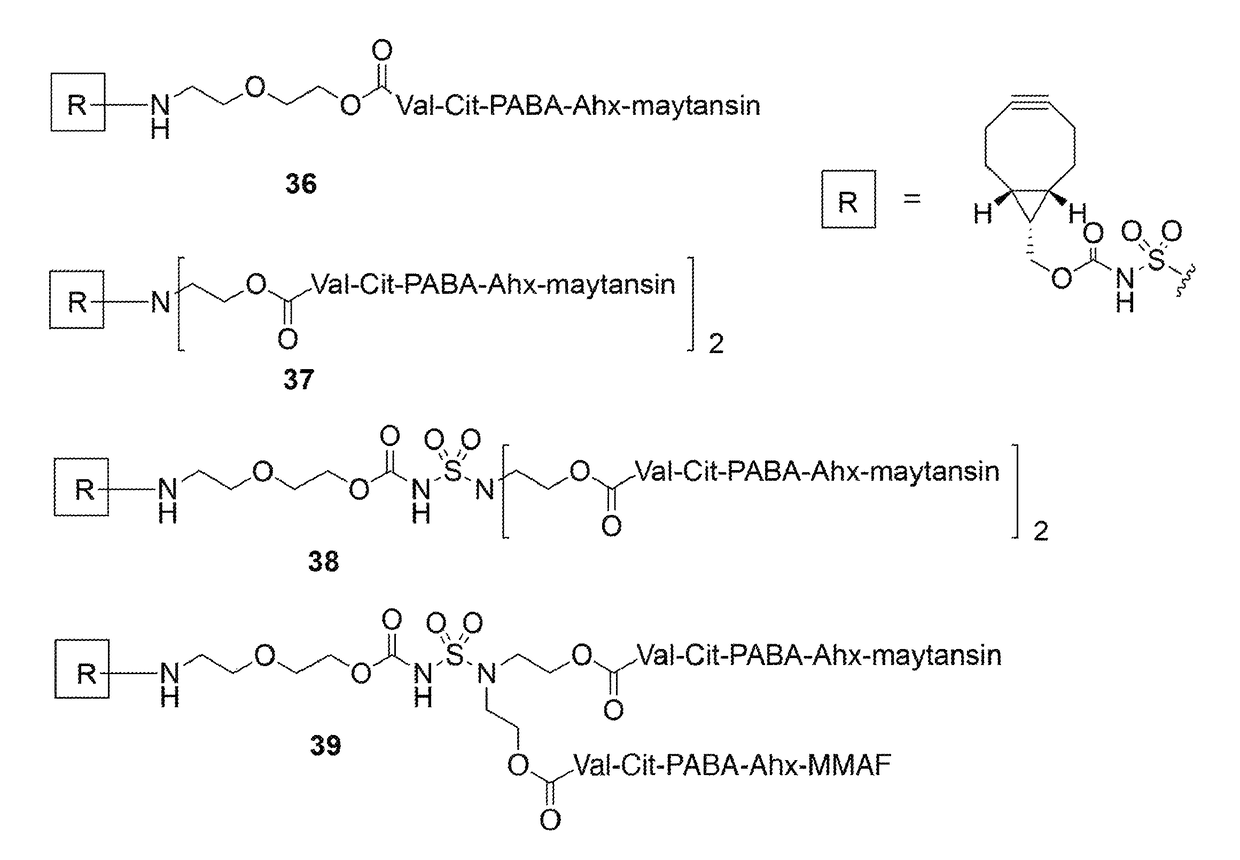
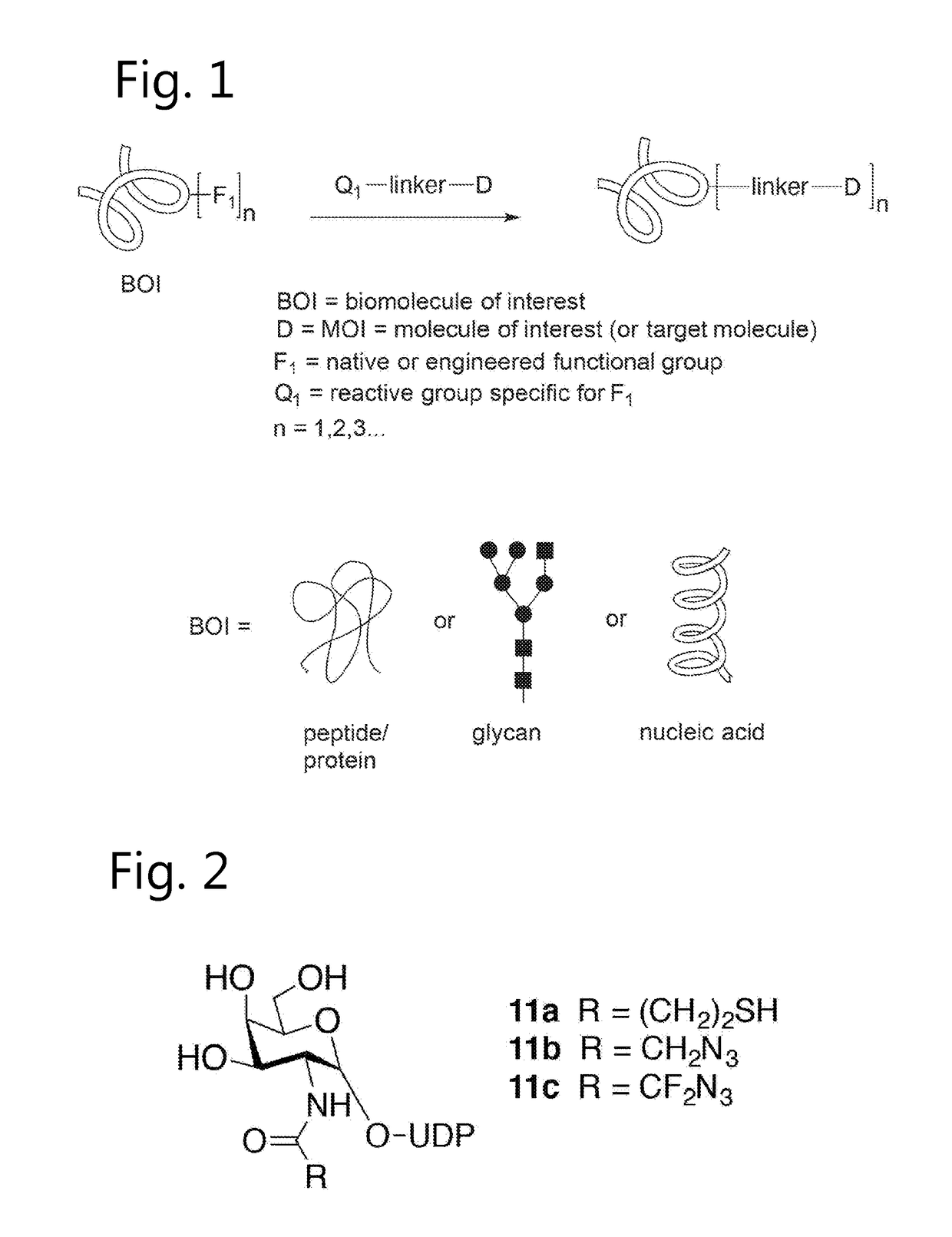
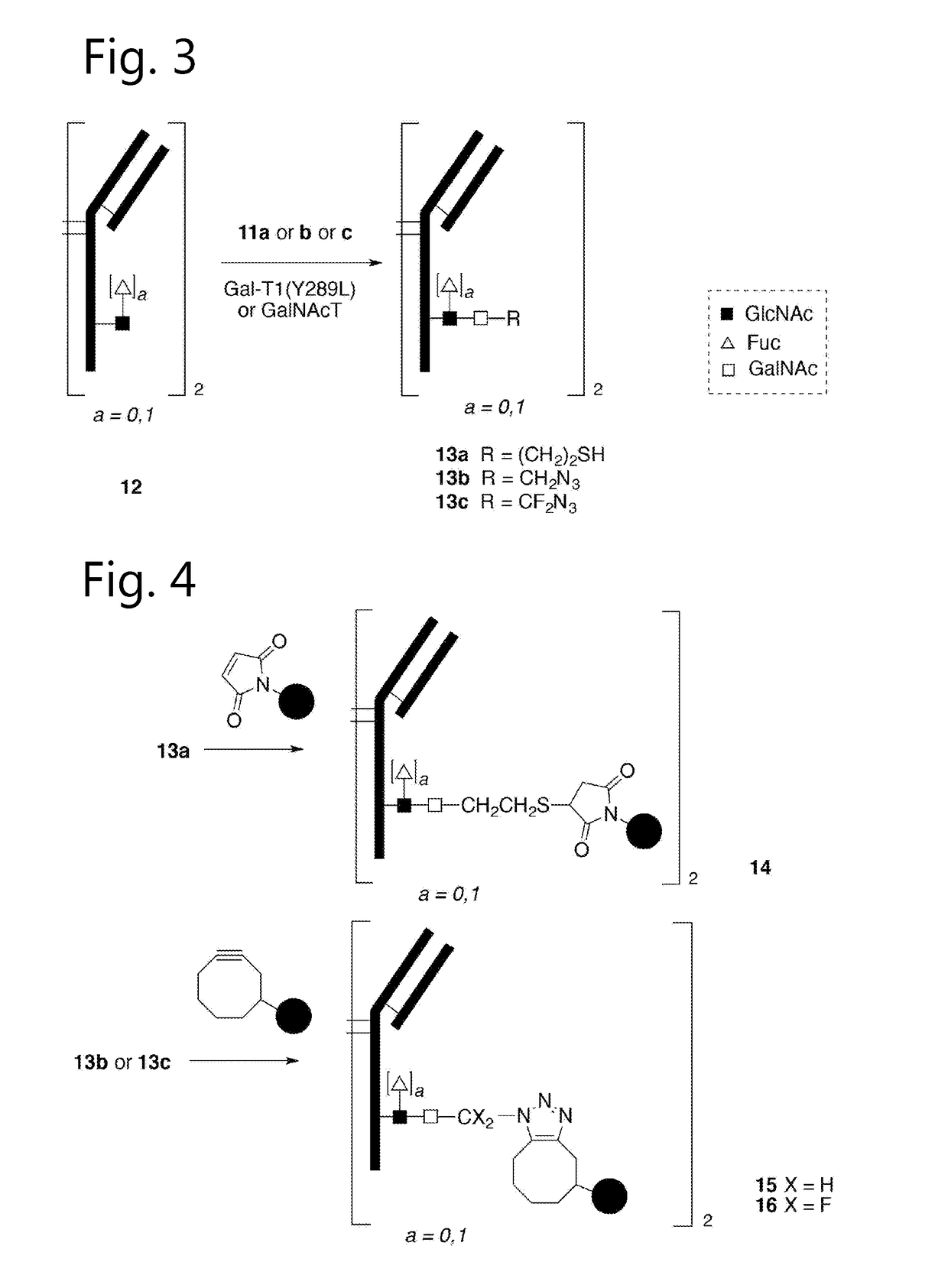
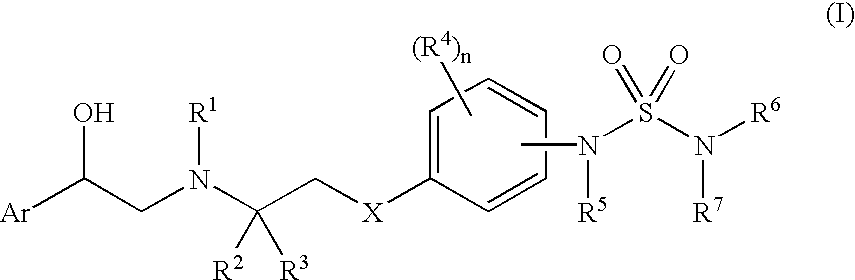
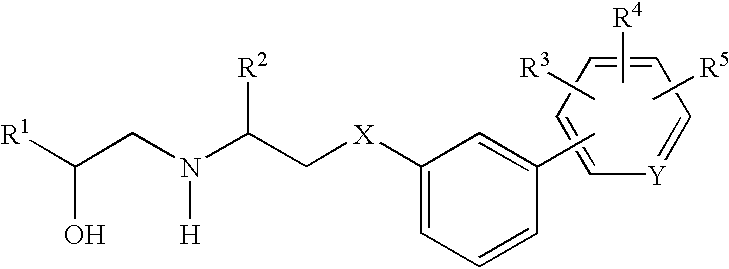
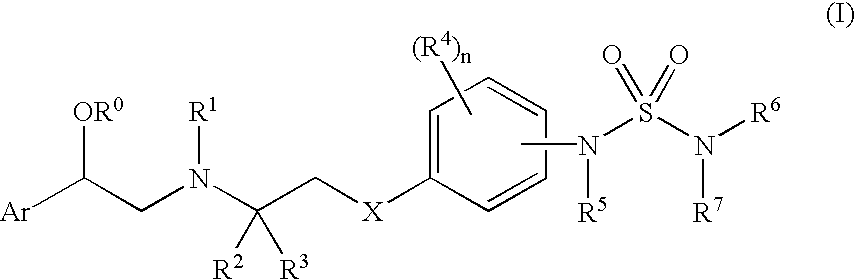
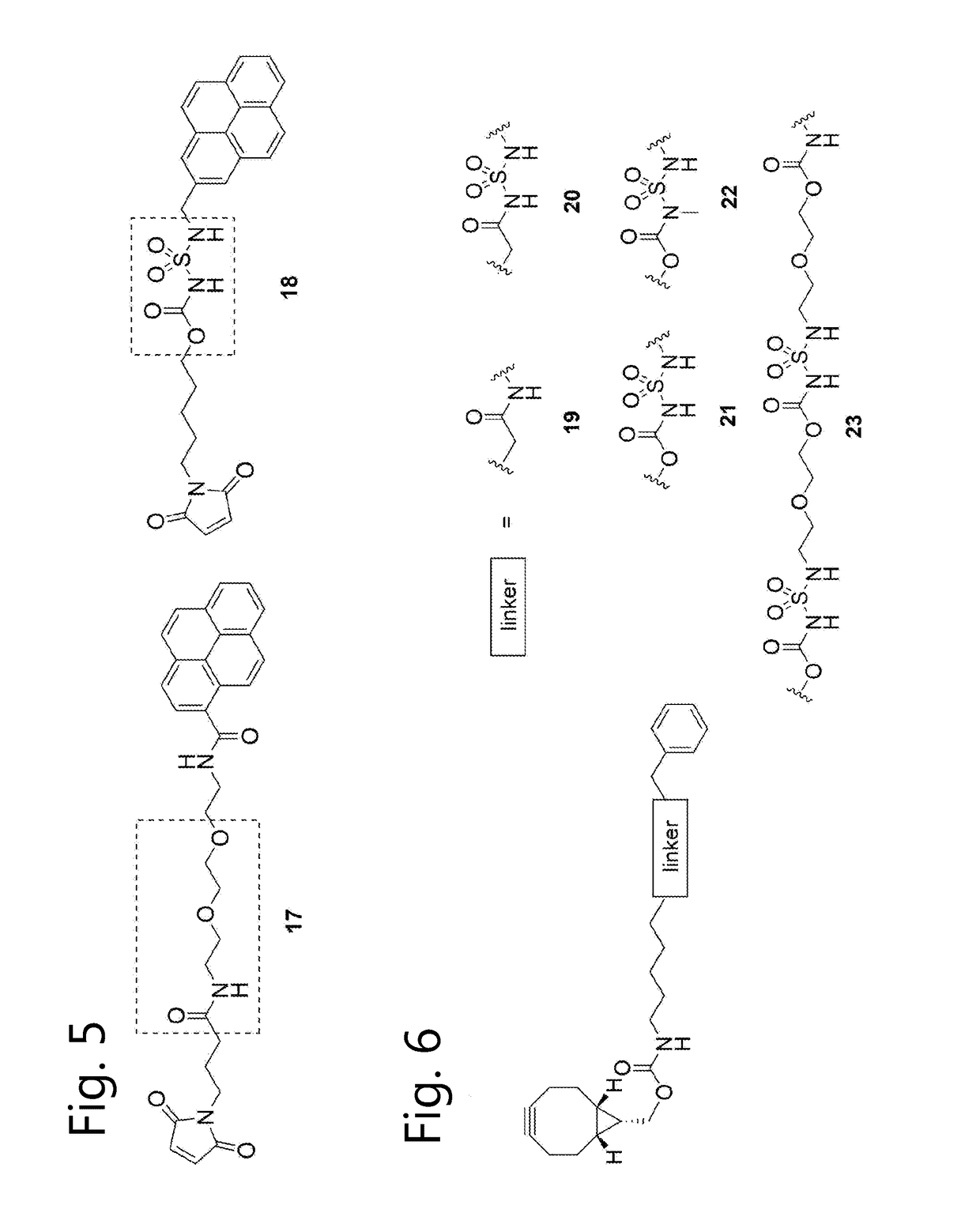
Sulfamide linker, conjugates thereof, and methods of preparation
ActiveUS20170072068A1Improve efficiencyConjugation efficiency of the sulfamide-containing maleimide 18 is higherImmunoglobulinsPharmaceutical non-active ingredientsChemical compoundSulfamide
The present invention relates to a compound comprising an alpha-end and an omega-end, the compound comprising on the alpha-end a reactive group Q1 capable of reacting with a functional group F1 present on a biomolecule and on the omega-end a target molecule, the compound further comprising a group according to formula (1) or a salt thereof:Said compound may also be referred to as a linker-conjugate. The invention also relates to a process for the preparation of a bioconjugate, the process comprising the step of reacting a reactive group Q1 of a linker-conjugate according to the invention with a functional group F1 of a biomolecule. The invention further relates to a bioconjugate obtainable by the process according to the invention.
Owner:SYNAFFIX
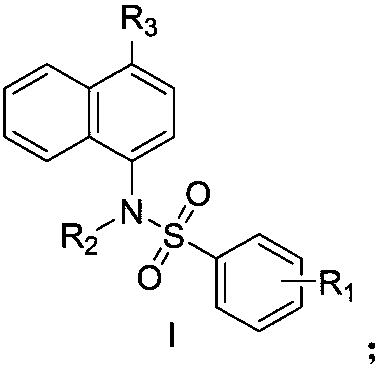
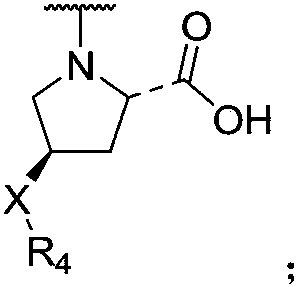
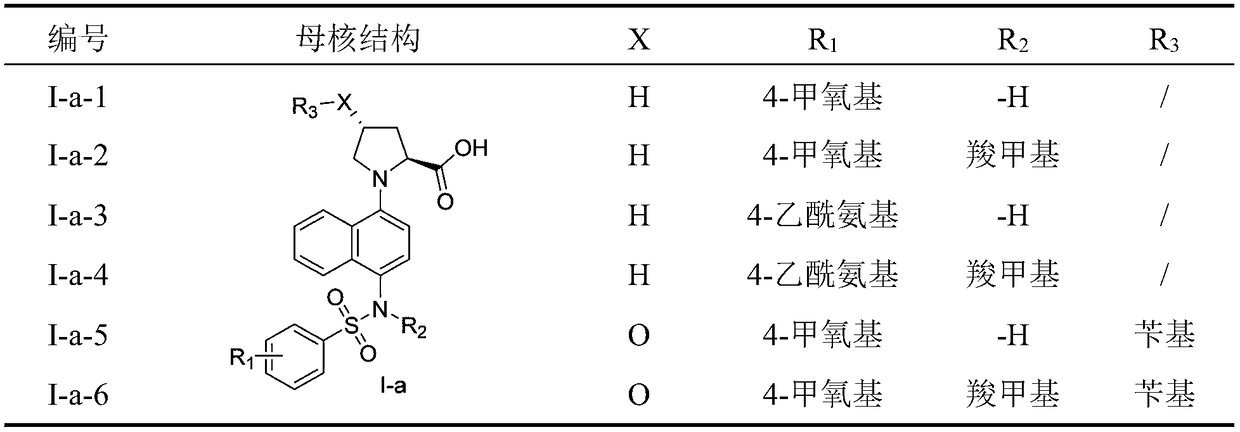
Naphthyl sulfamide amino acid derivative, preparation method and medical application thereof
ActiveCN108101821AReduce inflammatory damageImprove the inflammatory microenvironmentNervous disorderMetabolism disorderAnti-inflammatoryChronic renal disease
The invention discloses a naphthyl sulfamide amino acid derivative, a preparation method and medical application thereof. A naphthyl sulfamide amino acid derivative R3 substituent group represents a substituent group with an amino acid structure; the amino acid structure refers that the substituent group at least contains one carboxyl and one secondary amine or tertiary amine, and the substituentgroup is connected onto a mother nucleus through the secondary amine or the tertiary amine. The naphthyl sulfamide amino acid derivative provided by the invention can interfere with Keap1-Nrf2 combination and activate Nrf2, so that inflammatory injury is reduced, an inflammation microenvironment is improved, and the naphthyl sulfamide amino acid derivative has potential anti-inflammatory activity.The technician in the field knows that an Nrf2 activating agent can be used for inhibiting inflammatory reaction of diseases, so that the compound provided by the invention can be used for preparingan anti-inflammatory drug for treating diseases associated with inflammation, including chronic obstructive pulmonary diseases, Alzheimer diseases, Parkinson, atherosclerosis, chronic renal diseases,diabetes, intestinal inflammation, rheumatoid arthritis and the like.
Owner:CHINA PHARM UNIV
Method for synthesizing perfluoroalkyl sulfimide alkali metal salt and ionic liquid synthesized by same
ActiveCN101747244AQuick responseEnhance nucleophilic attack capabilitySulfonic acid amide preparationRubidiumDecomposition
The invention discloses a method for synthesizing perfluoroalkyl sulfimide alkali metal salt (M[Rf1SO2NSO2Rf2]) which is abbreviated to M[PFSI], wherein Rf1 and Rf2=CmF2m+1, m=1-8, M=Li, Na, K, Rb, and Cs. The method utilizes potassium (rubidium and cesium) salt of perfluoroalkyl sulfamide and perfluoroalkyl sulfonyl fluoride for reaction in the existence of potassium carbonate (rubidium and cesium), so that the potassium (rubidium and cesium) salt of the perfluoroalkyl sulfimide can be conveniently prepared with high yield which is 70-90%; the high-purity corresponding lithium (or sodium) salt (M[PFSI], M=Li and Na) can be obtained through double decomposition exchange reaction of the potassium (rubidium and cesium) salt and lithium perchlorate (or sodium) in aprotic polar solvent (such as acetonitrile, dimethyl carbonate, nitromethane and the like); and the hydrophobic functionalized ionic liquid composed of cations of sulfonium, ammonium or phosphorus with the [PFSI] through reaction of the prepared alkali metal salt and the sulfonium salt, ammonium salt or phosphorus salt of which the side chain contains functionalized functional groups.
Owner:武汉市瑞华新能源科技有限公司
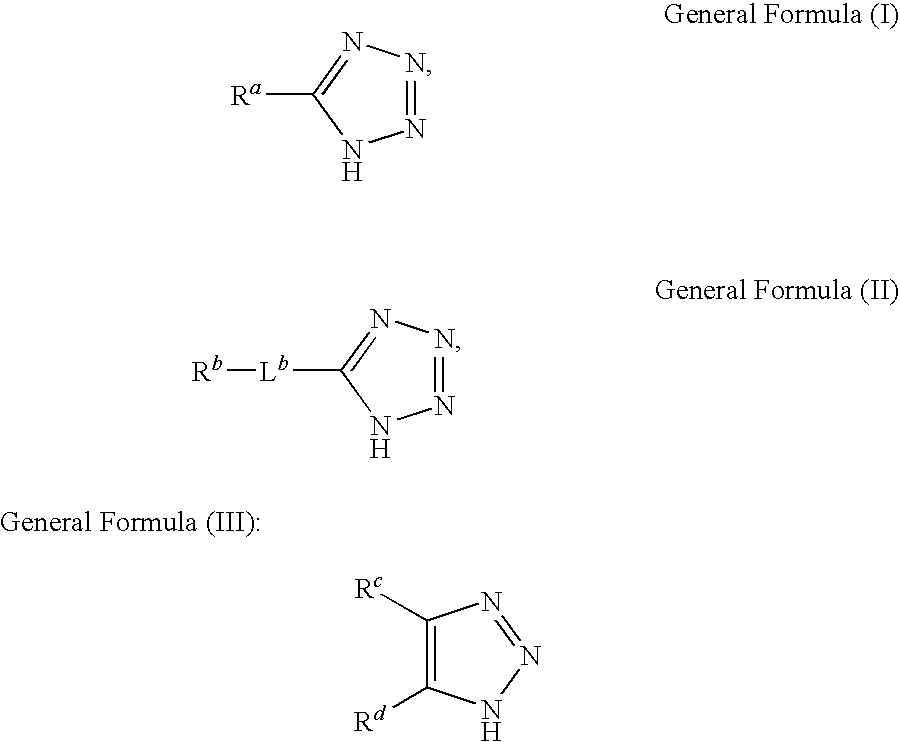
Polishing liquid for metals
InactiveUS20100330809A1Sufficient effectAvoid volatilityOther chemical processesSemiconductor/solid-state device manufacturingCarboxyl radicalTetrazole
A liquid for polishing metals, which is used in the chemical and / or mechanical flattening of a semiconductor device, the polishing liquid being characterized in that it comprises at least one member selected from the group consisting of tetrazoles or triazoles represented by any one of the following general formulas (I) to (III):wherein, Ra represents at least one substituent selected from the group consisting of a sulfo, an amino, a phosphono, a carbamoyl, a carbamide, a sulfamoyl, and a sulfonamide group; Rb represents at least one substituent selected from the group consisting of a hydroxyl, a carboxyl, a sulfo, an amino, a phosphono, a carbamoyl, a carbamide, a sulfamoyl, and a sulfonamide group; and Lb represents a divalent connecting group; and Rc and Rd each independently represent a hydrogen atom or a substituent, and at least one of Rc and Rd represent a hydroxyl, a carboxyl, a sulfo, an amino, a phosphono, a carbamoyl, a carbamide, a sulfamoyl, and a sulfonamide group or a group: -La-Re; wherein La represents a divalent connecting group; Re represents a hydroxyl, a carboxyl, a sulfo, an amino, a phosphono, a carbamoyl, a carbamide, a sulfamoyl or a sulfonamide group; R and R′ each independently represent a group selected from the group consisting of a hydrogen atom, alkyl groups and aryl groups; and R″ independently represents a group selected from the group consisting of alkyl groups and aryl groups.
Owner:FUJIFILM CORP
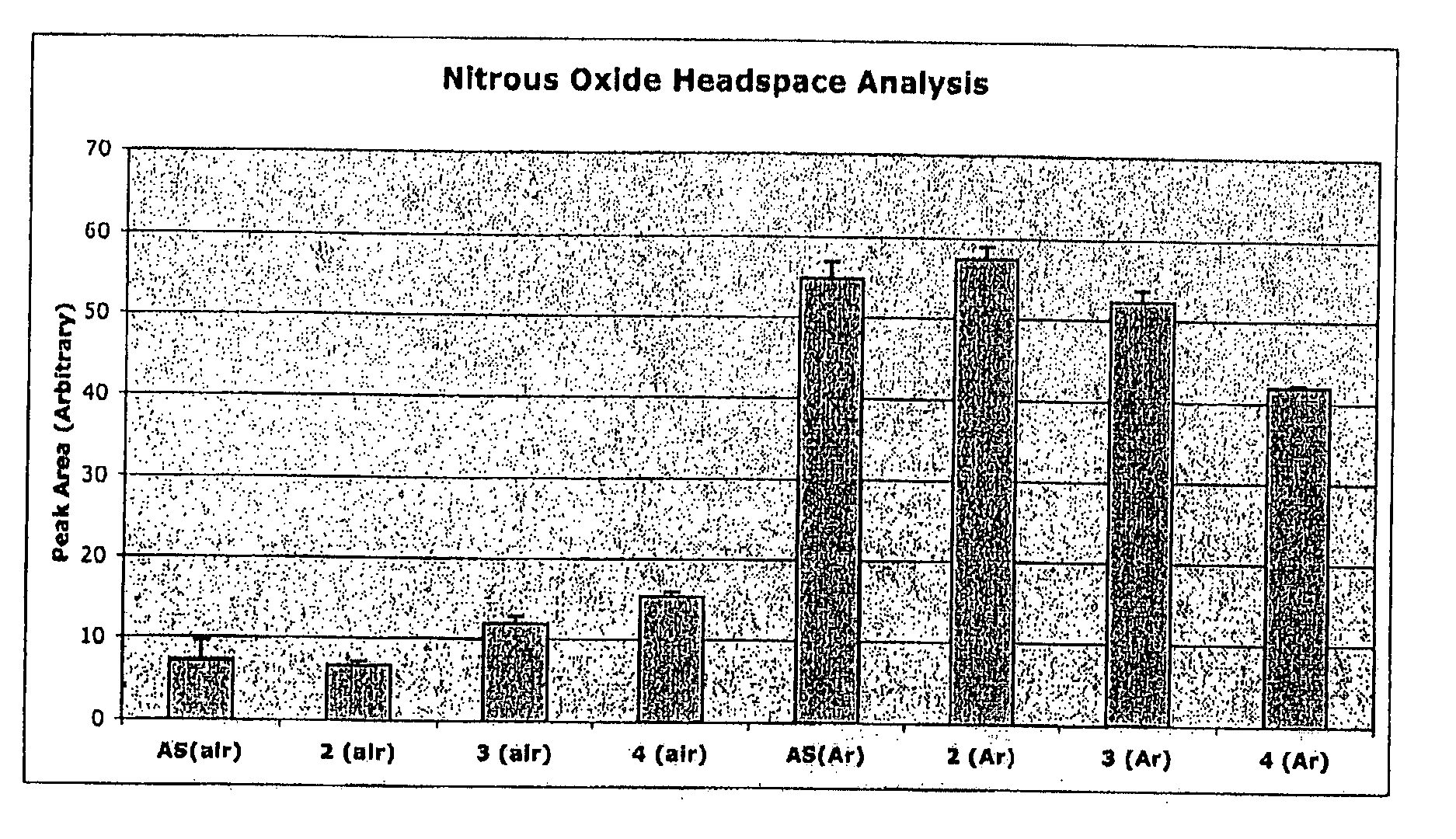
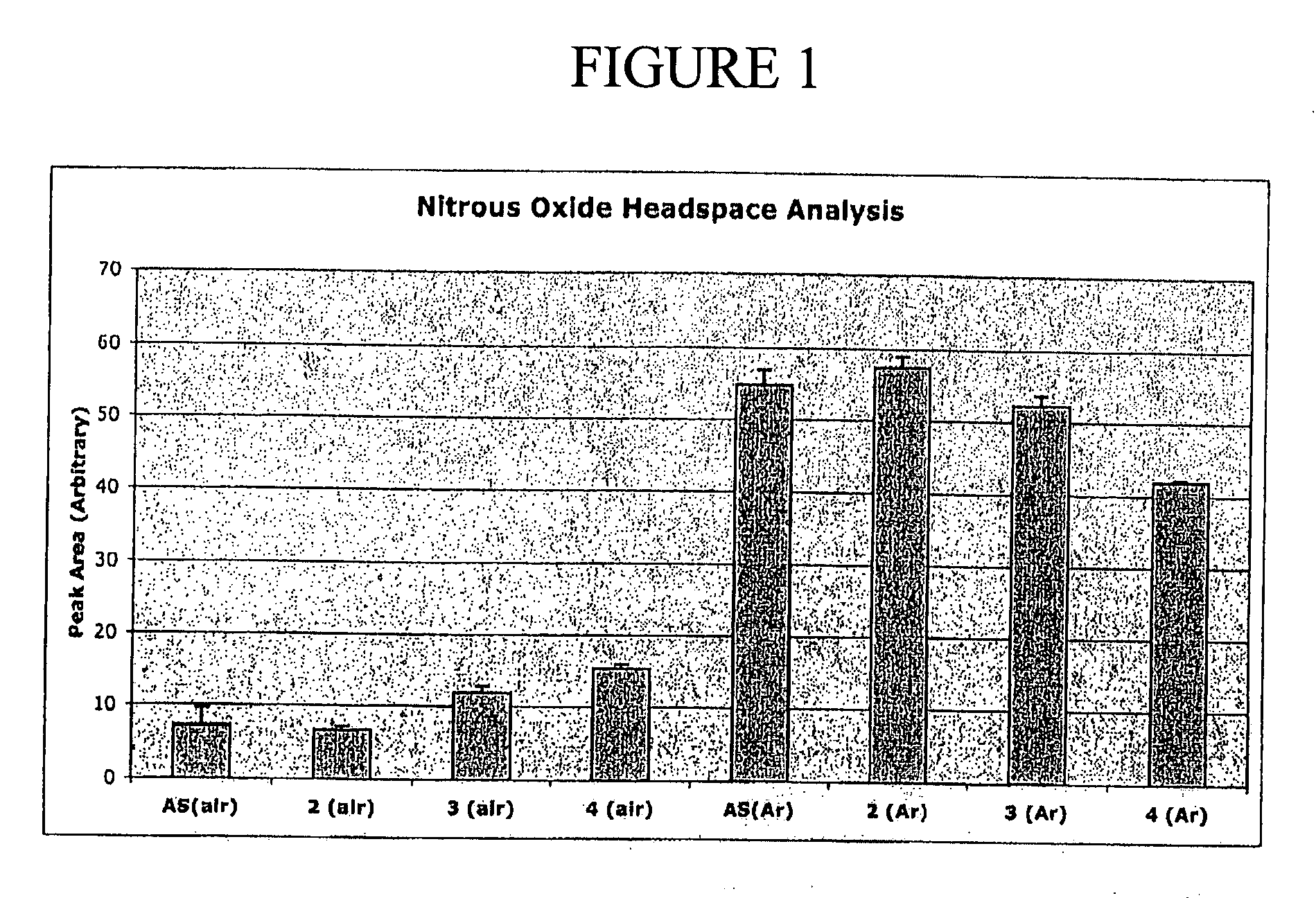
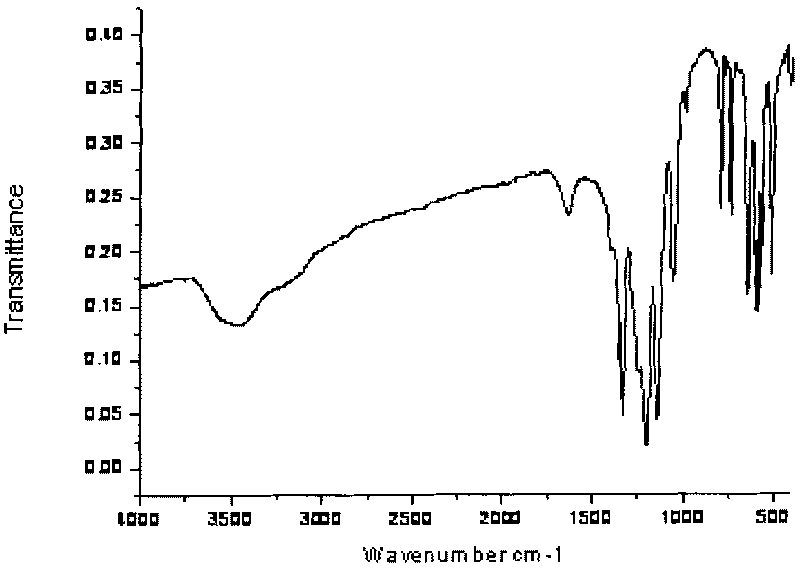
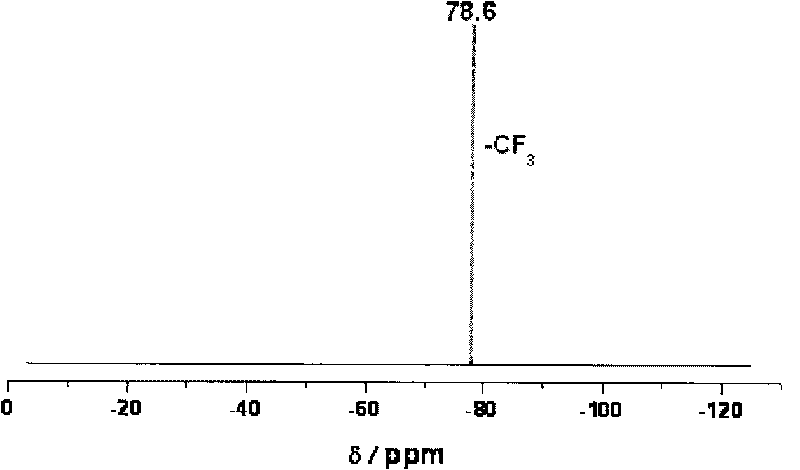
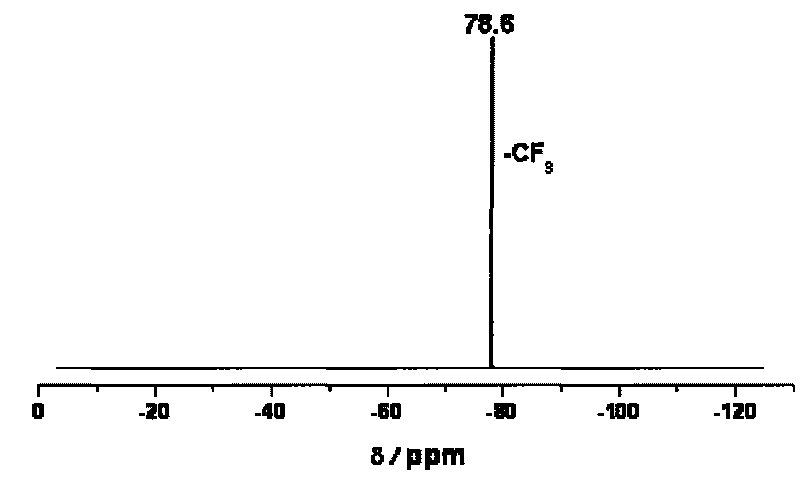
Trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound and preparation method thereof
ActiveCN102952139AIncrease polarityIncrease diversityNervous disorderOrganic chemistryPharmaceutical SubstancesMethyl palmoxirate
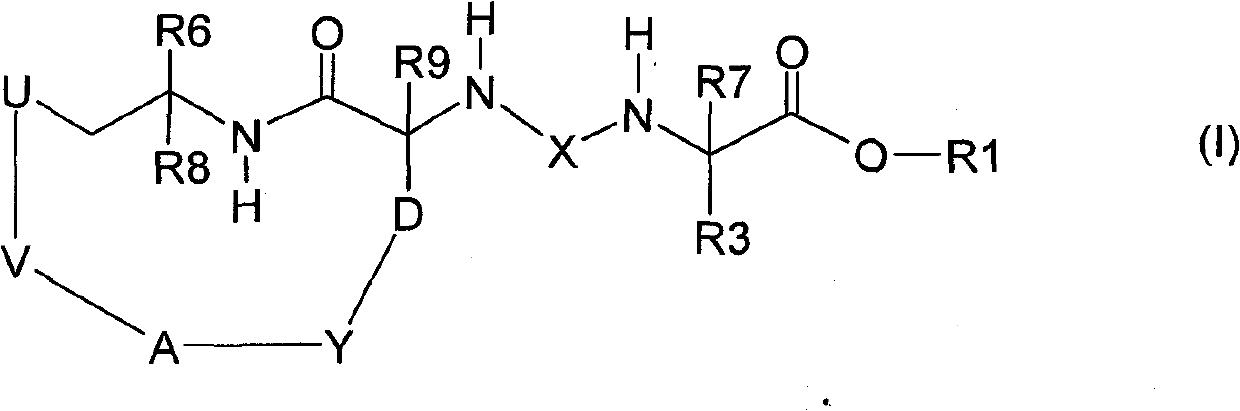
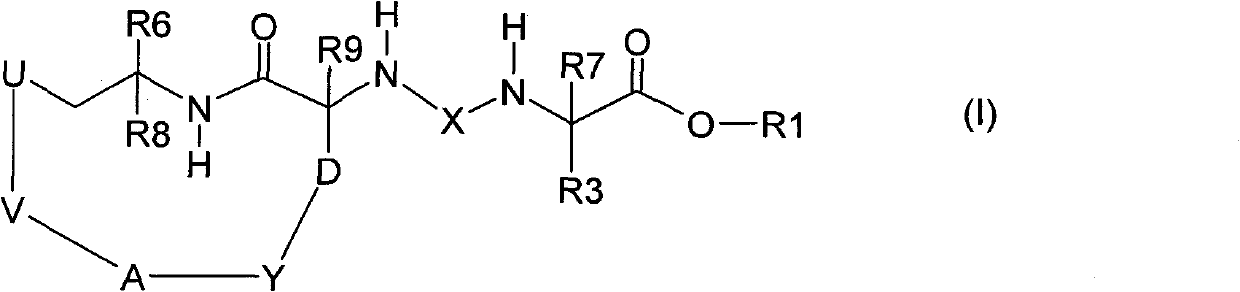
The invention relates to a trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound and a preparation method thereof, mainly solves the technical problem that the trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound is reported in no documents and realizes the screening on the structure-activity relationship of the medical activity of the trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound. The structural general formula is shown in the specification, wherein n=1 or 2; G is CH2 or an N atom; R1 and R2 are hydrogen, amide, sulfamide, urea, alkyl or aryl; the preferable amide is benzamide; the preferable sulfamide is methylsulfamide; the preferable urea is methylurea; and the preferable alkyl or aryl is one of C1-4 straight-chain or substituent-side-chain-containing alkyl and substituted aryl.
Owner:上海药明康德新药开发有限公司
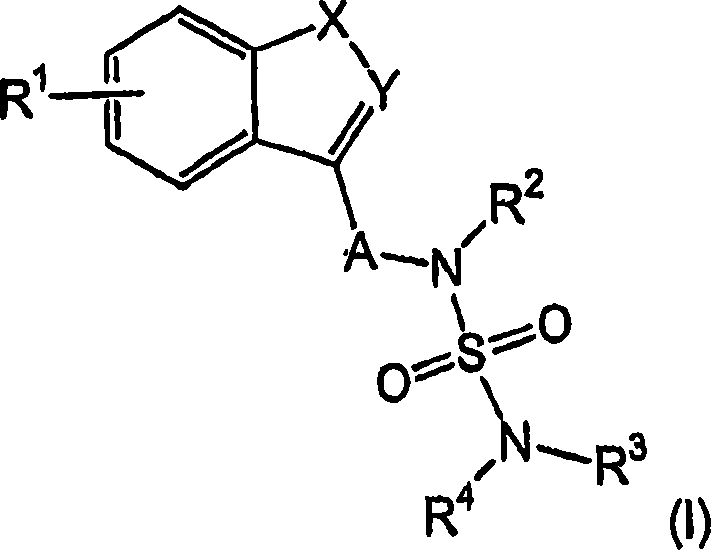
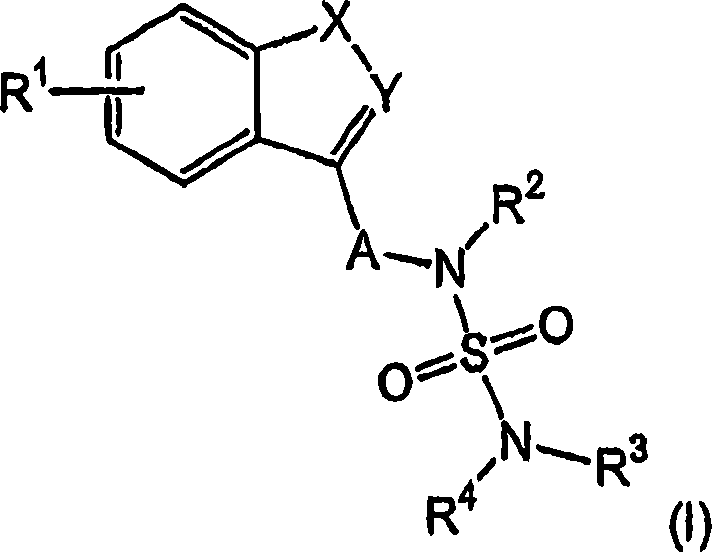
Novel benzo-fused heteroaryl sulfamide derivatives useful as anticonvulsant agents
The present invention relates to novel benzo-fused heteroaryl aminosulfonamide derivatives, pharmaceutical compositions containing them and their use in the treatment of epilepsy and related diseases.
Owner:JANSSEN PHARMA NV
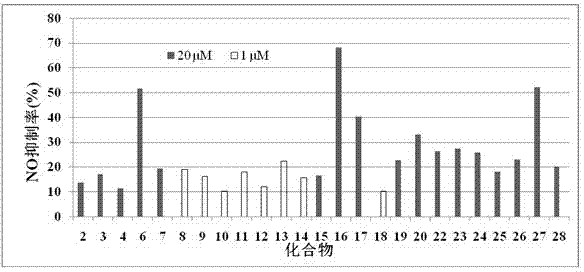
Sinomenine derivatives and their preparation methods and applications
InactiveCN102260212AUniqueNovel methodOrganic active ingredientsOrganic chemistryPolyethylene glycolTherapeutic effect
The invention discloses sinomenine derivatives, and a preparation method and application thereof. The sinomenine derivatives are sinomenine derivative monomers or dimers formed by connecting the 4-position or 6-position carbon (C) of the frameworks of sinomenine and the conventional sinomenine derivatives with linker through X, wherein X is an ether bond, an ester bond, an amido bond, a sulfamide bond, or an amine bond and the like, and the linker is an aliphatic compound, an aromatic compound or polyethylene glycol and the like. The method for preparing the sinomenine derivatives is novel and unique, and simple and practicable; the prepared sinomenine derivatives have high purity and yield; when macrophages RAW264.7 in vitro are adopted to evaluate the NO inhibiting activity and anti-inflammatory action in rats, the sinomenine derivatives have high bioactivity, can be applied to preparing anti-inflammatory medicines for rheumatoid arthritis, rheumatic arthritis and the like, immunosuppressive agents and health products, have wide application range and good treatment effect, and have good social benefit.
Owner:NANJING UNIV
Compound
InactiveUS20060074060A1Eliminate intrinsic oestrogenicityReduce conversionAntibacterial agentsOrganic active ingredientsMethyl palmoxiratePyrazole
There is provided a compound of Formula (I) wherein (I) R2 is selected from (i) an alkyloxyalkyl group (ii) a nitrile group, and wherein R2 is capable of forming a hydrogen bond (iii) alkylaryl group, wherein the aryl group is substituted by other than a C1-10 group (iv) alkenylaryl group wherein the aryl group is substituted (v) alkylheteroaryl group, wherein when heteroaryl group comprises only C and N in the ring, the aryl group is substituted by other than a methyl group (vi) alkenylheteroaryl group, (vii) ═N—O-alkyl or ═N—O—H group (viii) branched alkenyl (ix) alkyl-alcohol group (x) amide or alkylamide wherein (a) the alkyl of the alkylamide is —CH2— or —Ch2Ch2—, (b) the amide is di-substituted and / or (c) the amide is substituted with at least one of alkyl heterocycle group, alkenyl heterocycle group, alkylheteroaryl group, alkenylheteroaryl group, heteroaryl group, alkylamine group, alkyloxyalkyl group, alkylaryl group, straight or branched alkyl group, (xi) —CHO so that R1 together with R3 provide the enol tautomer (a); OR R2 together with R3 form (xii) a pyrazole wherein (a) R4 is ═N—O-alkyl or ═N—O—H group, (b) the pyrazole is substituted with one of alkyl-OH group, alkyl ester group, alkyloxyalkyl group, branched alkyl group, and an amide and / or (c) the 2 position is substituted with a group selected from —OH and —O-hydrocarbyl (xiii) a heteroaryl ring to provide a compound of the formula (b); (II) R2 is selected from groups capable of forming a hydrogen bond, a sulphamate group, a phosphonate group, a thiophosphonate group, a sulphonate group and a sulphonamide group; and (III) R3 is selected from —OH, ═O, or a C(═O)—mimetic.
Owner:STRIX LTD
Preparation method for celecoxib
The invention relates to a preparation method for celecoxib, and belongs to the field of chemical pharmaceuticals. The method comprises the step of performing cyclization reaction on 4, 4, 4-trifloro-1-(4-tolyl)-1, 3-butanedione and p-sulfamine phenylhydrazine or 4-sulfamine phenylhydrazine hydrochloride in a solvent to obtain a celecoxib coarse product, wherein the solvent for cyclization reaction is a low molecular organic acid or a low molecular organic acid aqueous liquor. According to the method, the product is high in yield, good in purity, easy to purity, good in quality and low in cost; and the method is environment-friendly in condensation process, and is suitable for large-scale industrial production.
Owner:HENAN DONGTAI PHARM
Preparation method of azacyclo
The invention discloses a preparation method of azacyclo. The preparation method comprises the following steps of: (1) reacting diethylenetriamine or triethylene tetramine with methylsulfonyl chloride to generate methanesulfonamide; (2) during two-phase reaction, cyclizing compound catalytic cyclization on methanesulfonamide and a compound having the structure shown in a chemical formula (a) under the composite catalysis of benzyl triethylanmine compound and 15-crown-5 at 90 DEG C under a backflow condition; and (3) removing methylsulfonyl from methanesulfonamide azacyclo and performing methylation treatment, wherein X represents bromine, iodine or sulphonate. The preparation method has the advantages that raw materials are low in cost, atom economy is high, and operation is easy and safe. The preparation method is suitable for industrial production.
Owner:浙江凯普化工有限公司
Composition 064
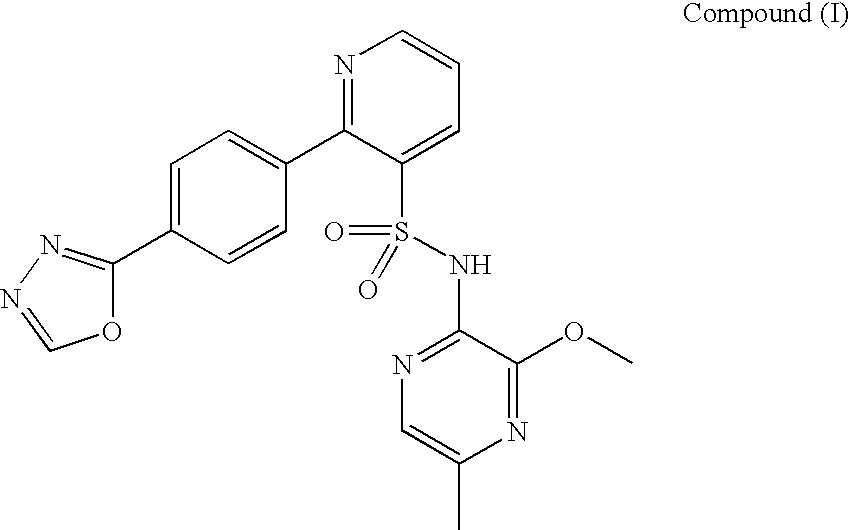
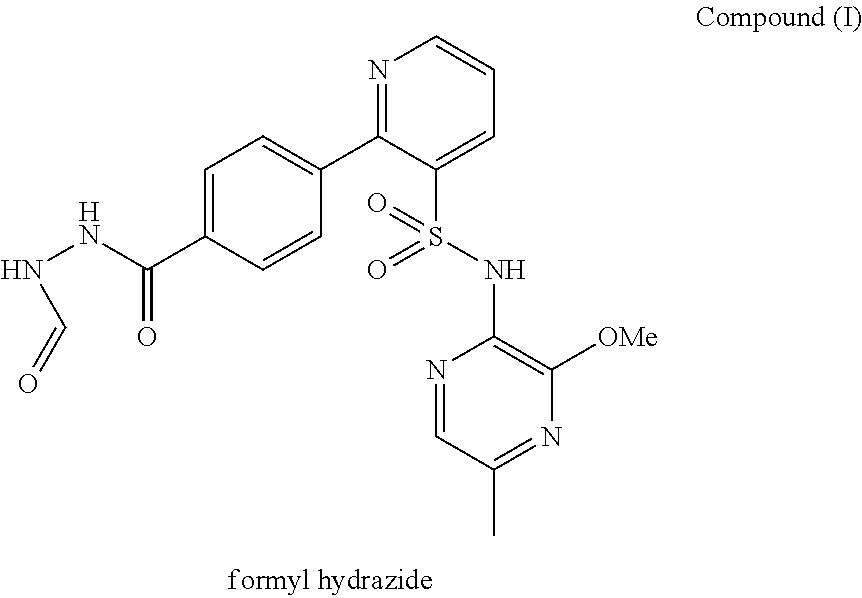
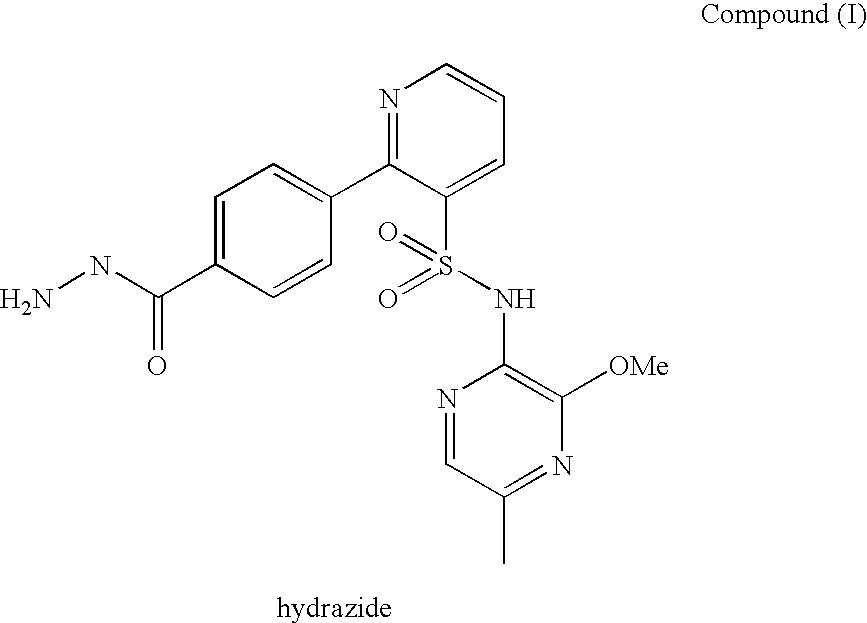
A pharmaceutical composition which comprises N-(3-methoxy-5-methylpyrazin-2-yl)-2-(4-[1,3,4-oxadiazol-2-yl]phenyl)pyridine-3-sulphonamide with mannitol and / or 5 microcrystalline cellulose is described.
Owner:ASTRAZENECA AB
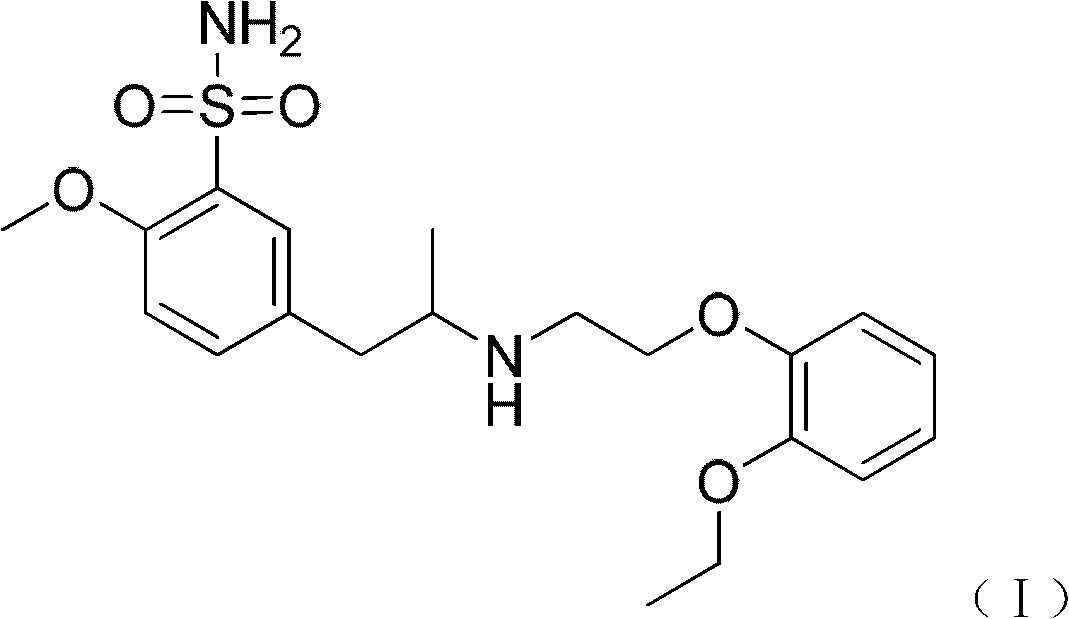
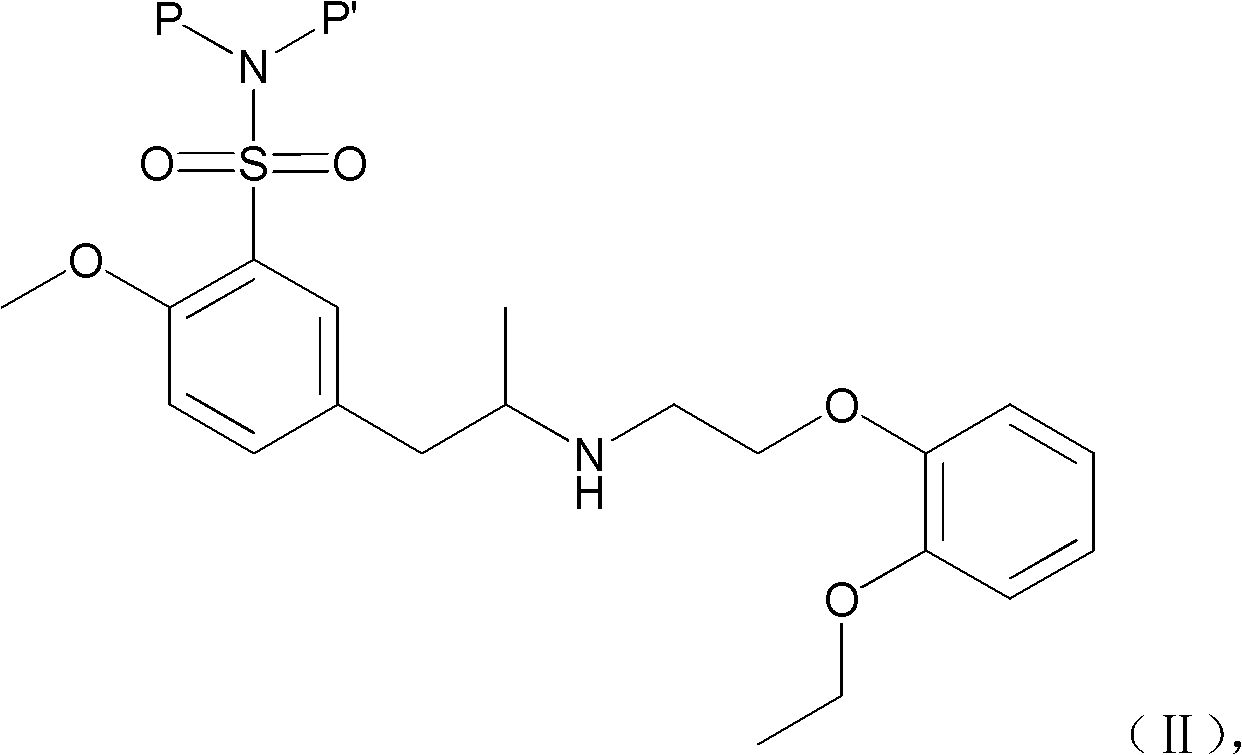
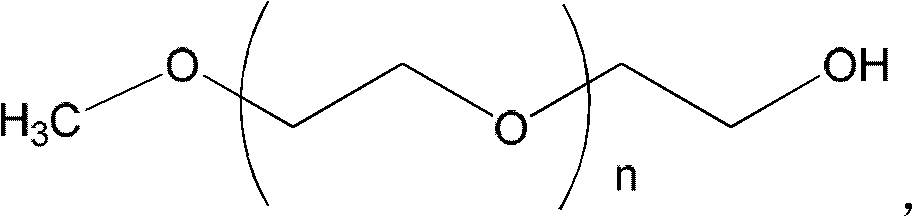
Combination of polyethylene glycol and tamsulosin and pharmaceutical compound comprising same
ActiveCN103127520APromote absorptionReduce transmittanceUrinary disorderPharmaceutical non-active ingredientsAlkyl transferTamsulosin
The invention provides a combination of polyethylene glycol and tamsulosin and a pharmaceutical compound comprising the combination which is as shown in a general formula (II). In the combination, a P and a P' are H or polyethylene glycol, and the P and the P' cannot be the H at the same time. The tamsulosin structurally contains a sulfamide group, the low-molecular-weight polyethylene glycol is introduced through an alkylation reaction, and therefore hydrophobicity of the tamsulosin is lowered, hydrophilism of the tamsulosin is increased, and the blood-brain barrier permeability of the tamsulosin is lowered. Therefore, the toxicity of the tamsulosin is lowered.
Owner:JENKEM TECH CO LTD TIANJIN
Macrocyclic urea and sulfamide derivatives as inhibitors of TAFIA
The invention relates to compounds of the formula (I) which are inhibitors of activated thrombin-activable fibrinolysis inhibitor. The compounds of the formula I are suitable for producing medicaments for prophylaxis, secondary prevention and treatment of one or more disorders associated with thromboses, embolisms, hypercoagulability or fibrotic changes.
Owner:SANOFI AVENTIS SA
Method for synthesizing 2-ethoxycarbonylaminosulfonyl-N,N-dimethyl nicotinamide
ActiveCN102382049ASimple and thorough separationHigh yieldOrganic chemistryEthyl chloroformateAqueous acetone
The invention relates to a novel method for synthesizing 2-ethoxycarbonylaminosulfonyl-N, N-dimethyl nicotinamide. 2-ethoxycarbonylaminosulfonyl-N,N-dimethyl nicotinamide is prepared by the following steps of taking liquid alkali as an acid binding agent; performing reaction of 2-aminosulfamide-N,N-dimethyl nicotinamide and ethyl chloroformate; adding 2-aminosulfamide-N,N-dimethyl nicotinamide into an acetone aqueous solution, adding ethyl chloroformate and liquid alkali into acetone aqueous solution while stirring; preserving heat till the completion of the reaction, distilling and recyclingacetone; filtering; separating; and drying to obtain the finished product. The product prepared by the method saves energy and reduces consumption and achieves the environmental safety; no pollutantssuch as carbon dioxide and salt-containing waste water are generated; and the yield and the content of the 2-ethoxycarbonylaminosulfonyl-N,N-dimethyl nicotinamide is high.
Owner:ANHUI FENGLE AGROCHEM
Sulfonic acid dimeric surfactant based on perfluoroolefine and preparation method thereof
ActiveCN102872751ARapid responseImprove surface activityTransportation and packagingMixingActive agentSurface-active agents
The invention discloses a sulfonic acid dimeric surfactant based on perfluorononylene and perfluorohexylene, and a preparation method of the surfactant. The surfactant is a compound with a general formula as described in the specification. As perfluor alkene oxygen phenyl serves as a lipophilic group, sulphonate serves as a hydrophilic group, and a lipophilic part is connected with a hydrophilic part by a sulfonamide bond, the surfactant is very high in surface activity and lower in critical micelle concentration. The preparation method takes the perfluorononylene or the perfluorohexylene as a raw material, and comprises the steps of condensing with phenol, chlorosulfonation, diamine condensing, and sulphur alkylation condensing. The preparation method has the characteristics of simple process, lower cost and the like, and has good application prospects.
Owner:江苏中丽新材料有限公司
Rubber sealing ring
The invention provides a rubber sealing ring. The material used by the rubber sealing ring is prepared from the following raw materials, in parts by weight: 80-90 parts of nitrile rubber; 5-7 parts of zinc oxide; 1-3 parts of an anti-aging agent; 0.5-1.5 parts of stearic acid; 90-110 parts of a high abrasion resistant carbon black; 2-4 parts of MDC sulphur; 0.5-1.5 parts of an accelerant; and 1-3 parts of N-cyclohexyl-2-benzothiazolesulfenamide. The rubber sealing ring provided by the invention can meet indicators stated by an SH / T0305-93 standard test method, and increases sealing performance in rubber seal adaptability tests of related petroleum products.
Owner:CHINA PETROLEUM & CHEM CORP
Construction and application of endoplasmic reticulum targeted nano drug delivery system
ActiveCN112472822APromote accumulationGood treatment effectMaterial nanotechnologyAntipyreticLysosomeReticulum cell
The invention provides construction and application of an endoplasmic reticulum targeted nano drug delivery system. By modifying sulfamide or sulfonylurea compounds with endoplasmic reticulum tendencyinto endoplasmic reticulum targeting nano-compounds constructed in a nano-carrier, the constructed novel endoplasmic reticulum targeting liposome can load water-soluble and fat-soluble drugs and quickly transfer the drugs to the endoplasmic reticulum part, so that accumulation of the drugs at the action part of the liposome is improved, and the bioavailability of the drugs is improved. The medicine treatment effect is enhanced, and the toxic and side effect is weakened; the delivery of nucleic acid drugs needing lysosome escape can be remarkably improved, and a new strategy is provided for exerting the curative effect of the drugs. The nano-carrier disclosed by the invention is not only limited to lipidosome, but also can be solid lipid nanoparticles, nano-emulsion, polymer micelles and the like. The endoplasmic reticulum targeted modification nano-carrier provided by the invention is novel in scheme and simple in preparation method, and has good industrial production and applicationprospects.
Owner:ZHEJIANG UNIV
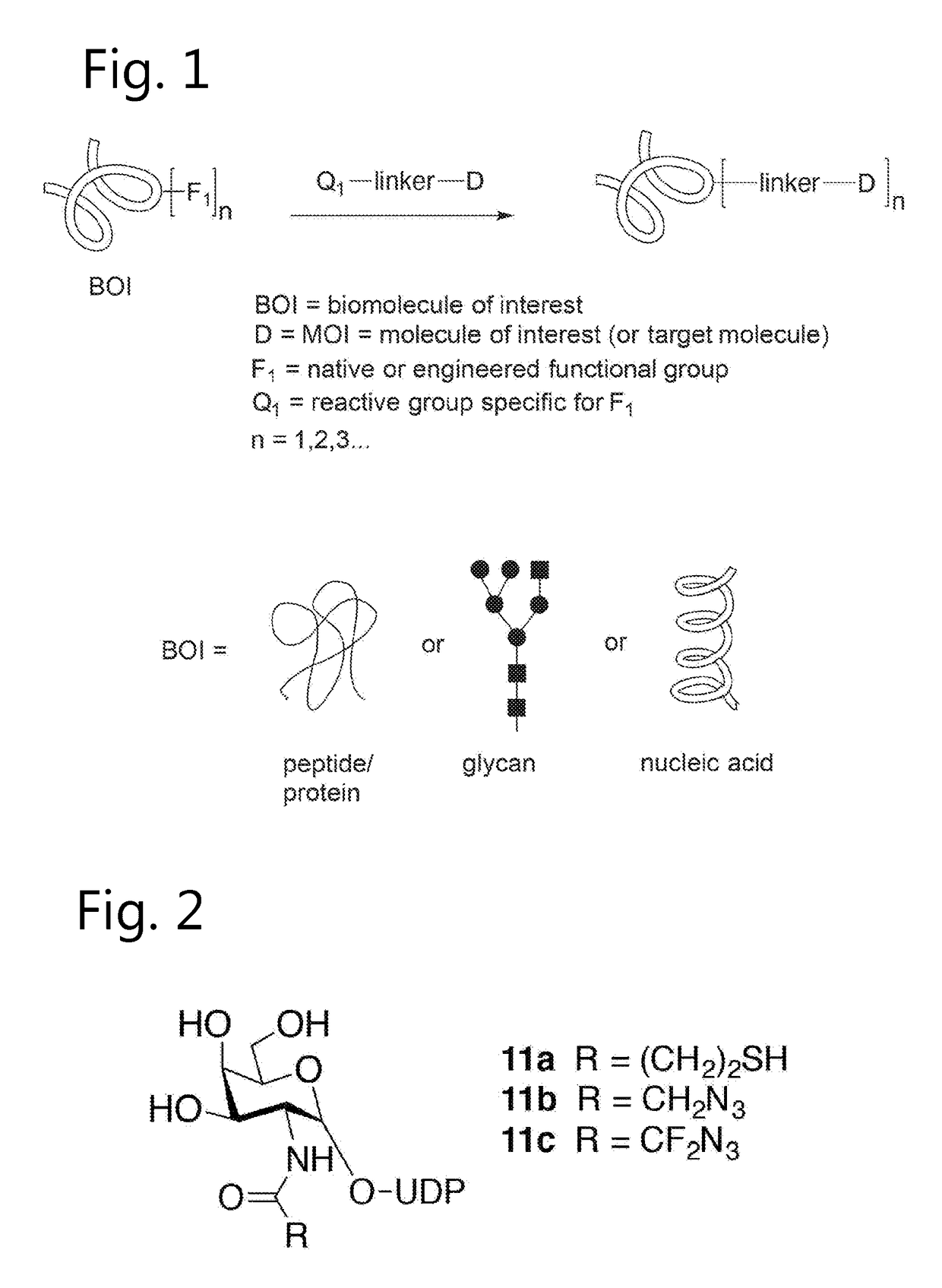
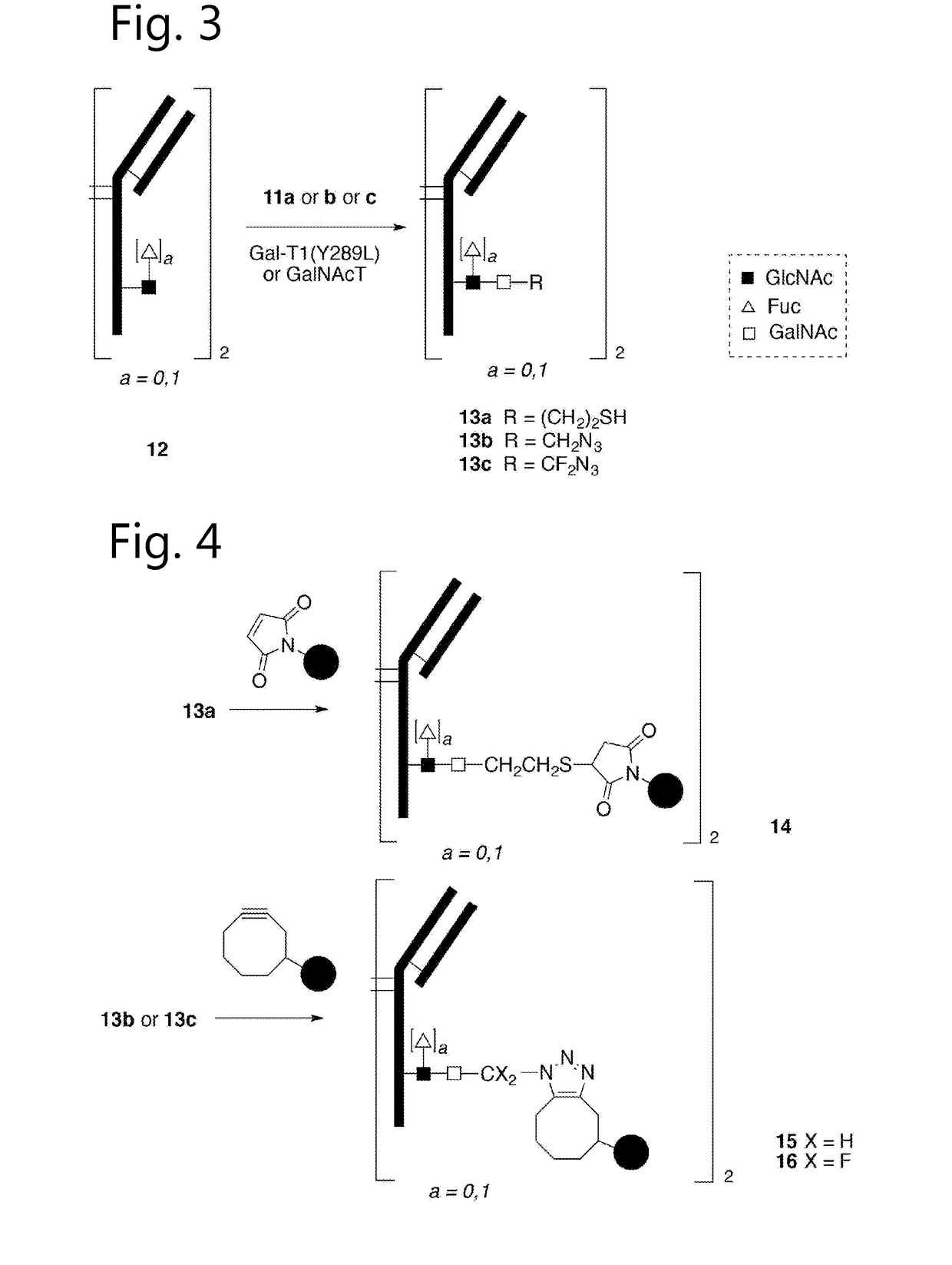
Bioconjugates containing sulfamide linkers for use in treatment
PendingUS20190262467A1Improve toleranceTreatment dosage can be reducedPharmaceutical non-active ingredientsAntineoplastic agentsSulfamideBioconjugation
The present invention is based on the surprising finding that the linker employed in a bioconjugate, such as an anti-body-drug conjugate, is not therapeutically inert but has an effect on the therapeutic index of the bioconjugate. The present invention thus concerns a method for increasing the therapeutic index of a bioconjugate and the bioconjugates for use in treatment, in particular cancer. The bioconjugates according to the invention have a sulfamide linker comprising a group according to formula (1).
Owner:SYNAFFIX
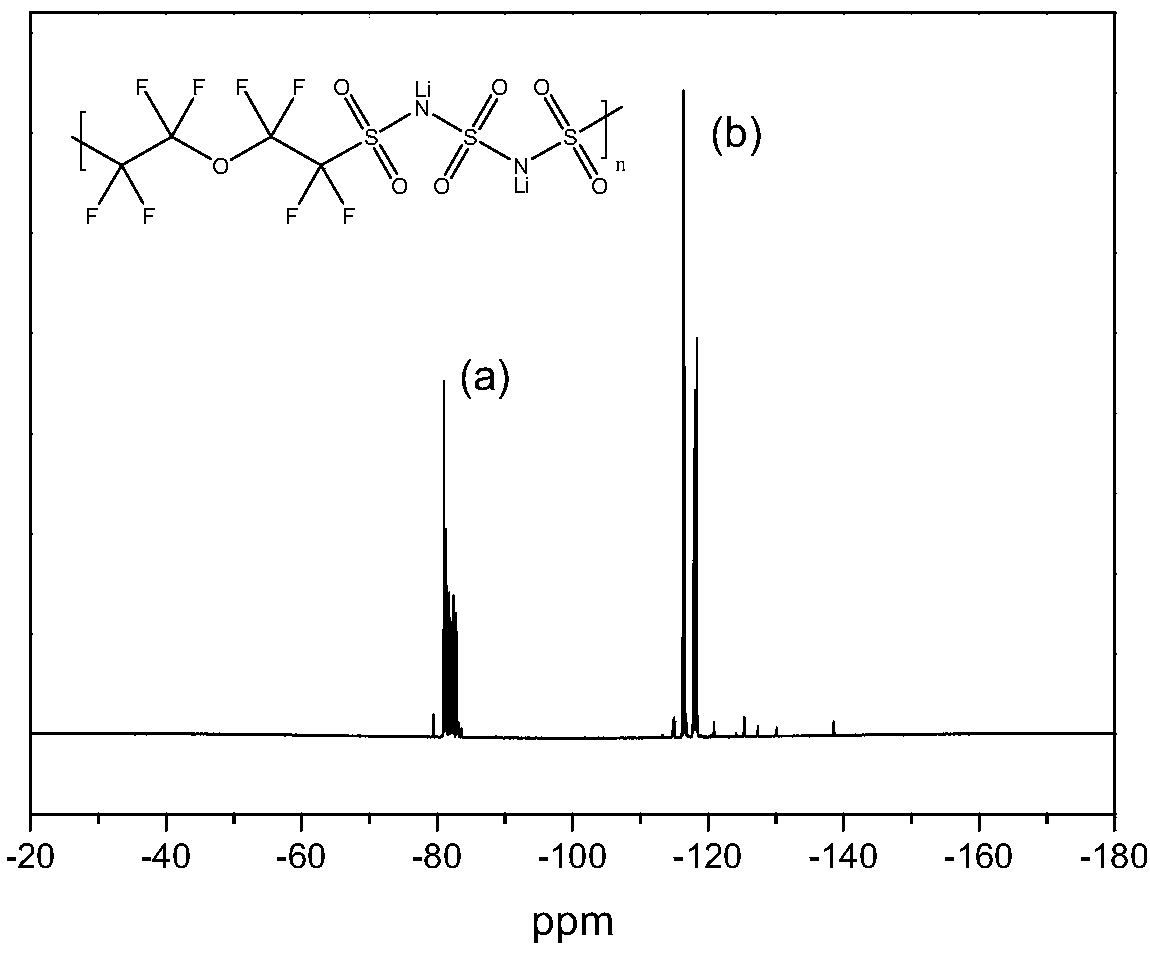
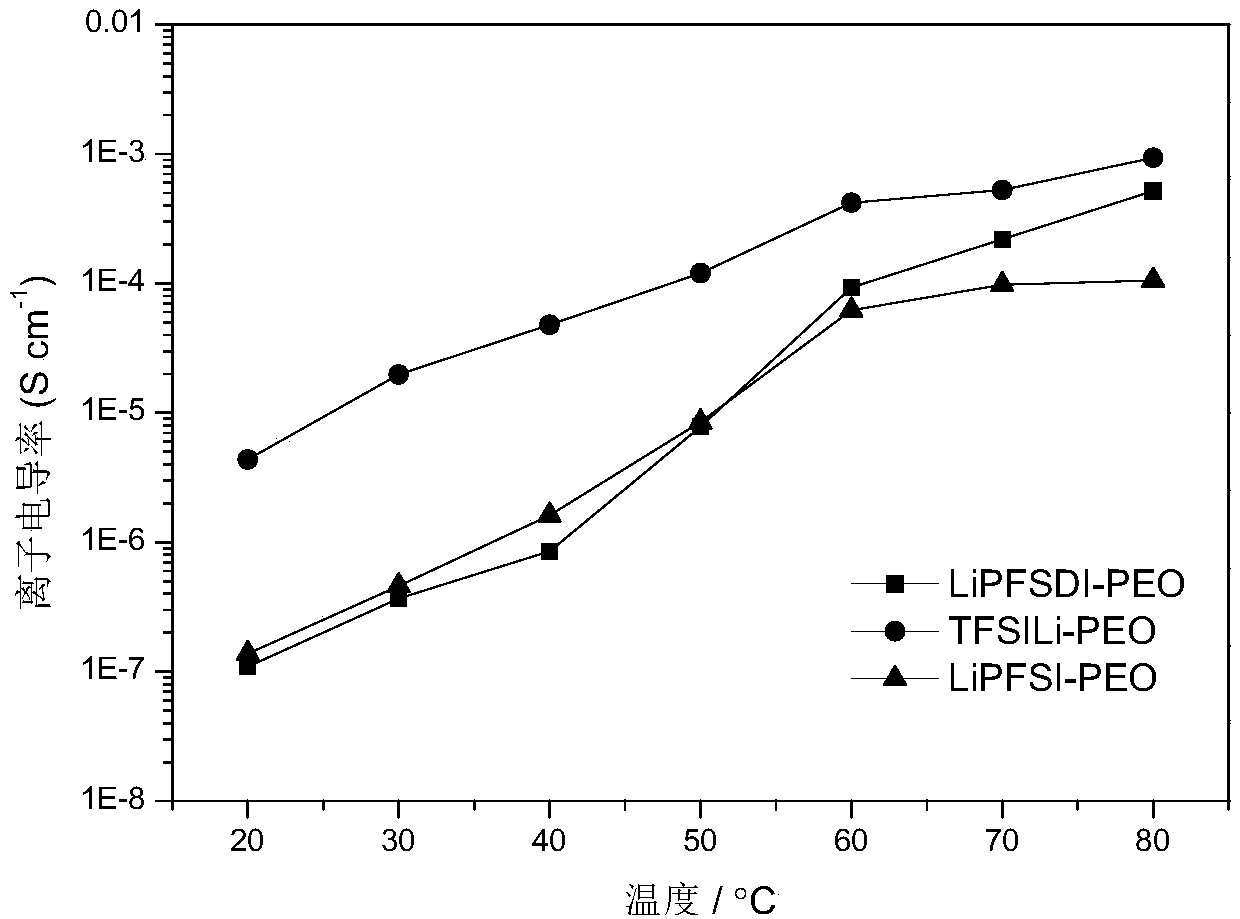
Novel conjugated fluorine-containing sulfimide single ion conductor polymer and preparation and application methods thereof
ActiveCN109456484AImprove ionic conductivityImprove securityCell electrodesSecondary cellsSolid state electrolyteElectrical conductor
The invention discloses a novel conjugated fluorine-containing sulfimide single ion conductor polymer and preparation and application methods thereof. The general formula of the novel conjugated fluorine-containing sulfimide single ion conductor polymer is shown as formula I. the preparation method of the novel conjugated fluorine-containing sulfimide single ion conductor polymer comprises dehalogenating and sulfonating perfluorochemicals and derivative monomers of the perfluorochemicals into perfluorochemical bisulfimides and derivatives of the perfluorochemical bisulfimides; then reacting the perfluorochemical bisulfimides and the derivatives with chlorine to graft chlorosulfonyl groups onto both ends of the perfluorochemical bisulfimides and the derivatives and then with sulfamides forpolycondensation under the action of acid-binding agent to obtain a perfuoropolymer containing a conjugated sulfimide structure. The prepared novel conjugated fluorine-containing sulfimide single ionconductor polymer is high in chemical stability and thermal stability, and with the unique conjugated sulfimide structure, achieves high ionic conductivity and high application values when applied tobattery materials such as lithium ion battery binders and solid and liquid eletrolytes. The preparation method of the novel conjugated fluorine-containing sulfimide single ion conductor polymer is fewin synthesizing processes, simple, low in cost and applicable to industrialized production.
Owner:宁波嘉玛材料科技有限公司
Sulfanilamide micromolecule surface modified polyamide composite membrane and preparation method thereof
ActiveCN112316752ACompensation process is cumbersomeCover costsMembranesSemi-permeable membranesPolymer scienceSulfanilamide
The invention provides a sulfanilamide micromolecule surface modified polyamide composite membrane and a preparation method thereof. The polyamide composite membrane comprises an ultra-filtration bottom membrane and a micromolecule modified polyamide layer, the polyamide layer covers the surface of the ultra-filtration bottom membrane, and functional groups of the small molecules are amino and sulfamido. According to the sulfanilamide micromolecule surface modified polyamide composite membrane, easily available functional micromolecule monomers with amino and sulfanilamide functional groups are grafted to the surface of the polyamide composite membrane through a secondary interface polymerization method to obtain a modified membrane, the hydrophilicity of the surface of the membrane is improved by utilizing the hydrophilicity of sulfanilamide groups, so that the mass transfer of water molecules is accelerated, the purposes of high flux and pollution resistance are achieved, and the water flux of the modified membrane can be increased by 38-65% compared with that of an unmodified polyamide membrane on the premise of keeping high rejection rate; and in addition, the existence of thesulfonamide groups provides more active N-chloro groups, so that the active chlorine resistance of the polyamide composite membrane is also improved.
Owner:TIANJIN POLYTECHNIC UNIV
Method for synthesizing benzene sulfonamide compounds
InactiveCN103819369AEfficient responseMild responseOrganic-compounds/hydrides/coordination-complexes catalystsSulfonic acid amide preparationBenzeneMethyl tertiary butyl ether
The invention relates to a method for preparing benzene sulfonamide compounds. In the method, a ternary catalyzing system of ethyltriphenylphosphonium bromide-silver compounds-porphyrin is adopted; the method for preparing N-tert-Butylbenzenesulfenamide from the reaction of methyl tertiary butyl ether with a weak reactivity and benzene sulfonamide compounds is realized; remarkably technical effects of preferable reaction temperature, high yield and good universality are achieved; moreover, as appropriate additives are added in the reaction, the collision between molecules is promoted and the reaction time is shortened; the method has favorable industrialization perspective and industrialized production value.
Owner:甘肃皓骏药业有限公司
Dihydrothiazolone compounds containing sulfamide and pharmaceutical compositions and use thereof
The invention provides dihydrothiazolone compounds containing sulfamide, represented by a formula (I), pharmaceutical compositions and use thereof. The compounds can be combined with proteins with bromodomain structural domains so as to adjust a downstream signal channel and exert a special function, and can be used for treating many diseases associated with bromodomain structural domains. The compounds can interfere combination of Brd4 with the bromodomain structural domain and an acetylized histone so as to down-regulate transcription of a cancer gene c-myc and associated target genes thereof, so that the compounds can become effective therapeutic drugs for treating tumors.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative, and pharmaceutical composition, preparation method and application thereof
ActiveCN111732575AGood antitumor pharmacological activityOrganic active ingredientsOrganic chemistryPhenylsulfonamidePharmaceutical medicine
The invention belongs to the technical field of biological medicine, and provides an N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative, and a pharmaceutical composition, a preparation methodand application thereof. The N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative is a compound with a structure of formula I, or a stereoisomer or pharmaceutically acceptable salt and isotope compound thereof. The structure of the formula I is shown in the specification; and in the formula I, R1 and R2 can form a substituted or unsubstituted piperidine or piperazine ring, and R3 representsa (1-methylpiperidine-4-yl)methyl group, a (2-oxopiperidine-4-yl)methyl group or a (4-oxocyclohexyl)methyl group. The N-(3-(pyrimidine-2-yl) phenyl) benzenesulfonamide derivative can be used as an effective c-Met inhibitor, and meanwhile, the pharmaceutical composition of the N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative has good multiple anti-tumor pharmacological activities.
Owner:北京鑫开元医药科技有限公司
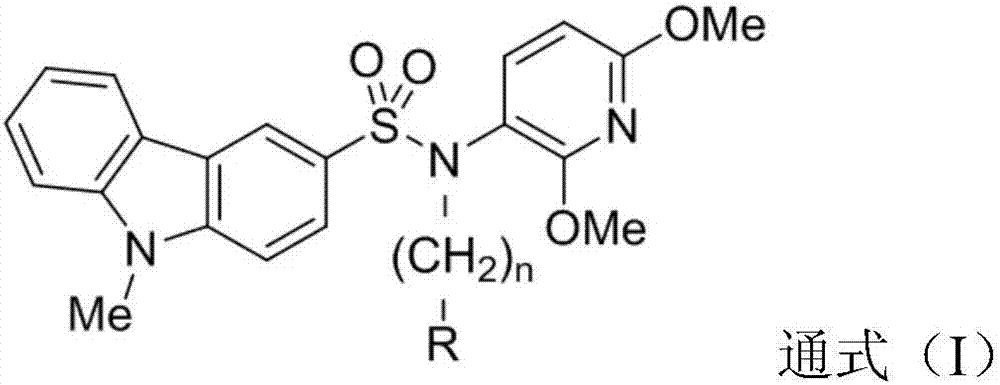
Carbazole sulfamide derivative or pharmaceutical slat thereof as well as preparation method and application thereof
ActiveCN107382967AHigh activityThe synthetic route is simpleOrganic active ingredientsGroup 5/15 element organic compoundsCarbazoleSide effect
The invention provides a carbazole sulfamide derivative or pharmaceutical slat thereof as well as a preparation method and an application thereof. The carbazole sulfamide derivative or the pharmaceutical slat thereof has the general formula (I). As a small-molecular tubulin inhibitor, the carbazole sulfamide derivative or the pharmaceutical slat thereof has an anti-tubulin function and significant anti-tumor activity, and meanwhile, the carbazole sulfamide derivative or pharmaceutical slat thereof is small in molecular weight, is simple to synthesize and has small toxic and side effects.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Application of benzene-sulfamide compounds in preparing anti-HIV-1(human immunodeficiency virus-1) drug
ActiveCN103893178AGood antiviral effectAntiviralsHeterocyclic compound active ingredientsPharmaceutical SubstancesMethyl palmoxirate
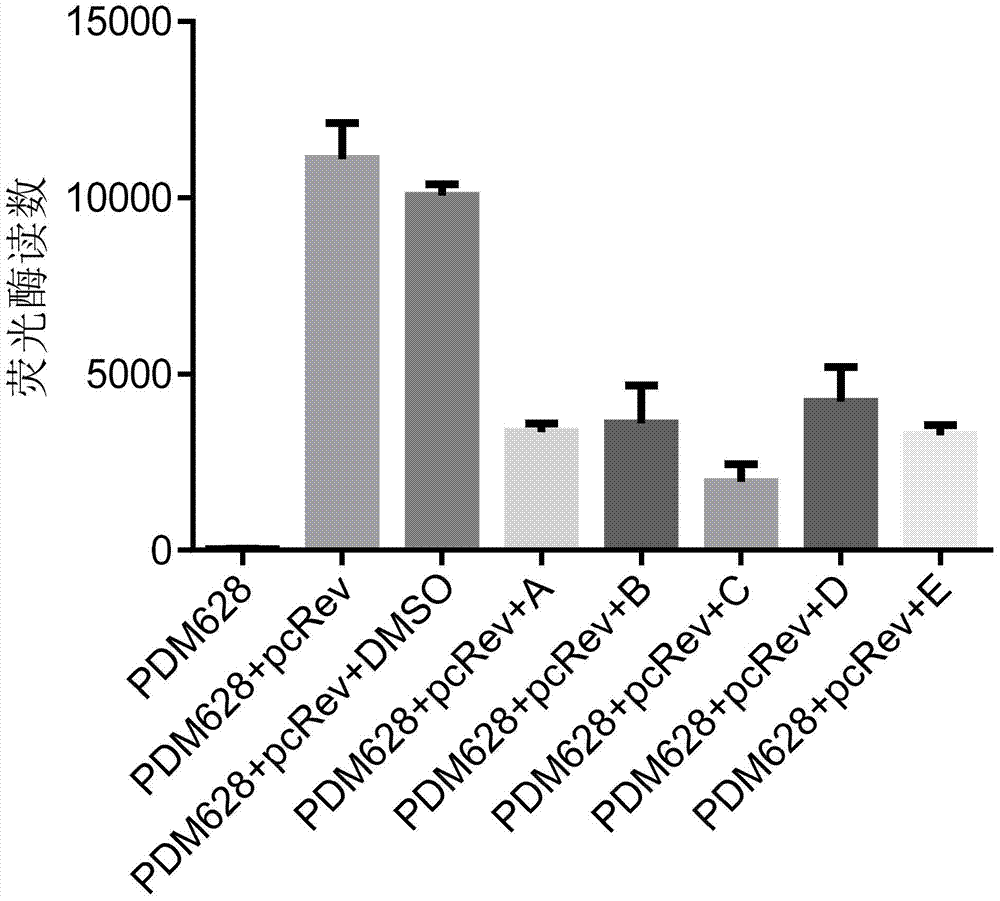
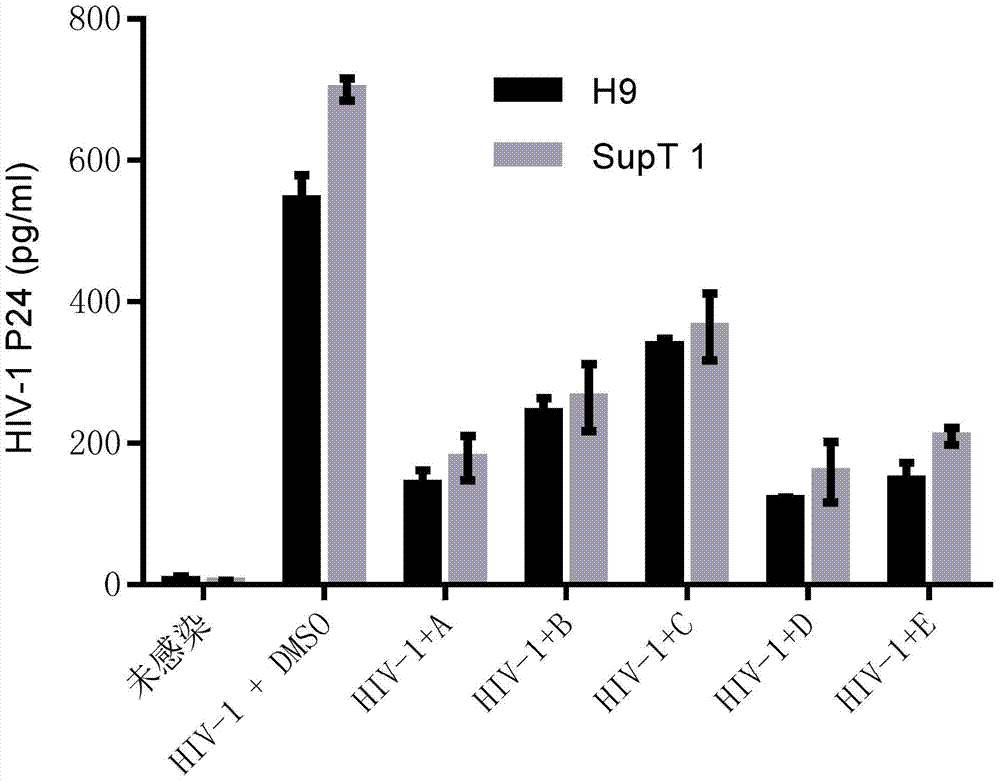
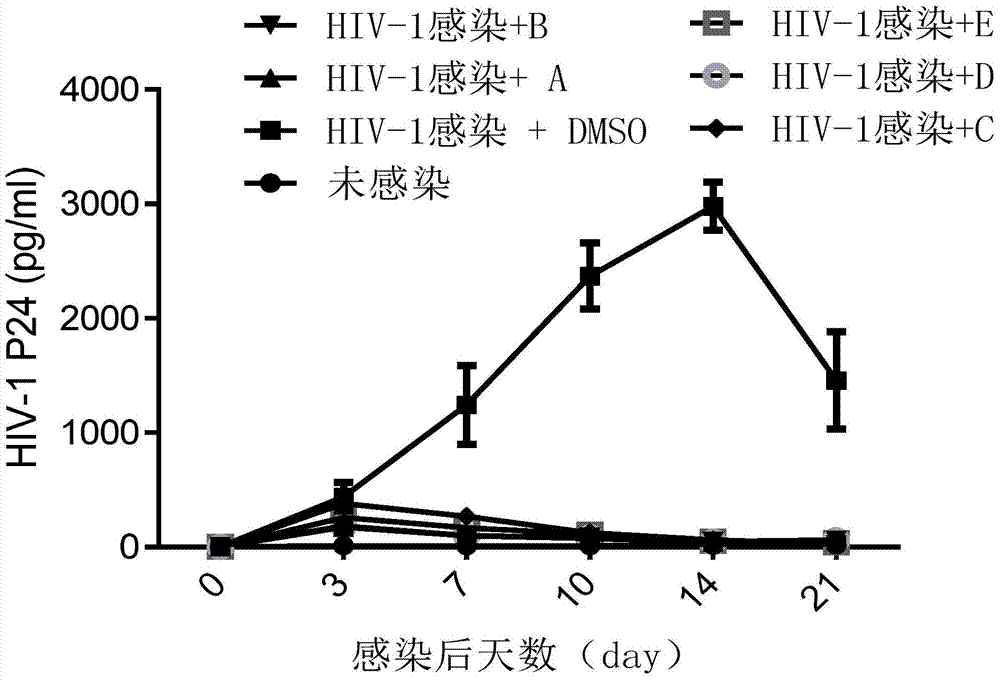
The invention discloses an application of benzene-sulfamide compounds in preparing an anti-HIV-1(human immunodeficiency virus-1) drug. A general formula of the benzene-sulfamide compounds is as shown in the specification, wherein X1-X4 are independently selected from halogen, H and -NO2, and have one -NO2 at the same time at most; X1-X4 are not halogen at the same time; R1 is selected from the substances as shown in the specification, wherein Y is C or N, n is an integer of 2-6, R3 is H or a statured chain hydrocarbon or a cyclic hydrocarbon with carbon number not exceeding 5, R4 is H or saturated chain hydrocarbons of C1-C4; R2 is selected from H, halogen or substance as shown in the specification; Y1 and Y2 are independently selected from H, halogen, and methyl. By applying the binding characteristic of Rev-RRE (response element), the inventor proves that the benzene-sulfamide compounds with the general formula can restrain Rev protein activity, causes expression quantity of luciferase in a screening system to reduce, and has a good antiviral effect. Moreover, powerful theoretical basis and practice basis are provided for further developing the antiviral drug, and therefore, the application has important development value and development significance.
Owner:SUN YAT SEN UNIV
Method for preparing pyrazole sulfamide type sodium, potassium and calcium metal complex by ultrasonic wave one-pot method and application
ActiveCN106866710AEasy to synthesizeHigh reaction yieldBiocideSodium organic compoundsOrganic synthesisPotassium
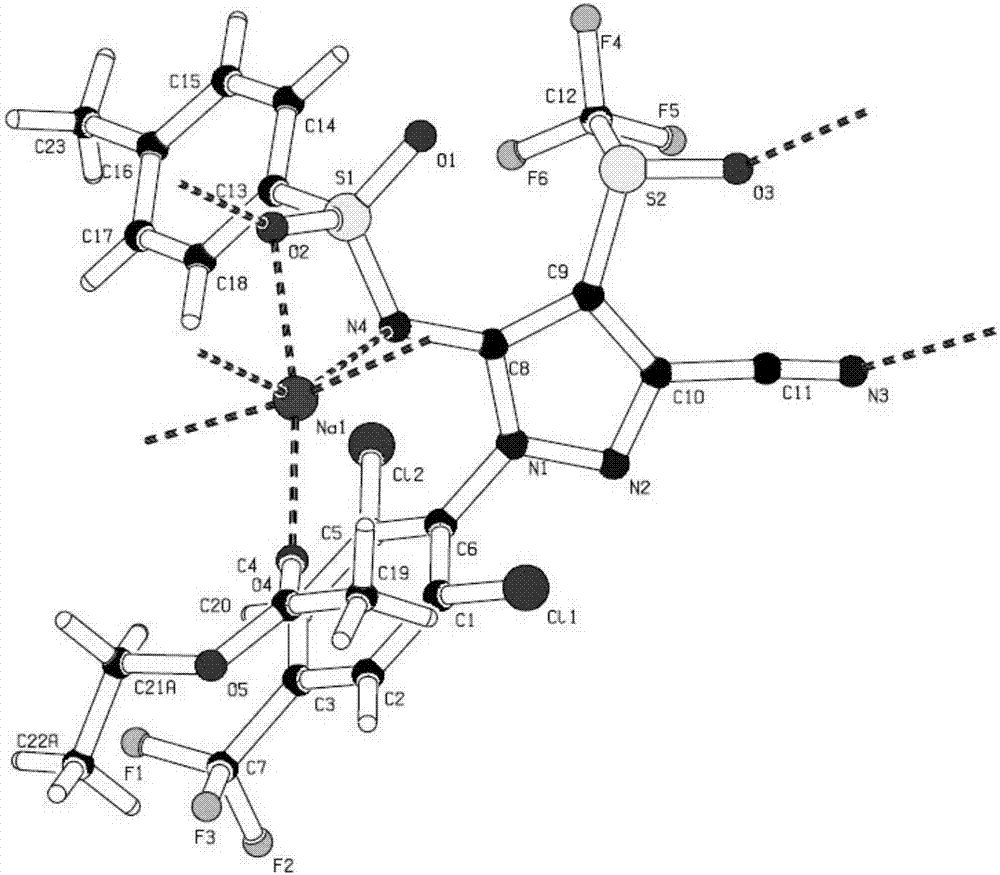
The invention belongs to the technical field of organic synthesis, and concretely discloses a method for preparing pyrazole sulfamide type sodium, potassium and calcium metal complexes by an ultrasonic wave one-pot method and application. Pyrazolone ring amidogen is subjected to structure modification and solvent participation; a series of pyrazole sulfamide type sodium, potassium and calcium metal complexes are synthesized by the ultrasonic wave one-pot method. The metal coordination modes of sodium, potassium, calcium and the like are diversified; the coordination with nitrogen atoms and oxygen atoms on sulfamide is realized; the coordination with oxygen atoms on a solvent can also be realized; metal on the potassium complex can also be coordinated with halogen atoms on substitutional groups; the synthetized sulfamide type metal complexes based on a pyrazole framework have excellent biological activity; particularly, high activity is shown in the insect killing and antibiosis aspects, so that the synthetized sulfamide type metal complexes based on the pyrazole framework have great research and development and application values.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
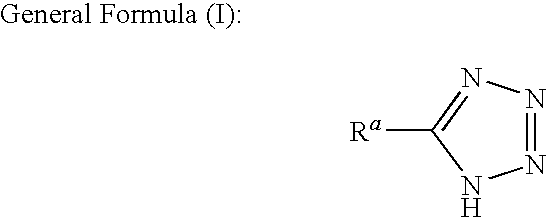
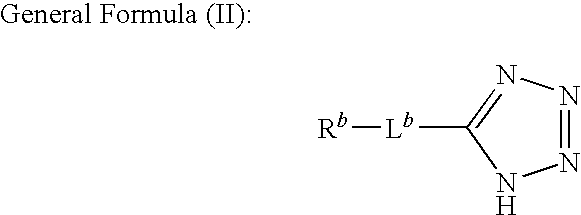
![Trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound and preparation method thereof Trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e716da64-c761-46e3-9894-0ae20f02892f/113110DEST_PATH_IMAGE008.PNG)
![Trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound and preparation method thereof Trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e716da64-c761-46e3-9894-0ae20f02892f/2011102521512100001DEST_PATH_IMAGE002.PNG)
![Trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound and preparation method thereof Trans-3a-fluoropyrrolidine[3,4-C]benzo cyclocompound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e716da64-c761-46e3-9894-0ae20f02892f/2011102521512100001DEST_PATH_IMAGE004.PNG)