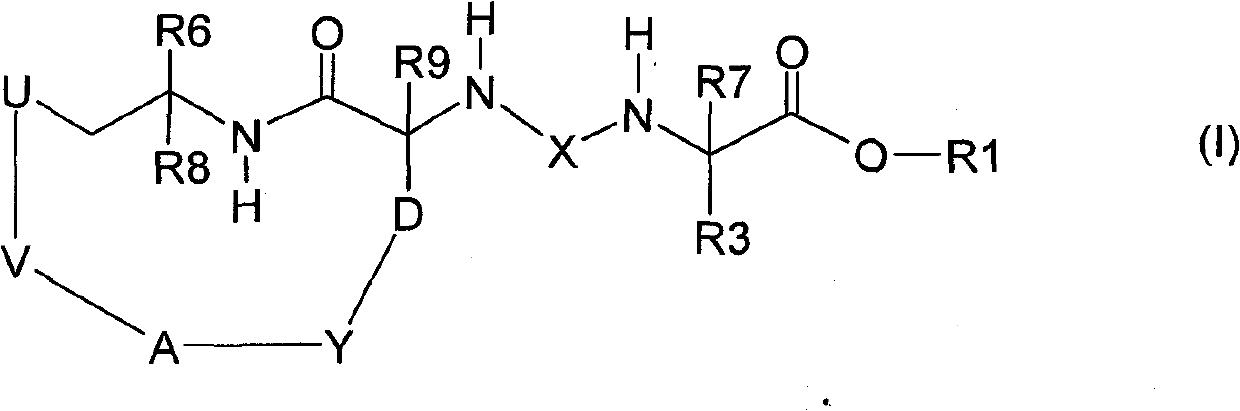
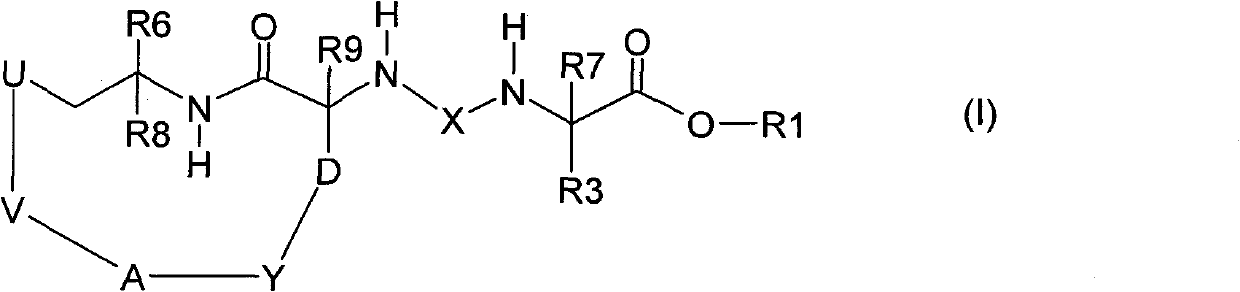
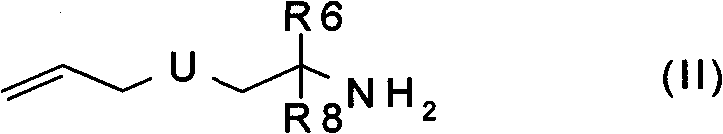Macrocyclic urea and sulfamide derivatives as inhibitors of TAFIA
A technology of heterocycles and compounds, applied in the field of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0307] (S)-6-amino-2-[3-((9S,12R)-9-isopropyl-11-oxo-2,7-dioxa-10-azabicyclo[12.2.2] Octadeca-1(17), 14(18), 15-trien-12-yl)ureido]hexanoic acid
[0308]
[0309] A. (S)-3-Methyl-2-{[1-phenylmethylene]amino}butan-1-ol
[0310] Add 2.64ml (2.78g, 26.16mmol) of benzaldehyde to a solution of 2.57g (24.91mmol) of L-valinol (L-valinol) in 28ml of toluene with stirring, and heat the mixture to reflux (using a water trap) for 1 hour . After cooling, it was concentrated and recrystallized from heptane. Suction filtration and drying under reduced pressure gave a colorless solid (3.74g).
[0311] 1 H-NMR (DMSO-d6, 400MHz) δ [ppm] = 8.23 (s, 1H), 7.77 (d, 2H), 7.45-7.40 (m, 3H), 4.49 (t, 1H), 3.68-3.31 (m , 1H), 3.48-3.40 (m, 1H), 2.96 (ddd, 1H), 1.94-1.81 (m, 1H), 0.88 (s, 5H).
[0312] B. (S)-1-allyloxymethyl-2-methylpropylamine
[0313] Add 1.25 g (60%, 31.36 mmol) of sodium hydride to 3.00 g (15.68 mmol) of (S)-3-methyl-2-{[1-phenylmethylene]amino}butan-1-ol in In 28 mL ...
Embodiment 2-1
[0335] (S)-6-amino-2-[3-((13S,16R)-13-isopropyl-15-oxo-2,11-dioxa-14-azabicyclo[16.2.2] Docos-1(21), 18(22), 19-trien-16-yl)ureido]hexanoic acid
[0336] A. (R)-1-(2-hept-6-enyloxyethyl)-2-methylpropylamine
[0337] Prepare 3.00 g (15.68 mmol) of (S)-3-methyl-2-{[1-phenylmethylene]amino}butan-1-ol (1-1A) in 28 ml dry THF under argon To the solution in , 1.50 g (60%, 37.51 mmol) of sodium hydride was added, and the mixture was stirred for 45 minutes. Add 2.83g (15.68mmol) of 7-bromohept-1-ene, then continue to stir overnight, carefully quench with 20ml of methanol, then add 300ml of 1N hydrochloric acid (pH=1), and stir at 40°C for 2h. The mixture was washed with dichloromethane, the aqueous phase was adjusted to pH 14 with 1N aqueous sodium hydroxide solution, and extracted 3 times with ethyl acetate. The combined organic phases were dried over sodium sulfate, filtered and concentrated. 0.91 g of crude product were obtained. The crude product was used in further reactions...
Embodiment 3-1
[0349] Example 3-1(S)-6-amino-2-[3-((E)-(9S,12R)-9-isopropyl-11-oxo-2,7-dioxa-10- Azabicyclo[12.2.2]octadec-1(17),4,14(18),15-tetraen-12-yl)ureido]hexanoic acid
[0350] The title compound was prepared in a manner analogous to Example 1-1, wherein the hydrogenation step was omitted and the final lyophilization with hydrochloric acid was not carried out, and was obtained directly as the trifluoroacetate salt. LC / MS (Method A): R t =0.89min, m / z: 491.2 [MH + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com