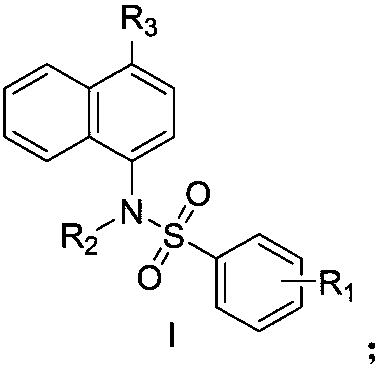
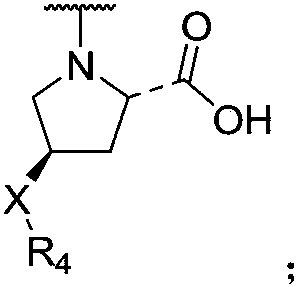
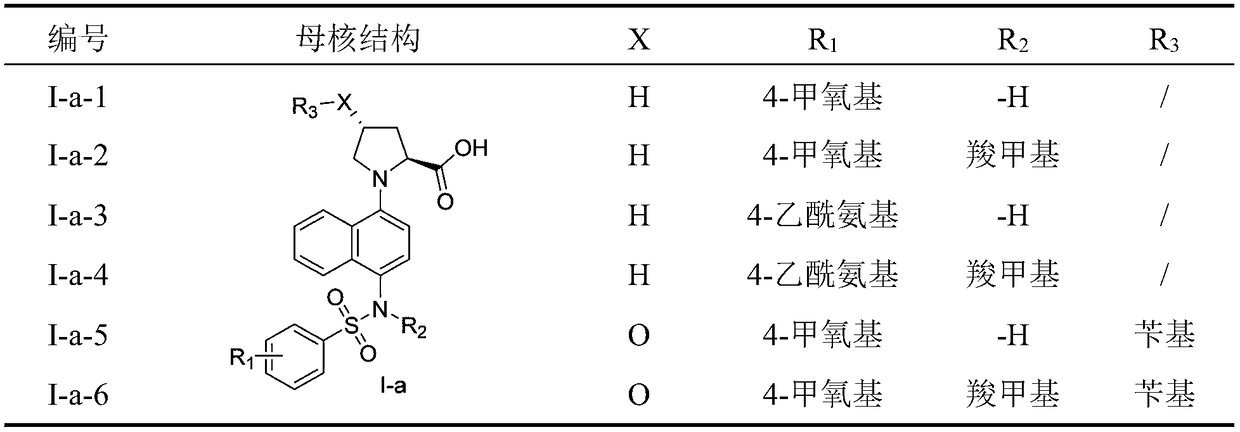
Naphthyl sulfamide amino acid derivative, preparation method and medical application thereof
A technology of naphthyl sulfonamide and amino acid, which is applied in the field of medicinal chemistry, can solve the problems that cannot, and rarely can, improve the etiology of inflammation, and achieve the effect of reducing inflammatory damage and improving the microenvironment of inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
[0055] (4-((4-methoxyphenyl)sulfonylamino)naphthalene-1-yl)-L-proline (I-a-1)
[0056] (1) 4-nitro-1-naphthol (1-1)
[0057] Dissolve 1-nitronaphthalene (8.5g, 49.1mmol) in 20.0mL DMSO (dimethyl sulfoxide), then dissolve potassium hydroxide (11.0g, 196.3mmol) in 10.0mL water, and add dropwise under ice-cooling In the reaction system, 10.0 mL of DMSO solution dissolved in tert-butanol peroxide (9.8 mL, 98.2 mmol) was added dropwise into the reaction system. After the dropwise addition, stir for about 10.0 min, remove the ice bath, and react at room temperature. React for 4h, add Na 2 S 2 o 3 (1.5g, 9.3mmol) was stirred for 1.0h, then 200.0mL of water was added, the pH was adjusted to 4 with dilute hydrochloric acid, extracted three times with 30mL of EA (ethyl acetate), the organic layers were combined, washed three times with saturated sodium chloride solution, Adjust the pH to 10 with 4M sodium hydroxide, wash the aqueous layer 3 times with EA, adjust the pH ...
Embodiment 2
[0067]
[0068] (4-((N-(carboxymethyl)-4-methoxyphenyl)sulfonylamino)naphthalene-1-yl)-L-proline (I-a-2)
[0069] The synthesis of 1-4 is the same as in Example 1
[0070] (1) (4-((4-methoxy-N-(2-methoxy-2-oxoethyl)phenyl)sulfonylamino)naphthalene-1-yl)-L-proline Methyl ester (2-1)
[0071]Compound (1-4) (200.0 mg, 454 μmol) was dissolved in 5.0 mL DMF, potassium carbonate (280.0 mg, 2.0 mmol) was added, and finally methyl bromoacetate (83.0 mg, 545 μmol) was added and stirred at room temperature. React for 4h, add water to the solution until the solid precipitates, extract three times with EA (30ml), combine the organic phases, and wash three times with saturated aqueous NACl solution (30ml), then wash the organic phase with anhydrous NaCl 2 SO 4 Drying, sand making, and column chromatography gave 190 mg of a light yellow oily substance with a yield of 82%. 1 H-NMR (300MHz, CDCl 3 )δ8.22 (d, J=8.8Hz, 1H), 8.08-7.94 (m, 1H), 7.69 (ddd, J=11.1, 8.8, 2.5Hz, 2H), 7.46 (s,...
Embodiment 3
[0075]
[0076] (4-((4-acetylaminophenyl)sulfonylamino)naphthalene-1-yl)-L-proline (I-a-3)
[0077] The synthesis of 1-3 is the same as in Example 1
[0078] (1) (4-((4-acetylaminophenyl)sulfonylamino)naphthalene-1-yl)-L-proline methyl ester (3-1)
[0079] The same synthesis method as compound (1-4), compound (1-3) (500mg, 1.67mmol) hydrogenolysis with 4-acetamidobenzenesulfonyl chloride (468mg, 2mmol), pyridine (404μL, 5.01mmol) as raw materials , 258 mg of white solid was obtained, the yield was 33%, m.p.109-112°C. 1 H-NMR (300MHz, C) δ8.17 (d, J = 7.8Hz, 1H), 7.84 (d, J = 7.8Hz, 1H), 7.62 (d, J = 8.5Hz, 2H), 7.56 (s, 1H), 7.48(d, J=8.6Hz, 2H), 7.39(s, 2H), 7.11(d, J=8.3Hz, 2H), 6.90(d, J=8.2Hz, 1H), 6.74(s, 1H), 4.47(s, 1H), 3.91(d, J=8.7Hz, 1H), 3.54(s, 3H), 3.15(s, 1H), 2.45(d, J=10.8Hz, 1H), 2.15( s,5H), 2.01(s,3H); EI-MS m / z: 468[M+H] + .
[0080] (2) (4-((4-acetylaminophenyl)sulfonylamino)naphthalene-1-yl)-L-proline (I-a-3)
[0081] With compound (I-a-1), wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com