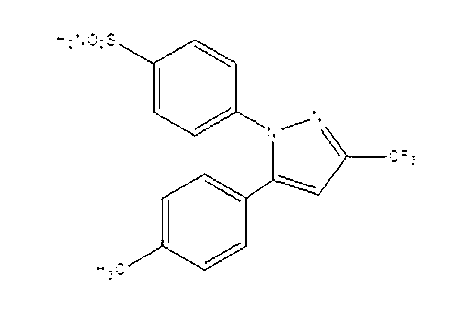
Preparation method for celecoxib
A technology of celecoxib and cyclization reaction, which is applied in the direction of organic chemistry, can solve the problems of poor quality, and achieve the effect of good quality, less waste, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Preparation of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione
[0015] Add 160 ml of methyl tert-butyl ether and 6.48 g (0.12 mol) of sodium methoxide to a 250 ml three-necked flask, and add 42.4 g (0.3 mol) of ethyl trifluoroacetate at a temperature of 25°C. Stir for 10 minutes. 13.4 g (0.3 mol) of p-methylacetophenone was slowly added dropwise, and the dropwise addition was completed in about 30 minutes. The temperature was raised to reflux for 12 hours and the reaction was completed. The solvent was distilled off under reduced pressure first under normal pressure to obtain a light yellow oily substance. Neutralize to PH4-5 with 10% hydrochloric acid. Extract three times with 20 ml of methyl tert-butyl ether, and combine the organic layers. The solvent was evaporated, and the crystals were left to stand to obtain 22.5 g of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione crystals, with a yield of 98.0%.
Embodiment 2
[0016] Example 2: Preparation of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione
[0017] Add 160 ml of methyl tert-butyl ether and 10.2 g (0.15 mol) of sodium ethoxide to a 250 ml three-necked flask, and add 42.4 g (0.3 mol) of ethyl trifluoroacetate at a temperature of 25°C. Stir for 10 minutes. 13.4 g (0.3 mol) of p-methylacetophenone was slowly added dropwise, and the dropwise addition was completed in about 30 minutes. The temperature was raised to reflux for 12 hours and the reaction was completed. The solvent was distilled off under reduced pressure first under normal pressure to obtain a light yellow oily substance. Neutralize to PH4-5 with 10% hydrochloric acid. Extract three times with 20 ml of methyl tert-butyl ether, and combine the organic layers. The solvent was evaporated, and the crystals were left to stand to obtain 22.8 g of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione crystals, with a yield of 99.1%.
Embodiment 3
[0018] Example 3: Preparation of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione
[0019] Add 160 ml of methyl ethyl ether and 15.36 g (0.16 mol) of sodium tert-butoxide to a 250 ml three-necked flask, and add 42.4 g (0.3 mol) of ethyl trifluoroacetate at a temperature of 25°C. Stir for 10 minutes. 13.4 g (0.3 mol) of p-methylacetophenone was slowly added dropwise, and the dropwise addition was completed in about 30 minutes. The temperature was raised to reflux for 12 hours and the reaction was completed. The solvent was distilled off under reduced pressure first under normal pressure to obtain a light yellow oily substance. Neutralize to PH4-5 with 10% hydrochloric acid. Extract three times with 20ml of methyl ethyl ether, and combine the organic layers. The solvent was evaporated, and the crystals were left to stand to obtain 22.7 g of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione crystals, with a yield of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com