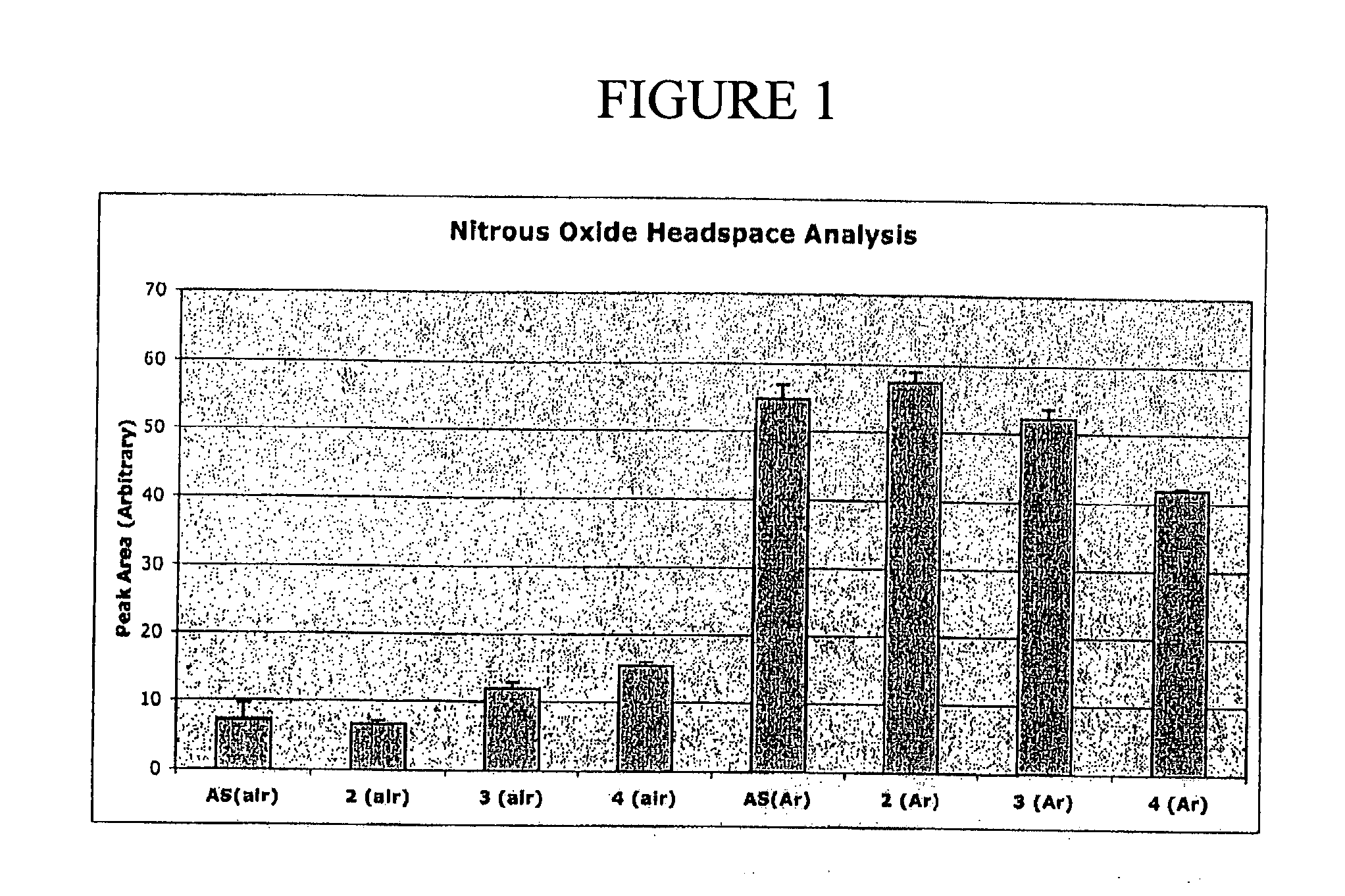
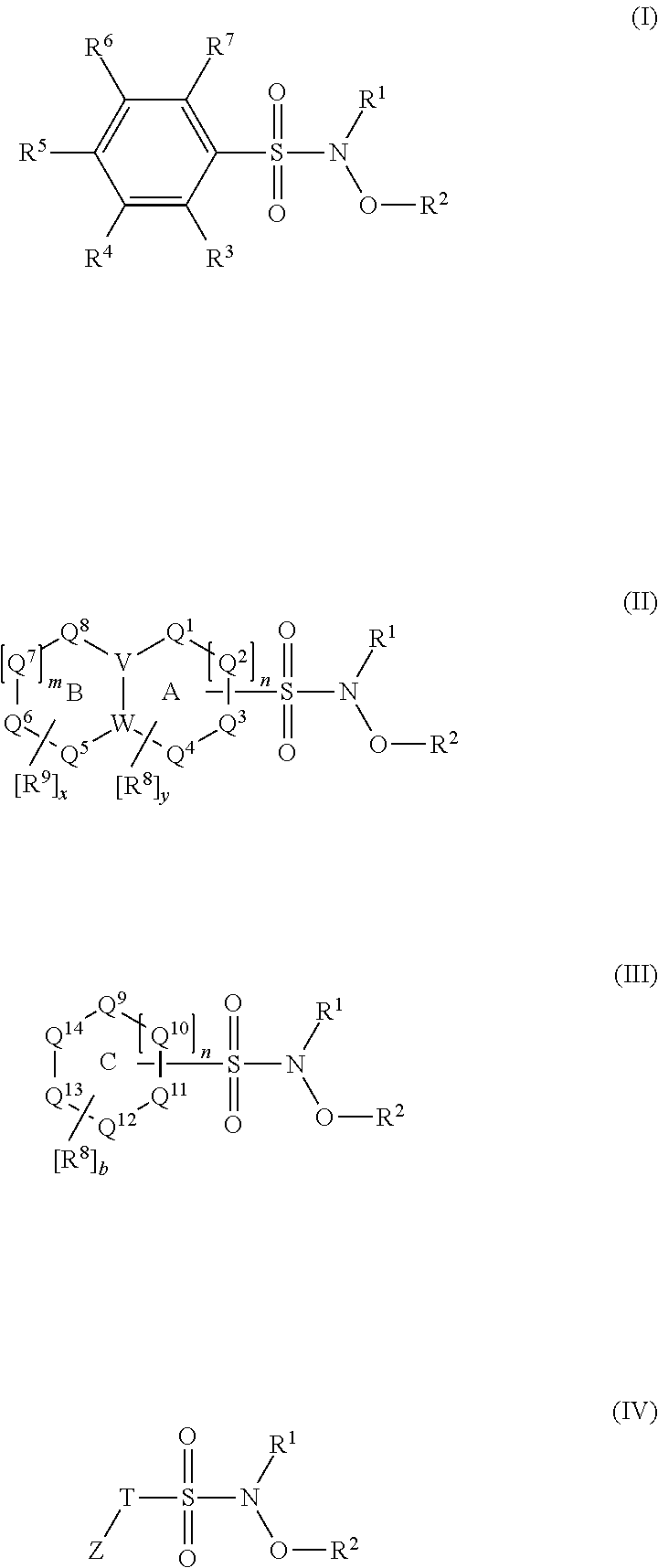
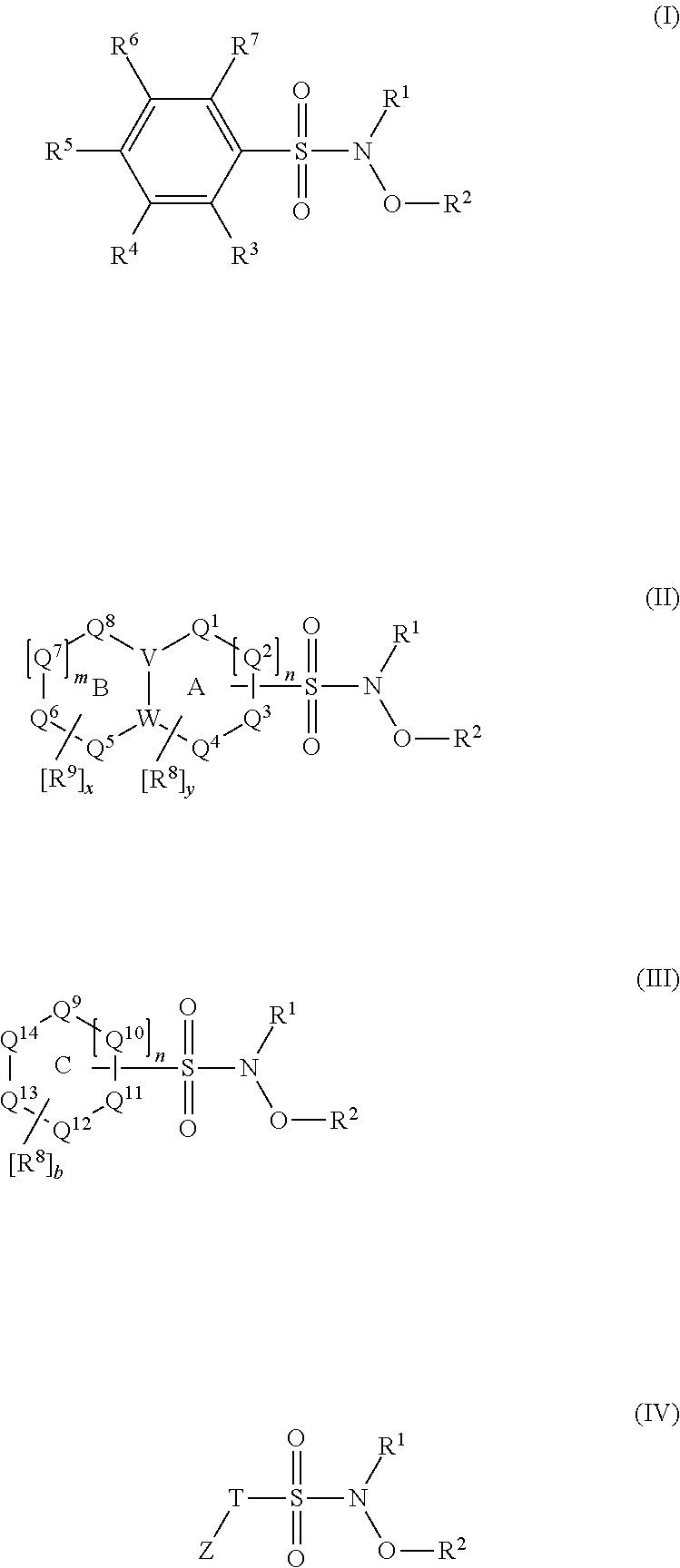
N-Hydroxylsulfonamide Derivatives as New Physiologically Useful Nitroxyl Donors
a technology of n-hydroxylsulfonamide and nitroxyl, which is applied in the field of n-hydroxylsulfonamide derivatives, can solve the problems of exercise intolerance, subsequent decline in heart function, and heart failure, and achieve the effects of increasing ischemia/reperfusion injury, controlling vascular tone, and protecting against myocardial ischemia/reperfusion injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compounds According to General Synthesis of Scheme A
[0116]The preparation of 2-bromo-N-hydroxy-benezene-sulfonamide is detailed below as a representative example of the synthetic method exemplified in Scheme A.
[0117]To a solution of hydroxylamine hydrochloride (0.82 g, 0.012 mol) in water (1.2 ml) at 0° C. was added a solution of potassium carbonate (1.6 g, 0.012 mol) in water (1.8 ml) dropwise maintaining an internal reaction temperature between 5° C. and 15° C. The reaction mixture was stirred for 15 minutes, whereupon THF (6 ml) and MeOH (1.5 ml) were added. 2-Bromobenzene sulfonyl chloride (1.51 g, 0.006 mol) was added portionwise maintaining a temperature below 15° C. and the reaction mixture was stirred at ambient temperature until complete consumption of the sulfonyl chloride was observed by TLC. The resulting suspension was concentrated to remove any volatiles and the aqueous suspension was extracted with diethyl ether (2×100 ml). The organic portion was dried...
example 2
Preparation of Compounds According to General Synthesis of Scheme B
[0131]The preparation of N-benzyloxy-2-bromo-benzenesulfonamide
is detailed below as a representative example of the synthetic method exemplified in Scheme B.
[0132]To a suspension of O-benzylhydroxylamine hydrochloride (3.75 g, 23.48 mmol) in MeOH (3 ml) and water (3.6 ml) was added a solution of potassium carbonate (3.24 g, 23.48 mmol) in water (3.6 ml), maintaining an internal reaction temperature below 10° C. The reaction mixture was stirred for 5 minutes, whereupon THF (12 ml) and 2-bromobenzene sulfonyl chloride (3 g, 11.74 mmol) were added. The reaction mixture was stirred at ambient temperature until complete consumption of the sulfonyl chloride was observed by TLC. The resulting suspension was concentrated in vacuo to remove any volatiles, and the aqueous suspension was extracted with diethyl ether (3×100 ml). The organic layer was dried over sodium sulfate, filtered and concentrated in vacuo to yield the crud...
example 3
Preparation of Compounds According to General Synthesis of Scheme C
[0137]The preparation of 4-Bromo-N-(tetrahydro-pyran-2-yloxy)-benzenesulfonamide
is detailed below as a representative example of the synthetic method exemplified in Scheme C.
[0138]To a solution of O-(tetrahydro-2H-pyran-2-yl)hydroxylamine (1.83 g, 15.65 mmol) in water (1.6 ml) at 0° C. was added a solution of potassium carbonate (1.1 g, 7.83 mmol) in water (2.4 ml) dropwise maintaining an internal reaction temperature below 10° C. After 15 minutes MeOH (2 ml) and THF (8 ml) were added was dropwise, followed by 4-bromobenzene sulfonyl chloride (2 g, 7.83 mmol) portionwise. The reaction mixture was stirred at ambient temperature until complete consumption of the sulfonyl chloride was observed by TLC. The resulting suspension was concentrated to remove any volatiles and the aqueous suspension was extracted with diethyl ether (3×100 ml). The organic portion was dried over sodium sulfate, filtered and concentrated in vacu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com