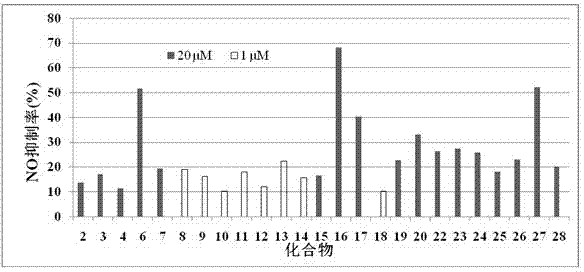
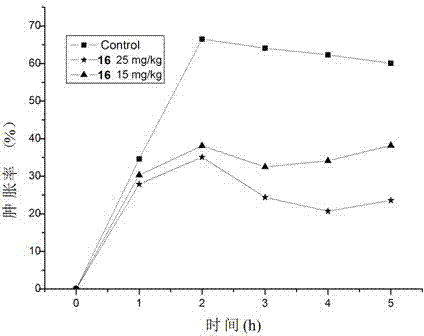
Sinomenine derivatives and their preparation methods and applications
A technology of sinomenine and its derivatives, which is applied in the field of macromolecular drug molecules, can solve problems such as structurally modified compounds not having ideal effects, and the mechanism of sinomenine is not completely clear, and achieve a wide range of applications, good curative effect, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0032] Example 14, 4'-Diethylene glycol-bisinomenine (compound 2).
[0033] The reaction formula of preparing 4,4'-diethylene glycol-bisinomenine is:
[0034]
[0035] According to the above reaction formula, compound 1 (329g, 1mmol) and diethylene glycol bis-p-toluenesulfonic acid (207mg, 0.5mmol) were dissolved in tetrahydrofuran (15mL), and then added acid-binding agent sodium hydroxide (60mg, 1.5mmol ). After reflux reaction 16h, thin-layer chromatography detects that reaction is complete, then removes reaction solvent with rotary evaporator, and gained oily matter is purified with silica gel column (eluent CH 2 Cl 2 / CH 3 OH / NH 4 OH, 100:9:1, v / v / v), the corresponding compound 2 was obtained with a yield of 63%.
[0036] Compound 2 was characterized, and the specific results were: 1 H NMR (300MHz, CDCl 3 )δ6.71(d, J=8.4Hz, 2H), 6.67(d, J=8.4Hz, 2H), 5.44(d, J=1.5Hz, 2H), 4.15-4.38(m, 6H), 3.97( t, J=4.8Hz, 4H), 3.75(s, 6H), 3.46(s, 6H), 3.16(t, J=4.2Hz, 2H), 2....
Embodiment 24
[0037] Example 24, 4'-ethylene glycol-bisinomenine (compound 3).
[0038] The reaction formula of preparing 4,4'-triethylene glycol-bisinomenine is:
[0039]
[0040] As in the above reaction formula, compound 3 was obtained by the preparation method of Example 1, and compound 3 was characterized, and the specific results were: 1 H NMR (300MHz, CDCl 3 )δ6.70(d, J=8.7Hz, 2H), 6.66(d, J=8.7Hz, 2H), 5.44(d, J=1.2Hz, 2H), 4.17-4.32(m, 6H), 3.77- 3.93(m, 8H), 3.74(s, 6H), 3.36(s, 6H), 3.17(br t, J=4.2Hz, 2H), 2.94-2.98(br, 2H), 2.73(dd, J=18.6 , 5.1Hz, 2H), 2.44-2.53(m, 4H), 2.40(s, 3H), 1.96-2.03(m, 4H), 1.86(td, J=12.9, 3.6Hz, 2H); 13 C NMR (75MHz, CDCl 3 )δ193.8, 152.4, 151.3, 147.6, 129.8, 129.7, 122.6, 115.0, 111.3, 70.9, 70.7, 70.5, 56.4, 55.6, 54.7, 49.6, 47.0, 45.7, 42.4, 40.7, 36.6, 24.5. MS m / z: 773 (M+H) + .
Embodiment 34
[0041] Example 34, 4'-tetraethylene glycol-bisinomenine (compound 4).
[0042] The reaction formula of preparing 4,4'-tetraethylene glycol-bisinomenine is:
[0043]
[0044] As in the above reaction formula, compound 4 was obtained by the preparation method of Example 1, and compound 4 was characterized, and the specific results were: 1 H NMR (300MHz, CDCl 3 )δ6.72(d, J=8.4Hz, 2H), 6.67(d, J=8.4Hz, 2H), 5.46(d, J=1.8Hz, 2H), 4.16-4.33(m, 6H), 3.67- 3.80(m, 12H), 3.75(s, overlapped, 6H), 3.47(s, 6H), 3.15(t, J=3.9Hz, 2H), 2.95-3.00(m, 4H), 2.72(dd, J= 18.3, 5.4Hz, 2H), 2.43-2.52(m, 4H), 2.41(s, 6H), 1.94-2.03(m, 6H), 1.85(td, J=13.2, 4.2, 2H); 13 C NMR (75MHz, CDCl 3 )δ 193.9, 152.3, 151.2, 147.4, 130.0, 129.9, 122.6, 115.3, 111.2, 70.8, 70.6, 70.5, 70.4, 56.3, 55.6, 54.6, 49.6, 46.9, 45.9, 42.5, 40.8, 36.8, 24.4 ESI-MS m / z: 817(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com