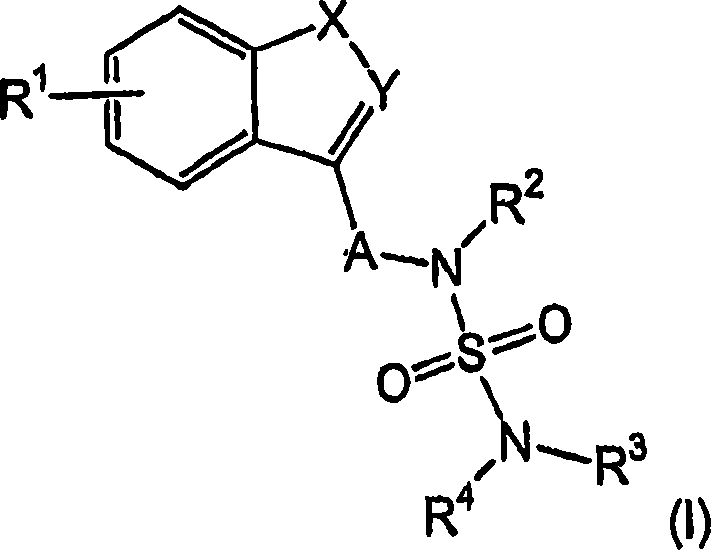
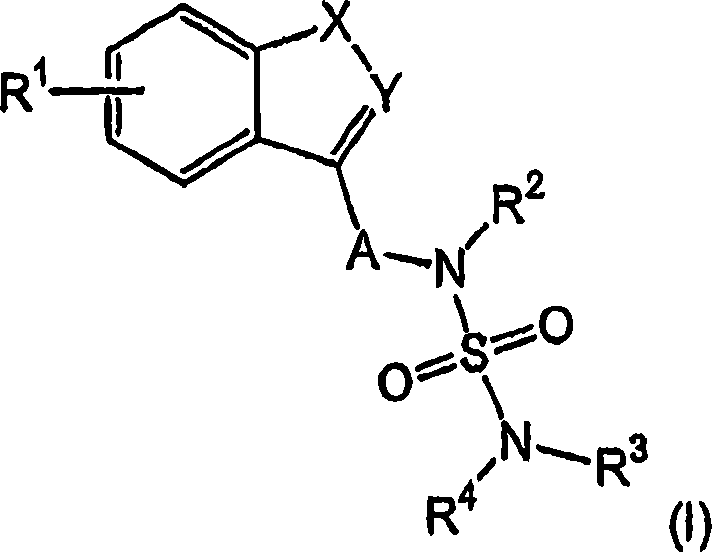
Novel benzo-fused heteroaryl sulfamide derivatives useful as anticonvulsant agents
A compound and a pharmaceutical technology, applied in the field of benzo-fused heteroaryl aminosulfonamide derivatives, can solve the problems of adverse side effects, weight loss, paresthesia and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0167] Where the preparation of the compounds of the invention results in mixtures of stereoisomers, these isomers may be separated by conventional techniques such as preparative chromatography. Compounds may be prepared in racemic form, or by enantiospecific synthesis or resolution as individual enantiomers. The compounds can be resolved into their individual enantiomers by standard techniques, such as first reacting with an optically active acid such as (-)-di-p-toluoyl-D-tartaric acid and / or (+)-di-p-toluoyl -L-tartaric acid) to form a diastereomer pair as a salt, followed by fractional crystallization and regeneration as the free base. Compounds can also be resolved by first forming the diastereoisomeric esters or amides, followed by chromatographic separation and removal of the chiral prosthetic group. Alternatively, the compounds can be resolved using a chiral HPLC column.
[0168] During any of the preparations of the compounds of the invention, it may be necessary to...
Embodiment 1
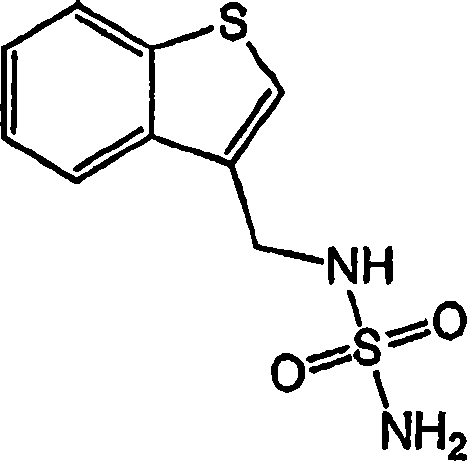
[0185] N-(Benzo[b]thiophen-3-ylmethyl)-sulfamic acid amide (compound #1)
[0186]
[0187] Thianthene-3-carbaldehyde (1.62 g, 10.0 mmol) was dissolved in absolute ethanol (50 ml). Sulfuramide (4.0 g, 42 mmol) was added and the mixture was heated to reflux for 16 hours. Cool the mixture to room temperature. Sodium borohydride (0.416 g, 11.0 mmol) was added, and the mixture was stirred at room temperature for 3 hours. The reaction was diluted with water (50ml) and extracted with chloroform (3 x 75ml). The extracts were concentrated and chromatographed (5% methanol / DCM) to afford the title compound (white solid).
[0188] 1 H NMR (DMSO-d 5 ): δ7.98 (1H, dd, J = 6.5, 2.3Hz), 7.92 (1H, dd, J = 6.6, 2.4Hz), 7.62 (1H, s), 7.36-7.45 (2H, m), 7.08 ( 1H, t, J=6.3Hz), 6.72(2H, s), 4.31(2H, d, J=6.3Hz).
Embodiment 2
[0190] N-[(5-Chlorobenzo[b]thiophen-3-yl)methyl]-sulfamic acid amide (compound #3)
[0191]
[0192] (5-Chloro-1-benzothiophen-3-yl)methanamine (0.820g, 4.15mmol) and sulfuramide (2.5g, 26mmol) were mixed in anhydrous dioxane (50ml), and the mixture was heated to Reflux for 4 hours. The reaction was cooled and diluted with water (50ml). The solution was extracted with chloroform (3 x 75ml). The extracts were concentrated and chromatographed (5% methanol / DCM) to afford the title compound (white solid).
[0193] 1 H NMR (DMSO-d 6 ): δ8.05(2H, m), 7.74(1H, s), 7.40(1H, d, J=6.5Hz), 7.07(1H, t, J=6.3Hz), 6.72(2H, s), 4.26 (2H, d, J=6.4Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com