N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative, and pharmaceutical composition, preparation method and application thereof
A technology for medicines and compounds is applied in the field of preparation of N-phenyl)benzenesulfonamide derivatives and pharmaceutical compositions to achieve the effect of good antitumor pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
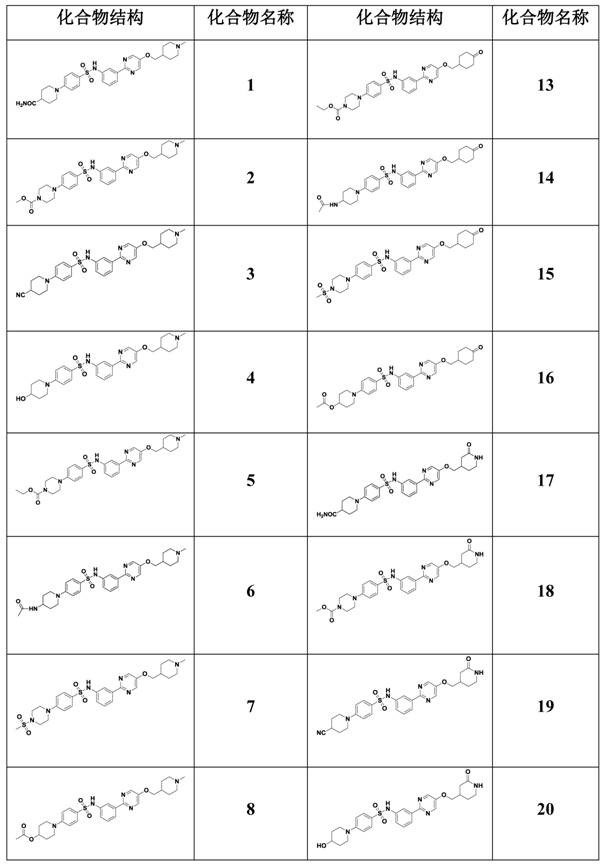
Image
Examples
Embodiment 1
[0056] 1-(4-(N-(3-(5-((1-methylpiperidin-4-yl)methoxy)pyrimidin-2-yl)phenyl)aminosulfonyl)phenyl)piperidine- 4-Formamide
[0057]
[0058] first step:
[0059] Compound 1a (57.4g, 200.0mmol), compound 1b (38.2g, 200.0mmol), K 2 CO 3 (30.4g, 220.0mmol) was dissolved in DMF (500ml), and reacted at 60°C for 8 hours, and the reaction was detected by thin layer chromatography (TLC). After the reaction was completed, part of the solvent was removed under reduced pressure, and water (500ml) was added. , was extracted twice with ethyl acetate (500ml), the organic layer was dried, concentrated and separated by chromatography to obtain 55.3g of compound 1c, with a yield of 69.4%. Compound 1c was an off-white solid.
[0060] Step two:
[0061] Compound 1c (39.8g, 100.0mmol) was dissolved in hydrogen chloride-ethyl acetate solution (500ml), stirred and reacted at 25°C for 8 hours, and detected by TLC. After the reaction was completed, the reaction was quenched with saturated sodium...
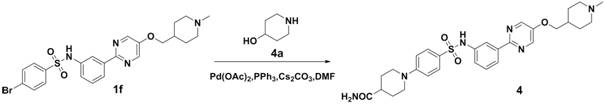
Embodiment 2
[0067] 4-(4-(N-(3-(5-((1-methylpiperidin-4-yl)methoxy)pyrimidin-2-yl)phenyl)aminosulfonyl)phenyl)piperazine- Methyl 1-formate
[0068]
[0069] Compound 1f was synthesized according to the method of the first step, the second step and the third step in Example 1.
[0070] Compound 1f (5.2g, 10.0mmol), compound 2a (1.4g, 10.0mmol), CsCO 3 (4.9g, 15.0mmol), PPh 3 (262.0mg, 1.0mmol), Pd(OAc) 2 (112 mg, 0.5 mmol) was dissolved in DMF (50 ml), heated to 100 ° C for 6 hours, after the reaction was completed, water (50 ml) was added to quench the reaction, ethyl acetate (100 ml) was extracted twice, the organic layer was dried, filtered, and column Chromatographic separation afforded 3.3 g of off-white solid, yield 56.8%, ESI(+) m / z=581.2.
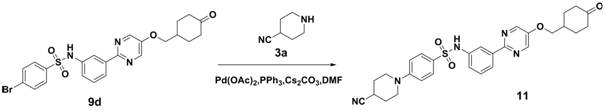
Embodiment 3
[0072] 4-(4-cyanopiperidin-1-yl)-N-(3-(5-((1-methylpiperidin-4-yl)methoxy)pyrimidin-2-yl)phenyl)amino Sulfonyl)phenyl)benzenesulfonamide
[0073]
[0074] Compound 1f was synthesized according to the method of the first step, the second step and the third step in Example 1.
[0075] Compound 1f (5.2g, 10.0mmol), compound 3a (1.1g, 10.0mmol), CsCO 3 (4.9g, 15.0mmol), PPh 3 (262.0mg, 1.0mmol), Pd(OAc) 2 (112 mg, 0.5 mmol) was dissolved in DMF (50 ml), heated to 100 ° C for 6 hours, after the reaction was completed, water (50 ml) was added to quench the reaction, ethyl acetate (100 ml) was extracted twice, the organic layer was dried, filtered, and column Chromatographic separation afforded 2.8 g of off-white solid, yield 51.3%, ESI(+) m / z=547.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com