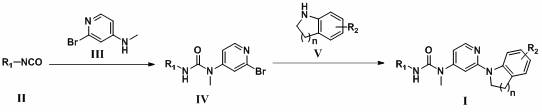
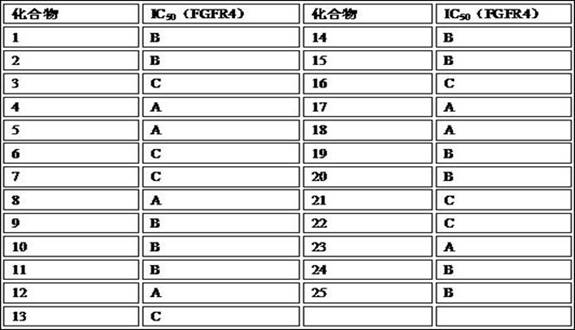
A kind of FGFR4 inhibitor, preparation method, pharmaceutical composition and application thereof
A technology of drugs and compounds, applied in the field of biomedicine, can solve the problems of inability to distinguish tumor cells, reduce adverse reaction rate, side effects, etc., and achieve the effect of improving therapeutic effect and good anti-tumor pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 3-(2,6-Dichlorophenyl)-1-(2-(6-methoxyindolin-1-yl)pyridin-4-yl)-1-methylurea
[0045]
[0046] Synthesis of compound 1c:
[0047] Dissolve compound 1a (37.4g, 200.0mmol), 1b (37.2g, 200.0mmol), potassium carbonate (41.4g, 300.0mmol) in acetonitrile (500mL), react at room temperature for 10 hours, monitor the reaction by TLC, and filter after the reaction , the filtrate was concentrated and separated by column chromatography to obtain 58.3 g of off-white (compound 1c), with a yield of 78.3%.
[0048] Synthesis of compound 1:
[0049] Compound 1c (3.7g, 10.0mmol), compound 1d (1.5g, 10.0mmol), cesium carbonate (6.5g, 20.0mmol), Pd 2 (dba) 3 (458mg, 0.5mmol), Xantphos (578mg, 1.0mmol) were dissolved in DMF (50mL), heated to 100°C and stirred for 6 hours, TLC monitored the reaction, after the reaction was completed, add water to quench the reaction, ethyl acetate (50mL each time) After two extractions, the organic layer was concentrated and separated by column chrom...
Embodiment 2
[0051] 3-(2,6-Dichlorophenyl)-1-(2-(6-fluoroindolin-1-yl)pyridin-4-yl)-1-methylurea
[0052]
[0053] The synthesis of compound 1c was carried out according to Example 1.
[0054] Synthesis of compound 2:
[0055] Compound 1c (3.7g, 10.0mmol), compound 2a (1.4g, 10.0mmol), cesium carbonate (6.5g, 20.0mmol), Pd 2 (dba) 3 (458mg, 0.5mmol), Xantphos (578mg, 1.0mmol) were dissolved in DMF (50mL), heated to 100°C and stirred for 6 hours, TLC monitored the reaction, after the reaction was completed, add water to quench the reaction, ethyl acetate (50mL each time) After two extractions, the organic layer was concentrated and separated by column chromatography to obtain 2.7 g of a light yellow solid (compound 2), with a yield of 62.8%. ESI(+) m / z=431.1.
Embodiment 3
[0057] 3-(2,6-Dichlorophenyl)-1-(2-(7-fluoroindolin-1-yl)pyridin-4-yl)-1-methylurea
[0058]
[0059] The synthesis of compound 1c was carried out according to Example 1.
[0060] Synthesis of compound 3:
[0061] Compound 1c (3.7g, 10.0mmol), compound 3a (1.4g, 10.0mmol), cesium carbonate (6.5g, 20.0mmol), Pd 2 (dba) 3 (458mg, 0.5mmol), Xantphos (578mg, 1.0mmol) were dissolved in DMF (50mL), heated to 100°C and stirred for 6 hours, TLC monitored the reaction, after the reaction was completed, add water to quench the reaction, ethyl acetate (50mL each time) After two extractions, the organic layer was concentrated and separated by column chromatography to obtain 2.0 g of a light yellow solid (compound 3), with a yield of 46.5%. ESI(+) m / z=431.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com