A kind of 4-pyrimidinecarboxamide compound, pharmaceutical composition, preparation method and application
A compound and pharmaceutical technology, applied in the field of biomedicine, can solve problems such as reducing the rate of adverse reactions, inability to distinguish tumor cells, and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
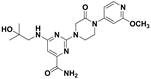
[0045] 6-((2-Hydroxy-2-methylpropyl)amino)-2-(3-oxo-4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)pyrimidine-4 -Formamide
[0046]
[0047] first step:
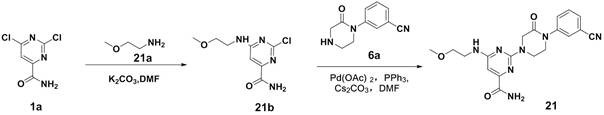
[0048] Compound 1a (38.2g, 200.0mmol), compound 1b (17.8g, 200.0mmol), K 2 CO 3 (30.4g, 220.0mmol) was dissolved in DMF (400ml), and reacted at 60°C for 8 hours, and the reaction was detected by thin layer chromatography (Thin Layer Chromatography, abbreviated as TLC). After the reaction was completed, water (300ml) was added, and ethyl acetate ( 300ml) extracted twice, the organic layer was dried, concentrated and separated by chromatography to obtain 40.3g of compound 1c with a yield of 82.6%. Compound 1c was an off-white solid.
[0049] Step two:
[0050] Compound 1c (2.4g, 10.0mmol), compound 1d (2.4g, 10.0mmol), palladium acetate (122mg, 0.5mmol), triphenylphosphine (262mg, 1.0mmol), cesium carbonate (3.9g, 12.0mmol) Dissolve in DMF (50ml), then raise the temperature to 100°C and stir for 8 hours, TLC detects the r...
Embodiment 2
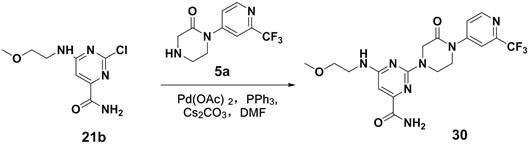
[0052] 2-(4-(3-aminocarbonylphenyl)-3-oxopiperazin-1-yl)-6-((2-hydroxy-2-methylpropyl)amino)pyrimidine-4-carboxamide
[0053]
[0054] Compound 1c was synthesized according to the method in the first step of Example 1.
[0055] Compound 1c (2.4g, 10.0mmol), compound 2a (2.2g, 10.0mmol), palladium acetate (122mg, 0.5mmol), triphenylphosphine (262mg, 1.0mmol), cesium carbonate (3.9g, 12.0mmol) Dissolve in DMF (50ml), then raise the temperature to 100°C and stir for 8 hours, TLC detects the reaction, after the reaction is completed, quench the reaction with water (40ml), then extract twice with ethyl acetate (50ml), and obtain by organic column chromatography 2.3 g of light yellow solid, yield 53.9%, ESI(+) m / z=428.2.
Embodiment 3
[0057] 6-((2-Hydroxy-2-methylpropyl)amino)-2-(3-oxo-4-(3-(trifluoromethoxy)phenyl)piperazin-1-yl)pyrimidine- 4-Formamide
[0058]
[0059] Compound 1c was synthesized according to the method in the first step of Example 1.
[0060] Compound 1c (2.4g, 10.0mmol), compound 3a (2.6g, 10.0mmol), palladium acetate (122mg, 0.5mmol), triphenylphosphine (262mg, 1.0mmol), cesium carbonate (3.9g, 12.0mmol) Dissolve in DMF (50ml), then raise the temperature to 100°C and stir for 8 hours, TLC detects the reaction, after the reaction is completed, quench the reaction with water (40ml), then extract twice with ethyl acetate (50ml), and obtain by organic column chromatography 3.0 g of light yellow solid, yield 64.2%, ESI(+) m / z=469.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com