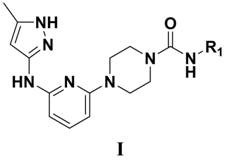
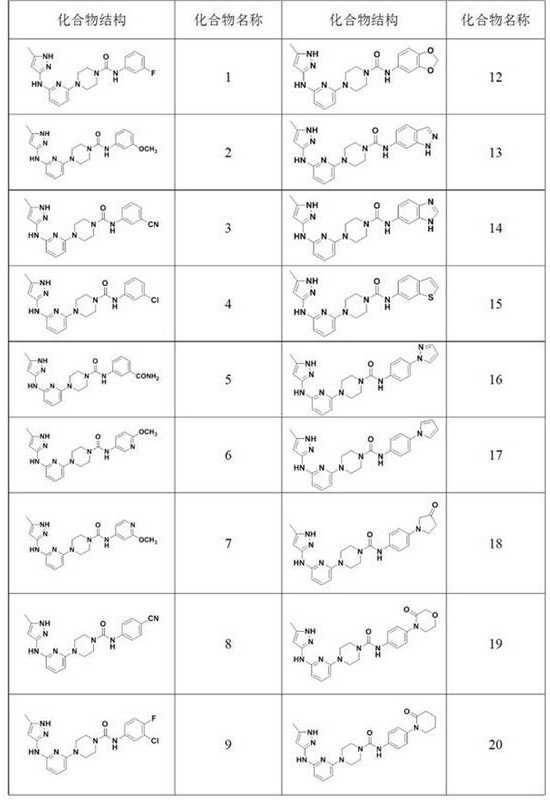
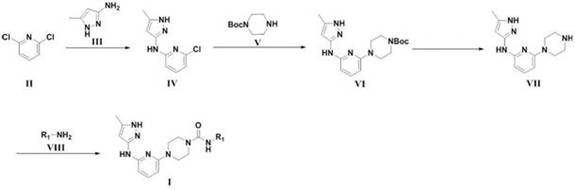
N-(5-methyl-1H-pyrazol-3-yl) pyridine-2-amine compound and preparation method thereof
A compound and hydrate technology, applied in organic chemistry, drug combination, pharmaceutical formula, etc., can solve the problems of cancer and other problems, and achieve the effect of low requirements for production equipment, simple operation and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] N-(3-fluorophenyl)-4-(6-((5-methyl-1H-pyrazol-3-yl)amino)pyridin-2-yl)piperazine-1-carboxamide
[0092]
[0093] first step:
[0094] Compound 1a (29.4g, 200.0mmol), compound 1b (19.4g, 200.0mmol), cesium carbonate (77.8g, 240.0mmol) were dissolved in N,N-dimethylformamide (DMF) (300mL), and the temperature was raised Stir at 80°C for 12 hours, monitor the reaction with TLC, cool down to room temperature after the reaction, add 300mL of water to quench the reaction, add ethyl acetate (400mLx2) for two extractions, combine the organic layers, dry, concentrate, and separate by column chromatography 28.5 g of compound 1c was obtained with a yield of 68.5%. Compound 1c is an off-white solid.
[0095] Step two:
[0096] Compound 1c (28.0g, 134.6mmol), compound 1d (30.1g, 161.5mmol), Pd(dppf)Cl 2 (5.8g, 8mmol), K 2 CO 3 (27.9g, 201.9mmol) was placed in DMF (200mL), heated to 100°C, stirred for 6 hours, monitored by TLC, cooled to room temperature after the reaction, ...
Embodiment 2
[0102] N-(3-methoxyphenyl)-4-(6-((5-methyl-1H-pyrazol-3-yl)amino)pyridin-2-yl)piperazine-1-carboxamide
[0103]
[0104] Compound 1f was synthesized according to the method of the first step, the second step and the third step in Example 1.
[0105] Dissolve compound 1f (258mg, 1.0mmol), compound 2a (123mg, 1.0mmol), triethylamine (303mg, 3.0mmol), CDI (162mg, 1.0mmol) in dichloromethane (30mL), stir at room temperature overnight , The reaction was monitored by TLC. After the reaction was completed, it was directly concentrated and separated by column chromatography to obtain 298 mg of an off-white solid (compound 2), with a yield of 73.2%, and ESI (+) m / z=408.2.
Embodiment 3
[0107] N-(3-cyanophenyl)-4-(6-((5-methyl-1H-pyrazol-3-yl)amino)pyridin-2-yl)piperazine-1-carboxamide
[0108]
[0109] Compound 1f was synthesized according to the method of the first step, the second step and the third step in Example 1.
[0110] Compound 1f (258mg, 1.0mmol), compound 3a (118mg, 1.0mmol), triethylamine (303mg, 3.0mmol), CDI (162mg, 1.0mmol) were dissolved in dichloromethane (30mL), stirred at room temperature overnight , The reaction was monitored by TLC. After the reaction was completed, it was directly concentrated and separated by column chromatography to obtain 254 mg of an off-white solid (compound 3), with a yield of 63.2% and ESI (+) m / z=403.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com