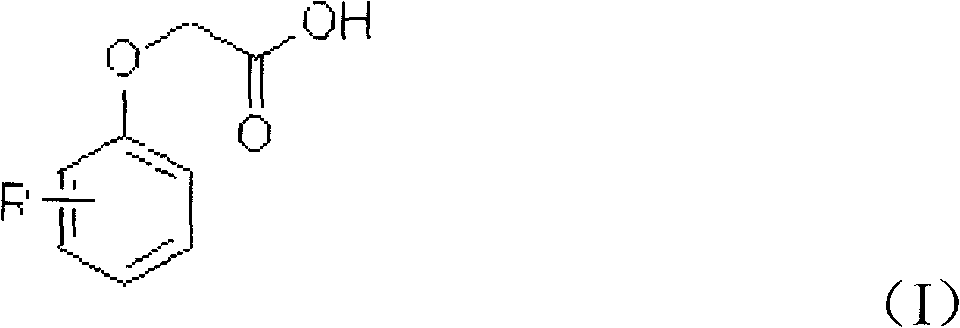
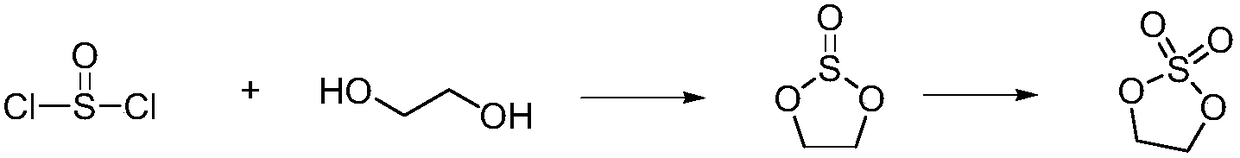
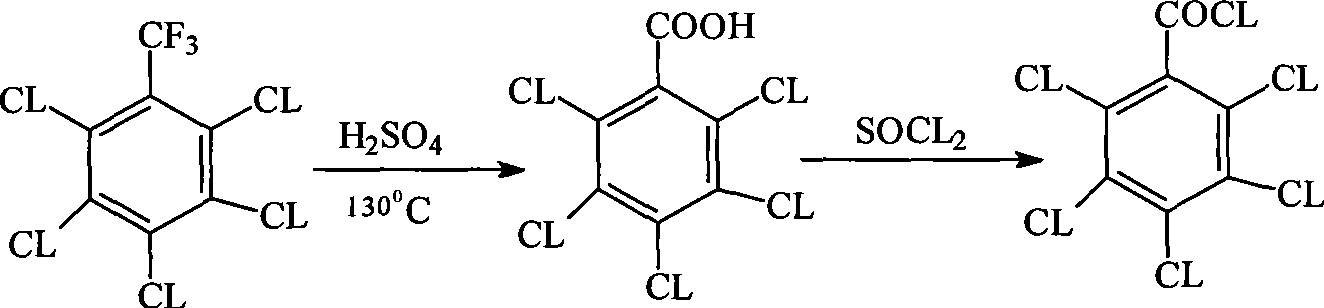
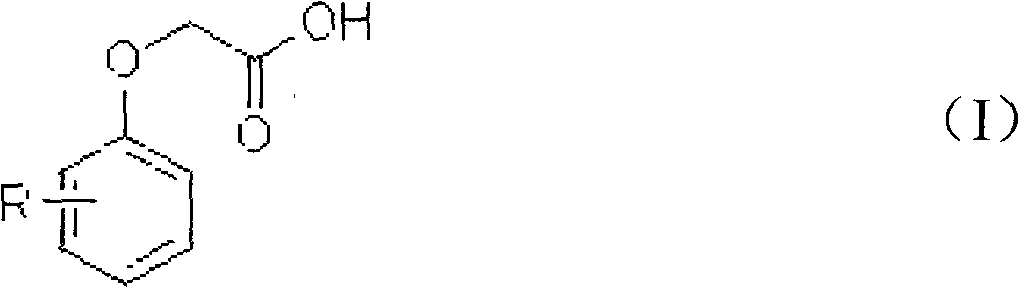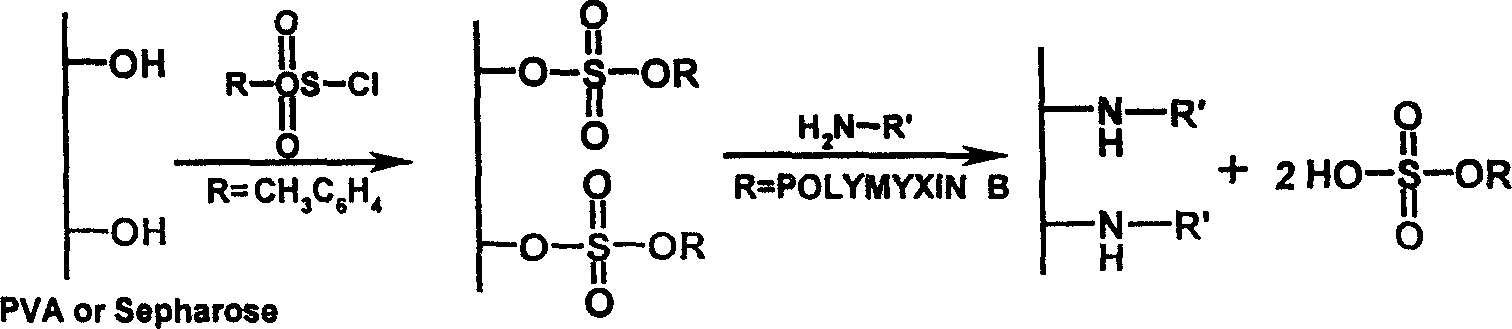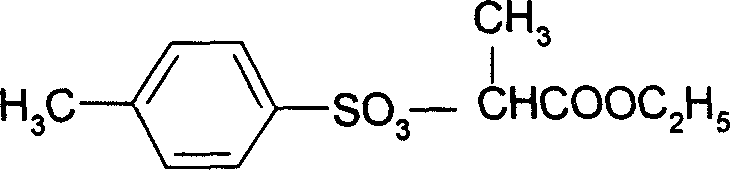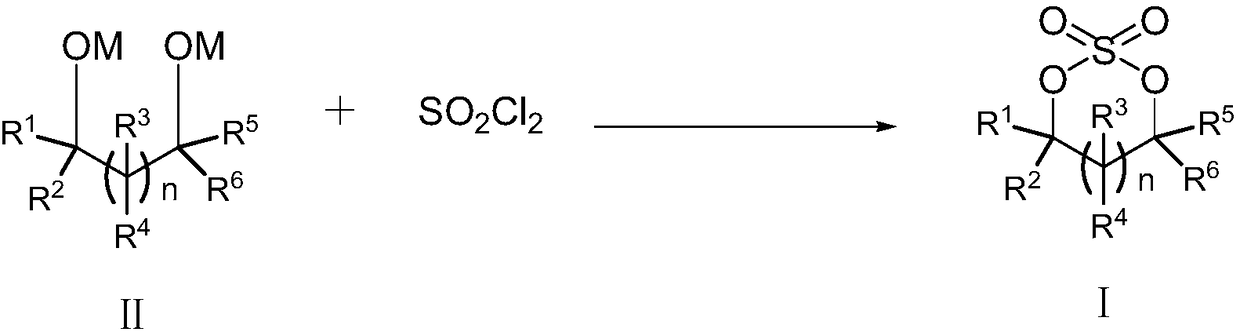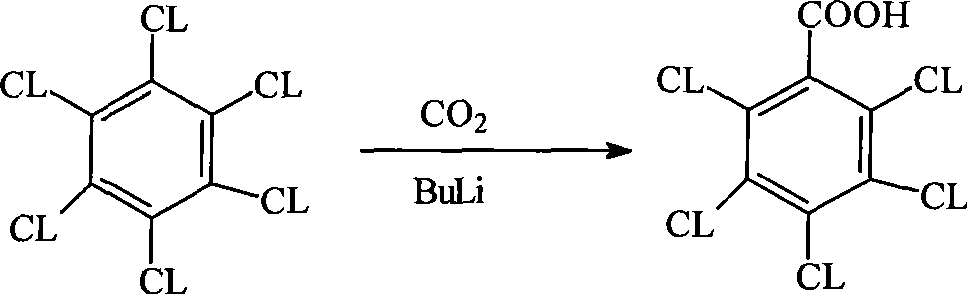Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
213 results about "Sulfuryl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sulfuryl chloride is an inorganic compound with the formula SO₂Cl₂. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature, as can be inferred from its rapid hydrolysis.
Chloration method for phenoxyacetic acid and derivatives thereof
InactiveCN102336654AEasy to industrializeOrganic compound preparationCarboxylic compound preparationChlorobenzeneIndustrial waste water
The invention provides a chloration method for phenoxyacetic acid and derivatives thereof, which comprises the following steps that: raw materials of the phenoxyacetic acid or the derivatives of the phenoxyacetic acid and chlorinating agents take reaction at a certain temperature in organic solvents under the effect of catalysts, wherein the chlorinating agents can be chlorine gas, sodium hypochlorite, calcium hypochlorite and sulfuryl chloride, the catalysts can be lewis acid and sulfurated substances, and solvents can be dichloromethane, dichloroethane, trichloromethane, carbon tetrachloride, formic acid, ethyl acetate, benzene, toluene, dimethylbenzene, chlorobenzene and o-dichlorobenzene. The method has the advantages that the operation can be carried out under the waterless condition, the generation of a large amount of industrial waste water is avoided, the used catalysts are safe and are easy to obtain, the solvents can be cyclically used, and the industrialization is easy.
Owner:DALIAN RES & DESIGN INST OF CHEM IND
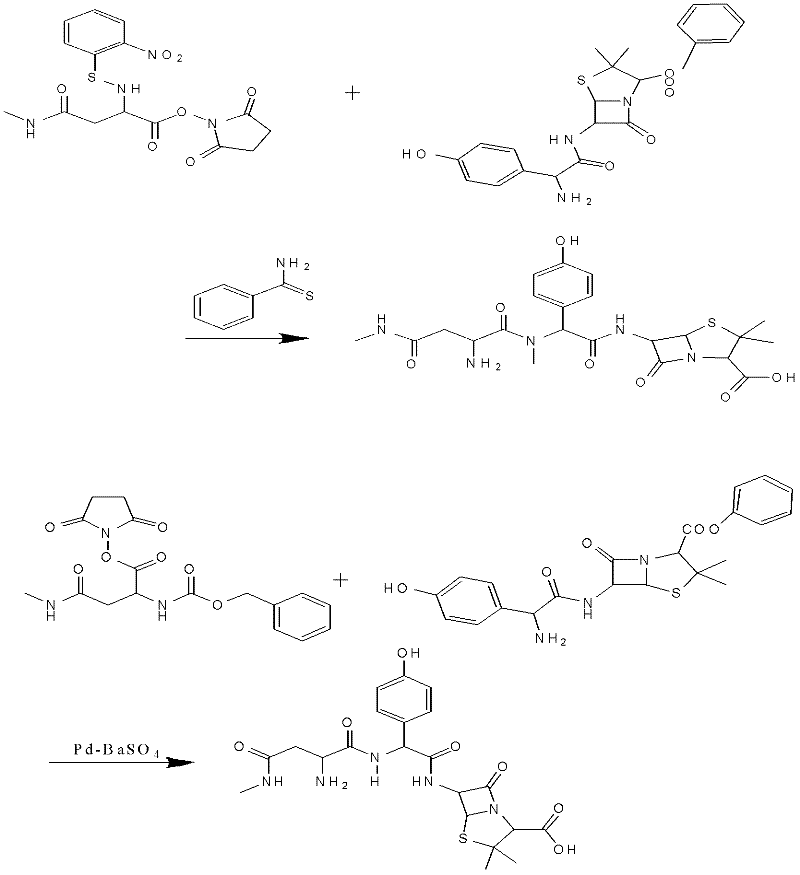
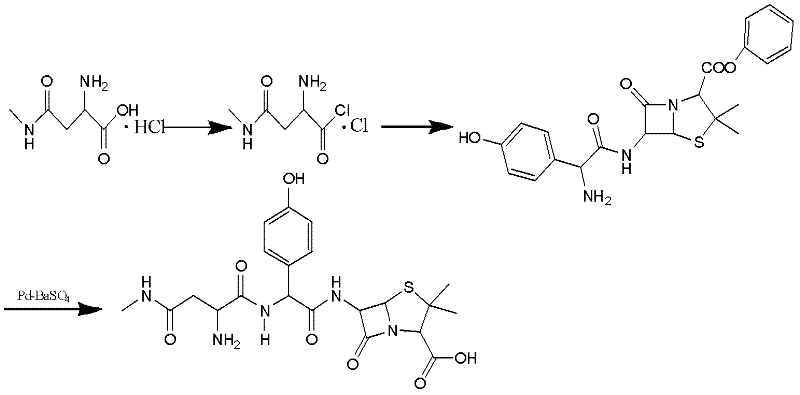
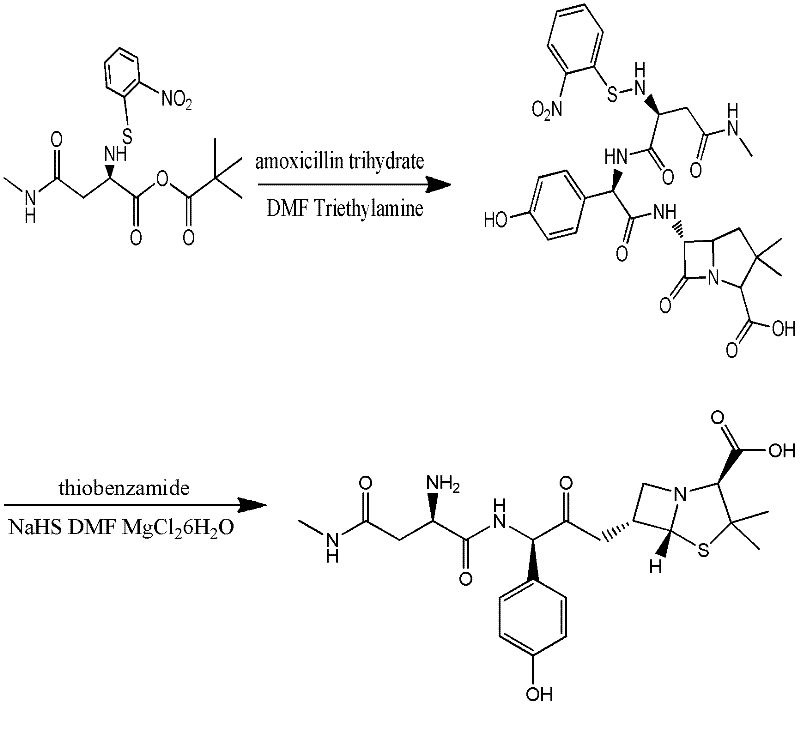
Preparation method for Aspoxicillin
The invention discloses a preparation method for Aspoxicillin. D-aspartic acid is added into a mixture liquid of sulfuryl chloride and carbinol under a low temperature of zero to prepare D-aspartic acid methyl ester hydrochloride; the obtained D-aspartic acid methyl ester hydrochloride and triethylamine are reacted in ethanol to obtain D-aspartic acid methyl ester educt; the D-aspartic acid methyl ester educt and methylamine aqueous liquid with a concentration of 40 percent are reacted in a room temperature to prepare aspartic formamide; the aspartic formamide, ethyl acetoacetate and potassium hydroxide are reacted in isopropanol to prepare diene salt (D-2-amino-3N-methylamino oxo-propionic acid diene formamide); the diene salt and pivaloyl chloride are reacted in acetone under the catalysis of pyridine to obtain active anhydride; then the active anhydride is condensed and further protected by deacidification to obtain a target product-crude product of Aspoxicillin. The preparation method for Aspoxicillin has the advantages of cheap and easily-obtained reagent, lower toxicity and lower environment pressure, stable and simple technological operation and high yield.
Owner:SOUTHWEST JIAOTONG UNIV
Process for the production of chlorinated alkanes
InactiveUS9067855B2Easy to transportEasy to utilizePreparation by halogen additionAlkaneRegioselectivity
Processes for the production of chlorinated alkanes are provided. The present processes comprise reacting one or more mono- and / or dichloroalkanes to form tri-, tetra- and / or pentachloroalkanes, with high regioselectivity. In those embodiments wherein a dichloroalkane is desirably utilized, it may advantageously be a vicinal dichloroalkane. Further, only one catalyst is utilized. The present processes make use of sulfuryl chloride as a chlorinating agent, rather than a gaseous chlorinating agent such as chlorine gas. Finally, the process uses lower intensity process conditions than at least some conventional processes, and thus, operating costs are saved.
Owner:BLUE CUBE IP
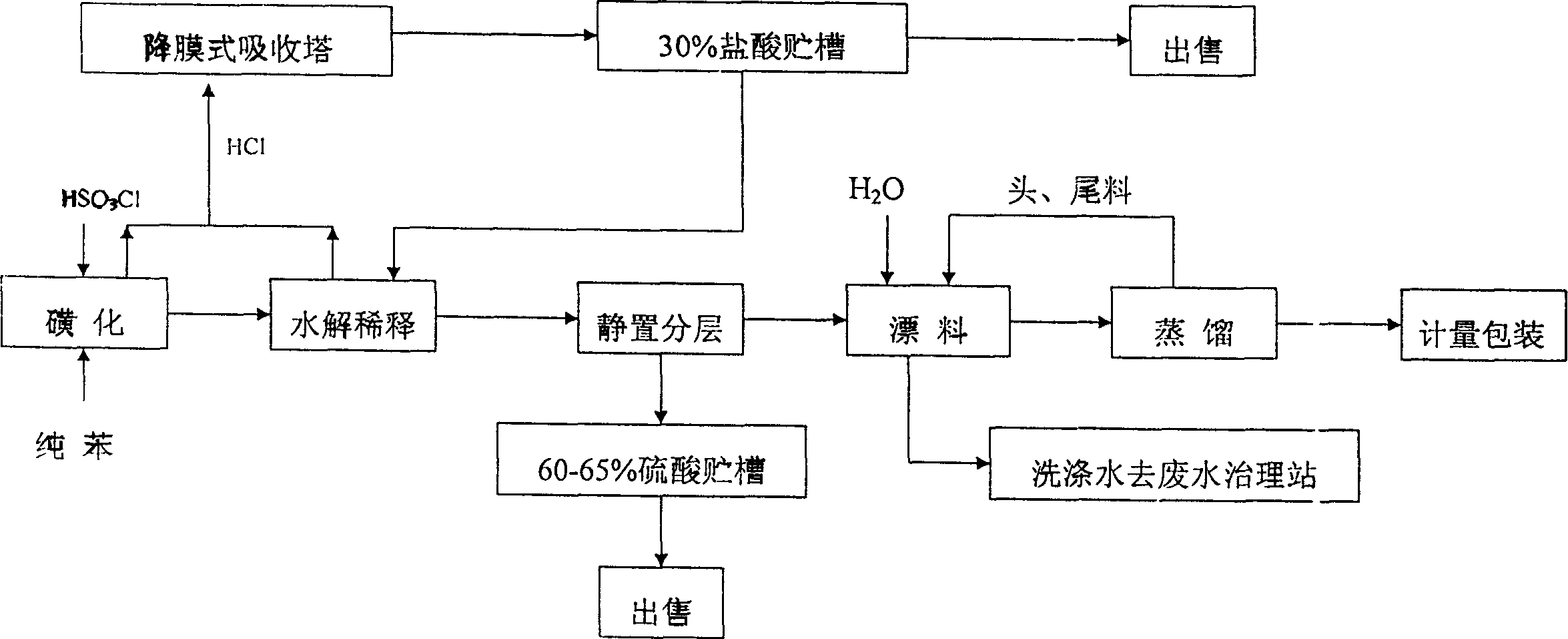
Production technology of phenyl sulfuryl chloride
The present invention provides a production method of benzenesulfonyl chloride. It is characterized by that said invention uses benzene and chlorosulfonic acid as raw material, and makes them undergo the processes of sulfonation reaction, hydrolytic dilution, standing still and layer separation and reduced pressure distillation so as to obtain the invented product. Said invention also provides the concrete steps of every process and its concrete requirements.
Owner:江苏康祥实业集团有限公司
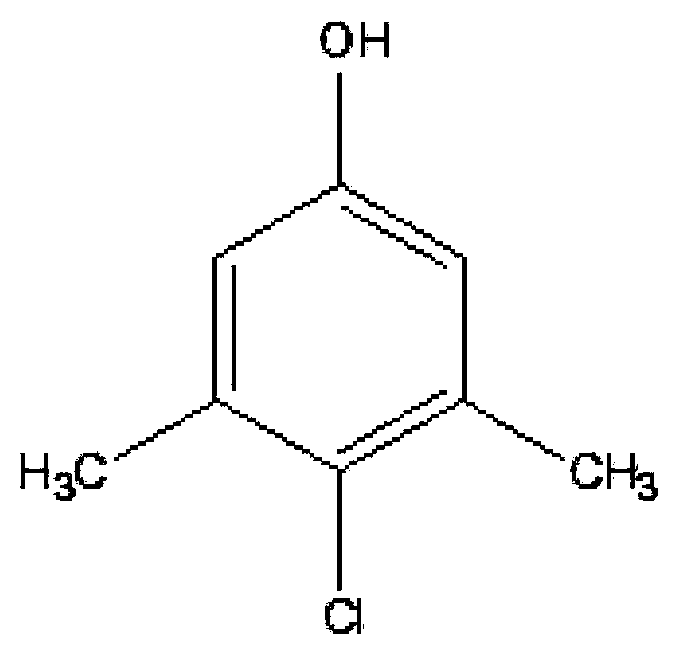
Green industrialized preparation method for 1-hydroxy-3,5-dimethyl-chlorobenzene
ActiveCN101823941AReduce usageHigh yieldOrganic chemistryOrganic compound preparationTemperature controlChlorobenzene
The invention discloses a green industrialized preparation method for 1-hydroxy-3,5-dimethyl-chlorobenzene. Water is used as a solvent; sulfuryl chloride or chlorine is used as a chlorinating agent; and a substrate 1-hydroxy-3,5-dimethyl benzene is subjected to chlorination reaction in a mode of multi-stage temperature control. By using the method, a catalyst with high cost and difficult reclamation can be saved, and the use of an organic solvent can be avoided, so that the discharge of waste gas (volatilization of the organic solvent) and waste substance (the catalyst) can be remarkably reduced during production and the industrialized production of the product is more environmentally-friendly and greener).
Owner:湖南瑞冠生物化工科技有限公司
Preparation method of vonoprazan fumarate
ActiveCN105085484AGet efficientlyLow costOrganic chemistryBulk chemical productionMethylene DichlorideVonoprazan
The invention discloses a preparation method of vonoprazan fumarate. The preparation method includes: S1, dissolving 5-(2-fluorophenyl)-1H-pyrrole-3-carboxaldehyde (I) in organic solvent, mixing with methylamine alcohol solution for 6-8h to generate imine, reducing with metal borohydride for 1-2h, and performing post-treatment to obtain a compound according to a formula II; S2, dissolving the compound prepared in the step S1 according to the formula II, in organic solvent, performing ice bathing and mixing with Boc anhydride to allow reaction for 1-2h, and performing post-treatment to obtain a compound according to a formula III; S3, dissolving the compound prepared in the step S2 according to the formula III, in organic solvent, adding sodium hydride and crown ether, adding 3-pyridine sulfuryl chloride, mixing for reaction for 1-2h, and performing post-treatment to obtain a compound according to a formula IV; S4, reacting the compound prepared in the step S3 according to the formula IV, in trifluoroacetic acid and methylene dichloride solution to obtain a compound according to a formula V; and S5, dissolving the compound prepared in the step S4 according to the formula V, in organic solvent to be salified with fumaric acid, thereby obtaining the vonoprazan fumarate (VI). The preparation method has few side reactions and high intermediate purity and allows simple post-treatment.
Owner:NANJING GRITPHARMA CO LTD
Preparation method for co-producing bis(chlorosulfonyl)imide and lithium bis(fluorosulfonyl)imide
InactiveCN111099566AReliable sourceImprove qualityNitrosyl chlorideAmidosulfonic acidImidePolymer science
The invention discloses a preparation method for coproducing bis(chlorosulfonyl)imide and lithium bis(fluorosulfonyl)imide, which comprises the following steps: S1, adding sulfuryl chloride into a first solvent, dropwisely adding octamethylcyclotetrasilazane for reaction, and carrying out purification to obtain bis(chlorosulfonyl)imide; S2, taking bis(chlorosulfonyl)imide and anhydrous hydrofluoric acid to react under the action of a catalyst to obtain bis(fluorosulfonyl)amide; and S3, taking the bis(fluorosulfonyl)amide and lithium fluoride to react in a second solvent, and carrying out purification to obtain the lithium bis(fluorosulfonyl)imide. According to the invention, proper raw materials are selected and matched with a solvent method to co-produce bis(chlorosulfonyl)imide and lithium bis(fluorosulfonyl)imide. The method has the advantages of simple preparation process, few and recyclable byproducts, high yield, no water participation in the production process, high purity of the obtained bis(chlorosulfonyl)imide and lithium bis(fluorosulfonyl)imide, no wastewater generation and green and environment-friendly process route, and is suitable for industrial production.
Owner:合肥利夫生物科技有限公司
Method for preparing graphene oxide grafted with thermotropic liquid crystal compound with end group containing epoxy group
InactiveCN102766265AWide variety of sourcesEasy to operateLiquid crystal compositionsEpoxyPtru catalyst
The invention discloses a method for preparing graphene oxide grafted with a thermotropic liquid crystal compound with an end group containing an epoxy group. P-phthanoyl chloride, p-hydroxybenzoic acid, thionyl chloride, diethylene glycol and glycidol are used as raw materials to react to obtain the thermotropic liquid crystal compound with the end group containing the epoxy group with pyridine and lauric acid butyltin as catalyst; thionyl chloride is added into graphene oxide mixed liquid formed by ultrasonic dispersion to react to obtain acyl chlorination graphene oxide; and acyl chlorination graphene oxide and thermotropic liquid crystal compound with the end group containing the epoxy group are used as raw materials and react with pyridine as catalyst to obtain graphene oxide grafted with the thermotropic liquid crystal compound with the end group containing the epoxy group. The graphite oxide is obtained by using Hummers oxidation method with chemical pure crystalline flake graphite as a raw material. Other chemical solvents are analytically pure. The raw materials are wide in source, simple in preparation process, free of pollution, low in cost and favorable for industrial large-scale production.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Process for the production of chlorinated alkanes
InactiveUS20140323775A1Efficient processingHigh selectivityPreparation by halogen additionAlkaneIodide
Processes for the production of chlorinated alkanes are provided. The present processes comprise catalyzing the addition of at least two chlorine atoms to an alkane and / or alkene with a catalyst system comprising one or more nonmetallic iodides and / or lower than conventional levels of elemental iodine and at least one Lewis acid. The present processes make use of sulfuryl chloride, or chlorine gas, as a chlorinating agent.
Owner:BLUE CUBE IP
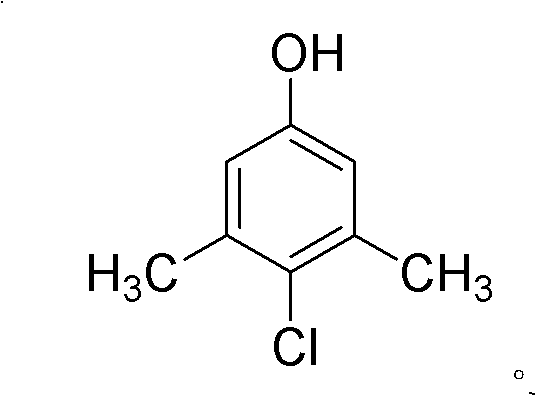
Preparation method of 3,5-dimethyl-4-chlorophenol
InactiveCN104326881AImprove conversion rateReduce generationOrganic chemistryOrganic compound preparationAluminium chlorideWater chlorination
The invention discloses a preparation method of 3,5-dimethyl-4-chlorophenol, which takes tetrachloroethylene as a solvent, benzyl thiophenol and aluminium chloride as cocatalysts, sulfuric chloride as a chloridizing agent, orientation chlorination is carried out through two phases of low-temperature chlorination and high-temperature chlorination, the mass ratio of tetrachloroethylene to MX is 0.5-4: 1; the mass ratio of the cocatalyst to MX is 2.5-6.5:1000; at low temperature chlorination phase, the mass ratio of sulfuric chloride dropping amount to MX is 0.9-1.2: 1; at high temperature chlorination phase, the mass ratio of sulfuric chloride dropping amount to MX is 0.1-0.2: 1; the temperature at the low temperature chlorination phase is controlled at 30-45 DEG C, chlorination is carried out for 4-6 hours; the temperature at high temperature chlorination phase is controlled at 50-65 DEG C, and chlorination is carried out for 1-2 hours, insulation reaction is carried out after the dropping process of sulfuric chloride is completed, tail gas is removed for 1-2 hours, and steps of water-washing layering, cooling and crystallizing, centrifuging and washing, and drying to obtain the product. According to the method, the conversion rate can reach more than 95%, finished product PCMX yield is increased, and the by-product can be effectively reduced.
Owner:RONGCHENG QINGMU CHEM MATERIALS
Process for the preparation of aromatic alpha-hydroxy ketones
InactiveCN102015603AOrganic compound preparationCarbonyl compound preparation by condensationHydrogen halideBromine
Process for the preparation of aromatic alpha-hydroxyketones (aromatic a- hydroxyketones) that does not require the use of chlorine, sulfuryl chloride or bromine and comprises the halogenation of an intermediate aromatic ketone with a hydrogen halide in the presence of an oxidising compound.
Owner:LAMBERTI SPA
Preparation process of chlorinated phenol
ActiveCN105777499AHas a positioning effectReduce consumptionOrganic chemistryOrganic compound preparationChlorinated phenolsEthyl Chloride
The invention discloses a preparation process of chlorinated phenol; phenol is used as a raw material, a mixture of arbitrary one of diphenyl sulfide and dimethyl sulfide, arbitrary one of acetic acid and toluene sulfonic acid and arbitrary one of aluminum trichloride and ferric trichloride is used as a catalyst, a chlorinated phenol crude product is generated through sulfuryl chloride chlorination, and a target product is obtained by melt crystallization. The mixed catalyst used in the reaction has a positioning function, the content of p-chlorophenol in the monochlorophenol mixture generated from the reaction is greater than 83%, the content of 2,4-dichlorophenol in dichlorinated phenol generated from the reaction is greater than 98%, the amount of trichlorinated phenol impurities generated from the reaction is reduced, and 2,4,6-dichlorophenol with the content greater than 99% and the total yield more than or equal to 98% is obtained without purification treatment; moreover, energy consumption is greatly reduced, and high-content p-chlorophenol and high-quality 2,4-dichlorophenol can be produced at the same time.
Owner:兰州诚胜化工科技有限公司
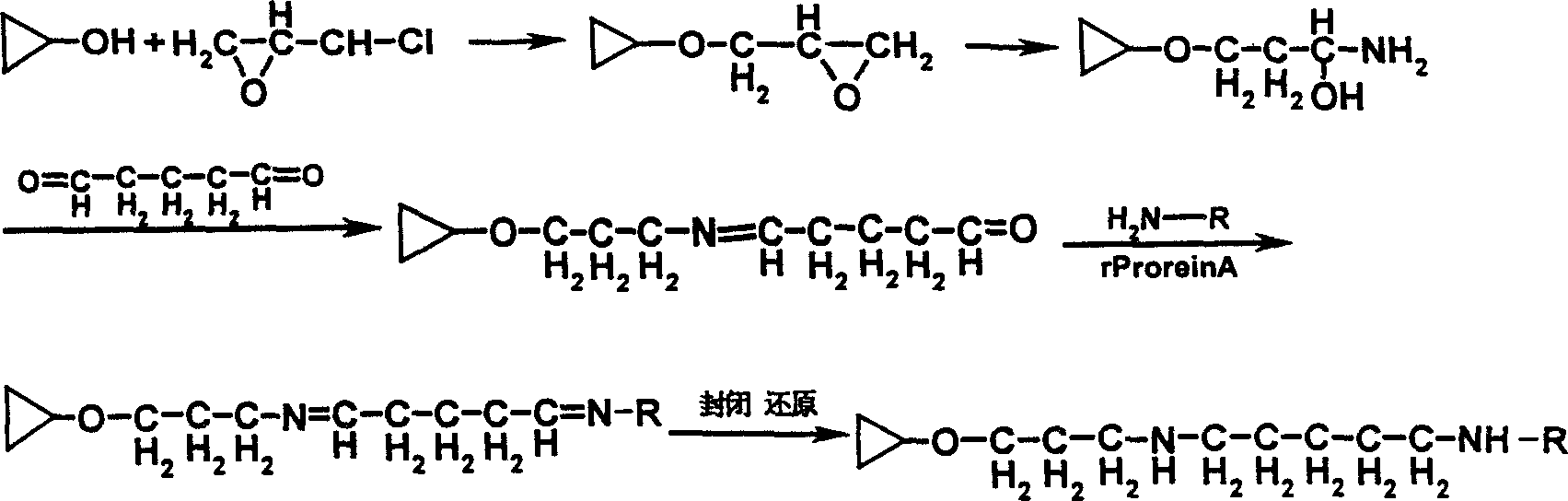
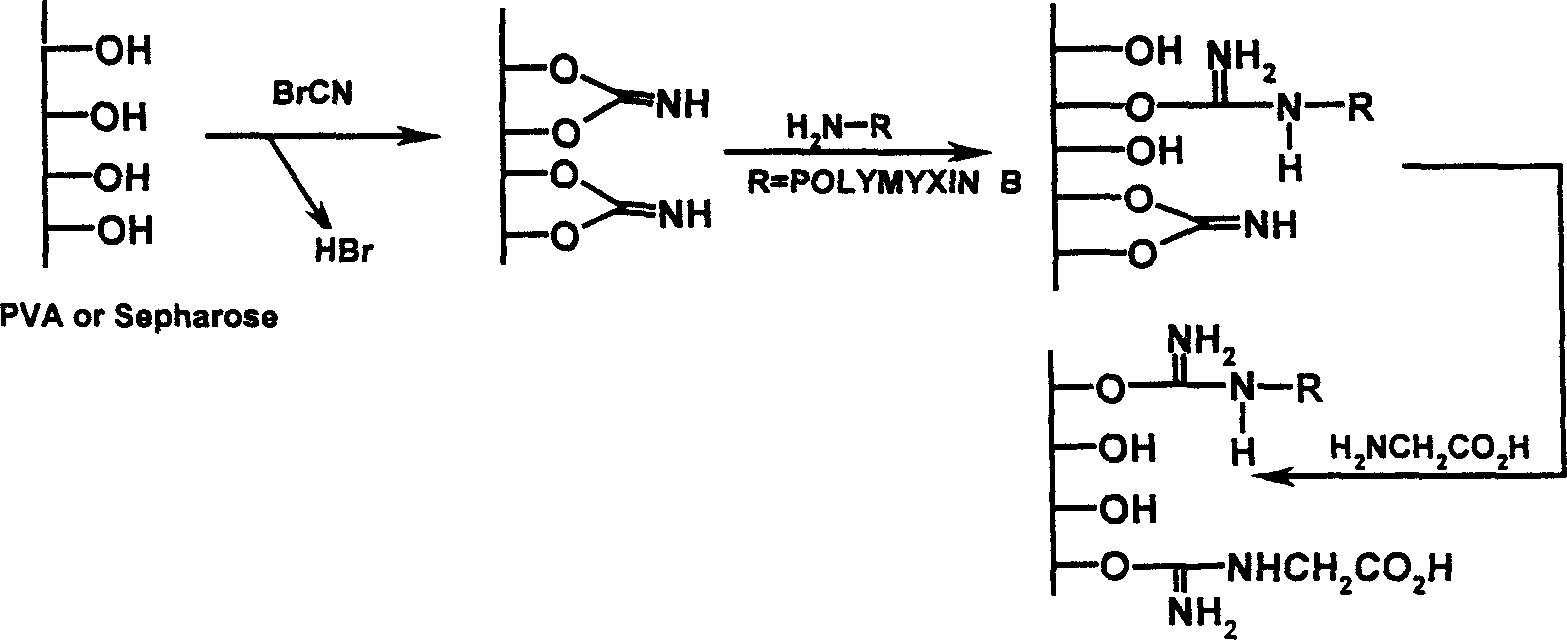
Endotoxin adsorptive material, preparing and use thereof
An endotoxic adsorpting method for treating endotoxemia and anaphylactic reaction caused by endotoxemia is prepared through activating the hydroxy group of agarose gel or DVA as carrier by cyanogen bromide or epoxy chloropropane or sulfuryl chloride, and adding polymyxin solution.
Owner:浙江科锐生物科技有限公司
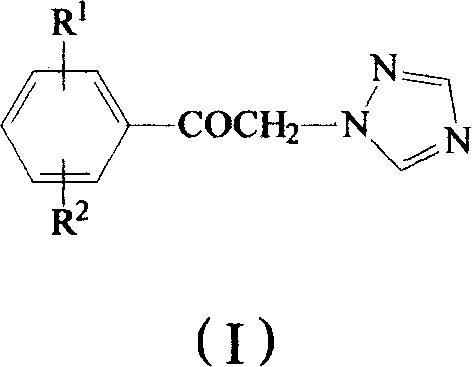
Method for preparing omega-(1H-1,2,4-triazol-1-yl)-arylethanone
ActiveCN101190900AHigh yieldSatisfied with the implementation effectOrganic chemistrySulfonateHalogen
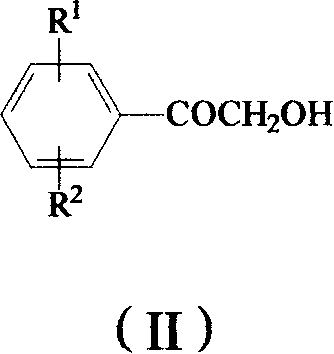
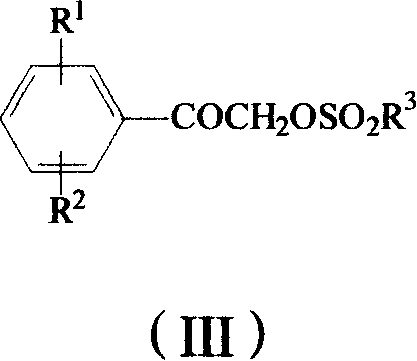
The invention provides a method to prepare omega-(1H-1, 2, 4- triazole-1-base) - arylethanone of formula (1) based on omega-hydroxyl arylethanone. The method comprises the steps that esterification reaction is done in the presence of alkali between the omega-hydroxyl arylethanone and at least one mole of sulfuryl chloride to generate corresponding arylacetyl methyl sulfonate and the arylacetyl methyl sulfonate in the presence of acid binding agent reacts with at least one mole of 1H-1, 2, 4- triazole or directly reacts with alkali metal salt of the 1H-1, 2, 4- triazole to generate the omega-(1H-1, 2, 4- triazole-1-base)- arylethanone, wherein, the Omega-(1H-1, 2, 4- triazole-1-base)- arylethanone substituted by aryloxy can be prepared by the etherification reaction of the omega-(1H-1, 2, 4- triazole-1-base)- arylethanone which can be substituted by halogen with potassium (sodium) phenate. The substituting groupR<1> and R<2> in the formula (I) structure are stated in the manual.
Owner:JIANGSU FENGDENG PESTICIDE
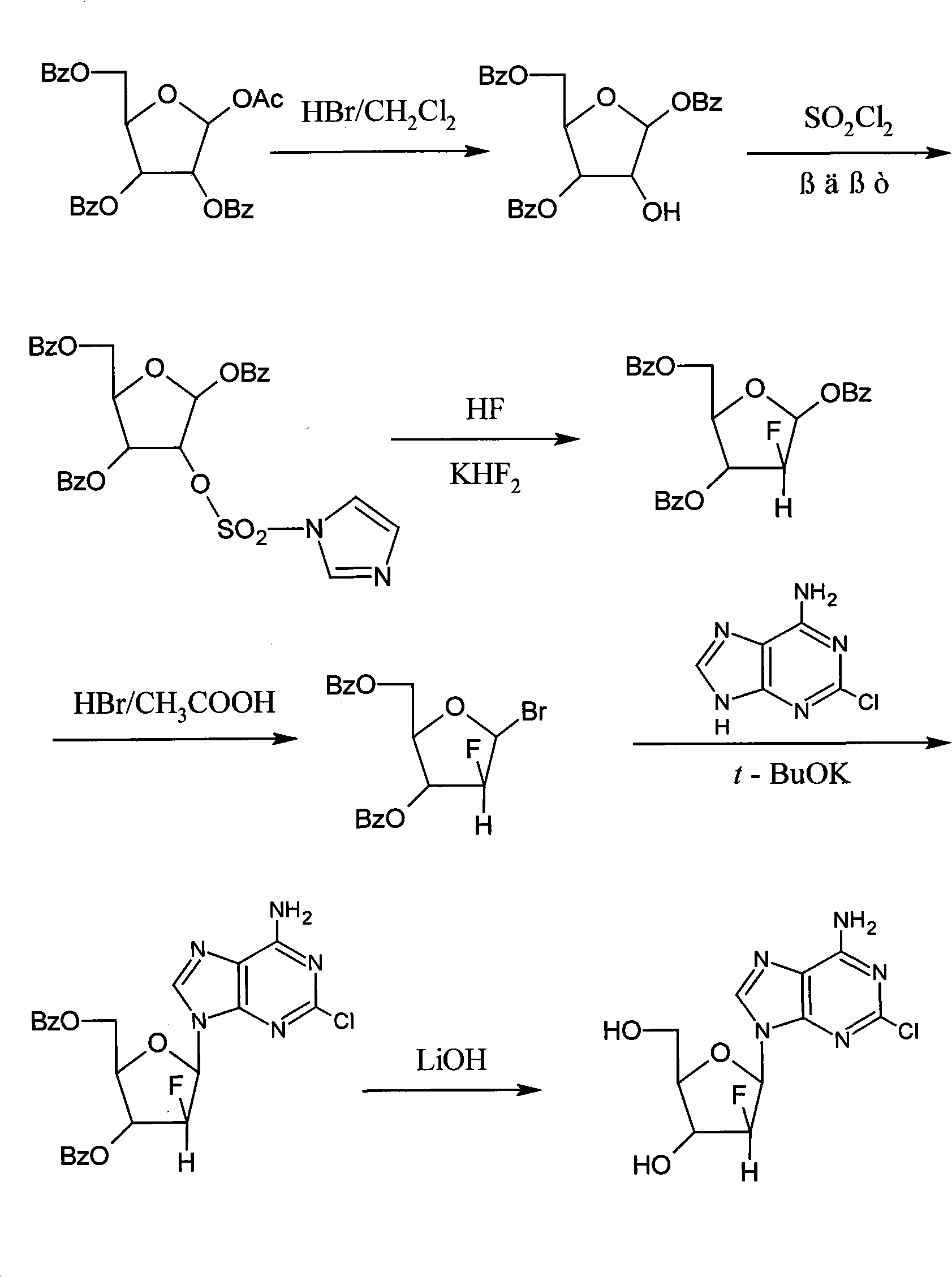
Method for synthesizing clofarabine
InactiveCN101265284AHigh selectivityHigh yieldSugar derivativesAntineoplastic agentsHydrobromidePhenacyl
The invention provides a preparation method of clofarabine, which includes allowing 1-acetyl-2,3,5-tri-o-benzoyl-Beta-D-ribofuranose as the initial raw material and dichloromethane solution of hydrobromide to perform the rearrangement reaction, reacting with sulfuryl chloride and imidazole, performing the fluoridation reaction in the presence of hydrogen fluoride aqueous solution and potassium hydrogen fluoride, and performing bromination reaction in acetic acid solution of hydrogen bromide, condensing with 2-chloro adenosine in alkaline condition, and removing benzoyl in the presence of lithium hydroxide to obtain clofarabine. Compared with prior art, the method has the advantages of high yield of each step, higher total yield, and easily realized industrialized production.
Owner:深圳万乐药业有限公司
Process for the production of chlorinated alkanes
InactiveUS20150045592A1High selectivityLow costPreparation by halogen additionAlkaneRegioselectivity
Processes for the production of chlorinated alkanes are provided. The present processes comprise reacting one or more mono- and / or dichloroalkanes to form tri-, tetra- and / or pentachloroalkanes, with high regioselectivity. In those embodiments wherein a dichloroalkane is desirably utilized, it may advantageously be a vicinal dichloroalkane. Further, only one catalyst is utilized. The present processes make use of sulfuryl chloride as a chlorinating agent, rather than a gaseous chlorinating agent such as chlorine gas. Finally, the process uses lower intensity process conditions than at least some conventional processes, and thus, operating costs are saved.
Owner:BLUE CUBE IP
Preparation method of fasudil hydrochloride
InactiveCN103044403ASolve problems that are difficult to obtain through suction filtrationReduce stepsOrganic chemistryIsoquinolineKinase
The invention belongs to the technical field of medicine, and particularly relates to a preparation method of a protein kinase inhibitor fasudil hydrochloride. According to the method, as 5-isoquinoline sulfuryl chloride hydrochloride is adopted to react with homopiperzine directly, the problem that 5-5-isoquinoline sulfuryl chloride is easy to hydrolyze is avoided; and as fasudil hydrochloride is obtained by a direct water phase evaporating method, the problem that the extraction filtration of fasudil hydrochloride is difficult is solved. The preparation method is simple to operate, and higher in product yield and product purity.
Owner:成都天翼医药科技有限公司
Chlorinating agents
InactiveUS20150057471A1Less-costly to mixAcceptable reaction ratePreparation by hydrogen halide split-offPreparation by halogen additionSulfuryl fluorideSulfuryl chloride
Owner:BLUE CUBE IP
High-molecular polymer containing perfluoro alkyl sulfimine side-chain and its synthesizing method
The present invention discloses one kind of polymer containing perfluoroalkyl sulfonyl imido side chain. It is synthesized through chlorosulfonating one of polyhydroxy ether phosphonitrile, crosslinked polystyrene and polystyrene with alkyl long side chain; grafting sulfuryl chloride group to the benzene ring; and final reaction with perfluoro sulfonamide. The obtained polymer containing perfluoroalkyl sulfonyl imido side chain has strong acidity and excellent heat stability, and excellent catalyzing effect on esterification, Dies-Alder reaction, Friedel-Crafts reaction, etc., and may be reused. Therefore, the present invention has latent value in use as green organic synthesis catalyst and electrolyte material. It has simple synthesis and low cost, and is favorable to industrial production.
Owner:HUAZHONG UNIV OF SCI & TECH
Apparatus and method for fabricating cathode collectors for lithium/oxyhalide electrochemical cells
InactiveCN101005132AElectrode rolling/calenderingFinal product manufactureLithiumConductive materials
An apparatus and method for fabricating continuous cathode collecters for use in lithium / thionyl chloride and lithium / sulfuryl chloride cells is described. The preferred electrically conductive material is acetylene black mixed with a polytetrafluroethylene (PTFE) binder in a dry, powderized form. The collector substrate is a nickel or stainless steel foil that has been expanded into a mesh or otherwise provided with perforations. A centering adjustment of the collector substrate controls loading of the electrically conductive mixture onto each side thereof. The dry, powdered electrically conductive mixture is then continuosly fed into the calender and formed into a collector structure by locking to itself through the collector substrate perforations before being cut to size.
Owner:WILSON GREATBATCH LTD
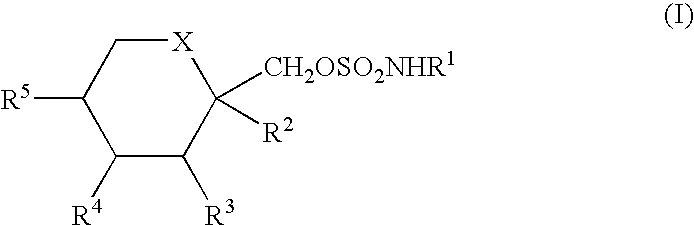
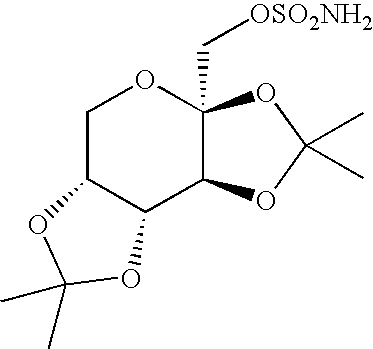
Process for the preparation of sulfamate derivatives
InactiveUS20050203287A1Simple and short routeReduce loadEsterified saccharide compoundsSugar derivativesHydrocarbon solventsHalogen
An improved process for the preparation of sulfamate derivatives such as topiramate is provided comprising (a) reacting an alcohol with sulfuryl chloride in the presence of an amine base and in a first halogenated hydrocarbon solvent selected from the group consisting of aliphatic halogenated hydrocarbon solvents having 1 to 12 carbon atoms and at least three halogen atoms, aliphatic halogenated hydrocarbon solvents having 2 to 12 carbon atoms and less than three halogen atoms, aromatic halogenated hydrocarbon solvents having 6 to 18 carbon atoms and mixtures thereof to produce a chlorosulfate intermediate and (b) reacting the chlorosulfate intermediate with an amine of the formula R1NH2, wherein R1 is hydrogen or an alkyl from 1 to 4 carbon atoms, in a second halogenated hydrocarbon solvent selected from the group consisting of aliphatic halogenated hydrocarbon solvents having 1 to 12 carbon atoms and at least three halogen atoms, aliphatic halogenated hydrocarbon solvents having 2 to 12 carbon atoms and less than three halogen atoms, aromatic halogenated hydrocarbon solvents having 6 to 18 carbon atoms and mixtures thereof.
Owner:GLENMARK PHARMACEUTICALS LIMITED
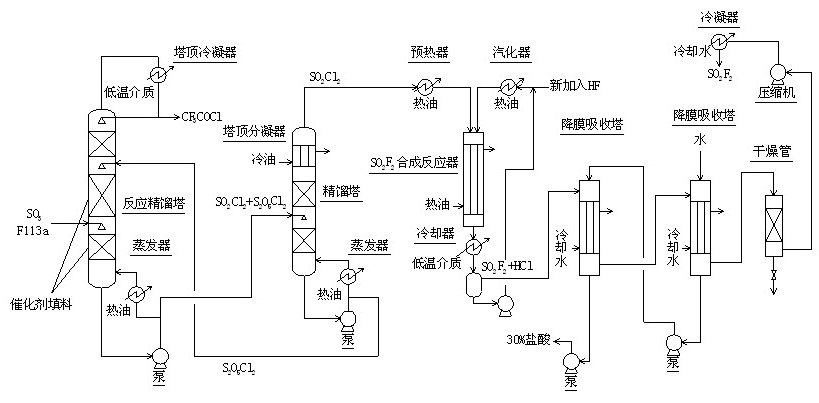
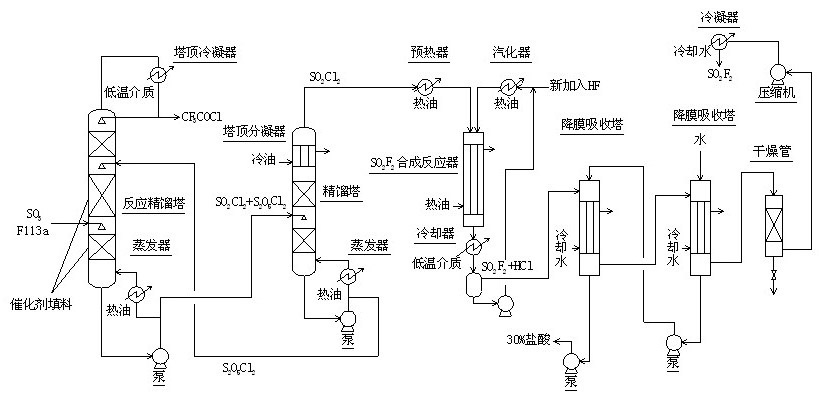
Method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride
InactiveCN102351681AHigh reaction yieldThree wastes lessSulfur-halogen-hydrogen-oxygen compoundsCarboxylic acid halides preparationReaction temperatureTrifluoroacetyl chloride
The invention relates to a method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride, which comprises the following process: continuously introducing sulfur trioxide and trifluorotrichloroethane (F113a) into a reaction rectifying tower containing a catalyst and a filler thorough the lower part in the tower according to a mol ratio of 1:1, wherein the temperature of the tower is controlled to range between 120 DEG C and 130 DEG C, and the reflux ratio at the top of the tower is 2.5-3; introducing tower bottoms into a sulfuryl chloride separating tower for rectification and separation, wherein the tower temperature is 145-150 DEG C, and the reflux ratio is 0.5-1.0; returning pyrosulfuryl chloride in the separating tower to the middle upper part of the reaction rectifying tower, heating the sulfuryl chloride rectified off from the top of the tower to 150 DEG C, and then introducing the rectified sulfuryl chloride and preheated recycled and newly-added hydrogen fluoride gas into a reactor containing a palladium / charcoal catalyst, wherein the reaction temperature is controlled to range between 150 DEG C and 160 DEG C; separating out unreacted hydrogen fluoride from the reaction product through a cold trap, and returning to the reactor for further use; and then absorbing and separating hydrogen chloride through a falling film, drying, compressing, and condensing to obtain the sulfuryl fluoride. The invention has the advantage of easy acquisition of raw materials, and the sulfuryl chloride byproduct can be directly used for the synthesis of sulfuryl fluoride.
Owner:ZHEJIANG UNIV +1
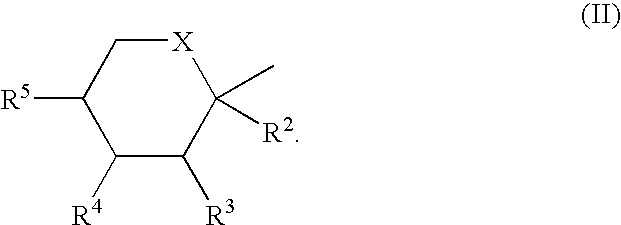
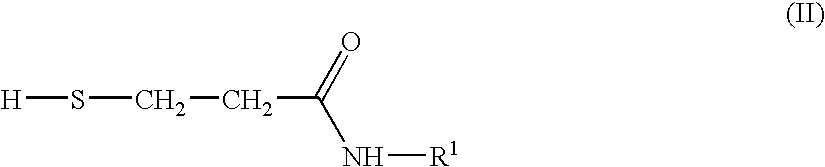
Preparation of N-Substituted Isothiazolinone Derivatives
InactiveUS20080227986A1Less harmful to the environmentCost-effectiveOrganic compound preparationCarboxylic acid amides preparationIsothiazolinoneManufacturing cost reduction
Provided is a process for the preparation of an N-substituted isothiazolinone derivative having the general formula (I), comprising reacting N-substituted 3-mercaptopropionamides of formula (II) or N,N′-bis-substituted 3,3′-dithiodipropionamides of formula (III) with sulfuryl chloride in the absence of solvents. Also provided is a process for the preparation of a compound having the general formula (III), comprising reacting a methyl ester of formula (IV) with an amine of formula (V) in a solvent of methanol. As no addition solvent is used in the process of the invention, the cost of manufacturing and pollution to the environment can be reduced.
Owner:BEIJING TIANQING CHEM CO LTD
Preparation method of parachlorometaxylenol
InactiveCN103351283AReduce usageReduce volatilityOrganic chemistryOrganic compound preparationTetrachloroethyleneOrganic solvent
The invention discloses a preparation method of parachlorometaxylenol. The method includes: adding a proper amount of tetrachloroethylene or dichloroethane into acidic water to prepare a composite solvent, i.e. an M system, letting a chlorinating agent sulfuryl chloride and a substrate 3, 5-dimethylphenol undergo a chlorination reaction in the M system so as to produce the target product parachlorometaxylenol. The method not only can avoid use of catalysts that are expensive and are difficult to recover, but also can reduce the use of organic solvents, and reduce energy consumption during production. At the same time, the method can improve the conversion per pass of the product and the first-time qualification rate. Moreover, the process also can significantly reduce the exhaust gas and waste in the production process, such as volatilization of organic solvents, discharge of inactivated metal catalysts, and can improve the production environment, thus being conducive to environmental protection.
Owner:JIANGSU HUANXIN NEW MATERIAL CO LTD
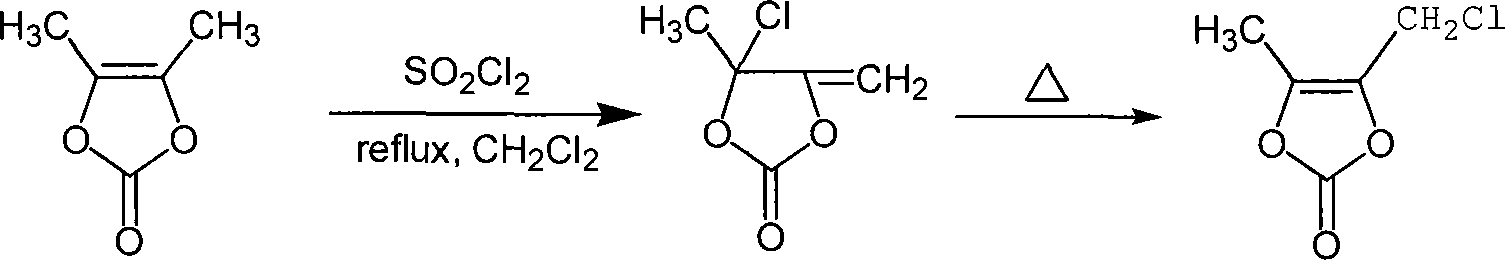
Chemosynthesis method of 4-chloromethyl-5-methyl-1,3-dioxy heterocyclic pentene-2-ketone
InactiveCN101250178AReasonable process conditionsPromote safe productionOrganic chemistryCyclopenteneChemical synthesis
The invention provides a chemical synthesis method of 4-chloromethyl-5-methyl-1, 3-dioxa cyclopentene-2-ketone, which comprises using 4, 5-dimethyl-1, 3-dioxa cyclopentene-2-ketone as raw material and uses sulfuryl chloride as chlorinating agent to process reflux reaction in organic solvent for 1-3h, evaporating out the solvent, rearranging for 1-4h at 70-100DEG C, and separating the reaction liquor after the rearrangement to obtain the 4-chloromethyl-5-methyl-1, 3-dioxa cyclopentene-2-ketone. The chemical synthesis method has the advantages of reasonable process conditions, reliable and safe production, high reaction total yield, low production cost, little discharge of three wastes and significant pratical, social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH +1
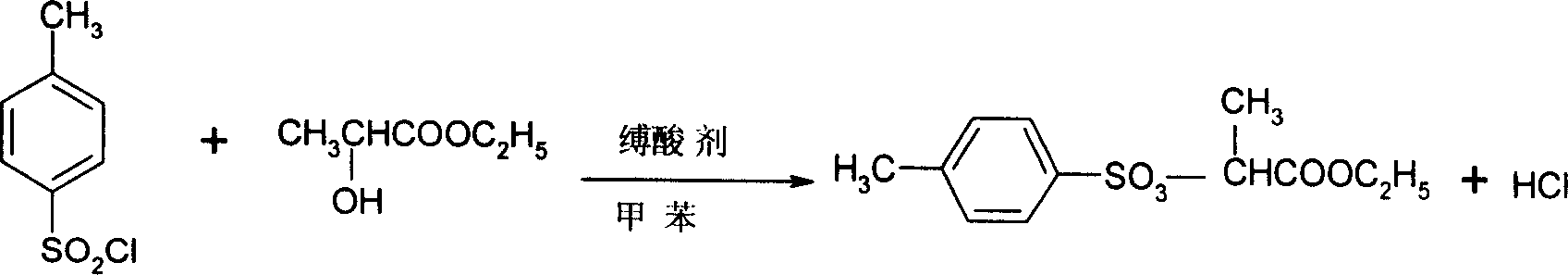
Preparation method of 2-(4'-methyl benzene sulfonyl) ethyl propionate
A process for preparing 2-(4'methylbenzosulfonyl) ethyl propionate includes such steps as adding toluene, P-toluene sulfuryl chloride and sodium hydroxide or potassium carbonate or CaO into reactor, dripping ethyl L-lactate at a temp lower than 10 deg.C, reaction, adding water, stirring, regulating pH=6-7, removing lower-layer water, and negative-pressure distilling to remove toluene and obtain product.
Owner:陈克付
Cyclic sulphate preparation method
The invention relates to the field of organic synthesis and especially relates to a cyclic sulphate preparation method. The sulphate preparation method disclosed by the invention comprises the step ofreacting a compound shown in formula II with sulfuryl chloride under the reaction solvent existence condition to prepare and obtain a compound shown in formula I. The sulphate preparation method disclosed by the invention has the characteristics of brief steps, small side reaction, simpleness in aftertreatment, small three wastes, low manufacturing cost, suitability for industrial large-scale production and the like.
Owner:SHANGHAI CHEMSPEC CORP +1
Method for preparing 2-chlorine-5 chloromethyl thiazole
InactiveCN104119291AShort reaction timeExperiment operation is simpleOrganic chemistryIsomerizationSodium sulfocyanate
The invention relates to a method for preparing 2-chlorine-5 chloromethyl thiazole. The method comprises the following steps of: preparing 2-chlorine-5 chloromethyl thiazole by taking 2,3-dichloropropene, sodium sulfocyanate, sulfuryl chloride sulfuryl chloride and the like as main raw materials and changing a reaction solvent, reaction temperature and the like; purifying the product. An experimental result indicates that the method in which 2-chlorine-5 chloromethyl thiazole with the purity of 99% is prepared from 2,3-dichloropropene and sodium sulfocyanate through a one-pot process sequentially including substitution reaction, isomerization reaction and chlorination-cyclization reaction by taking methylbenzene as a solvent has the advantages of simple operation, few side reactions and high efficiency.
Owner:SHAOXING UNIVERSITY
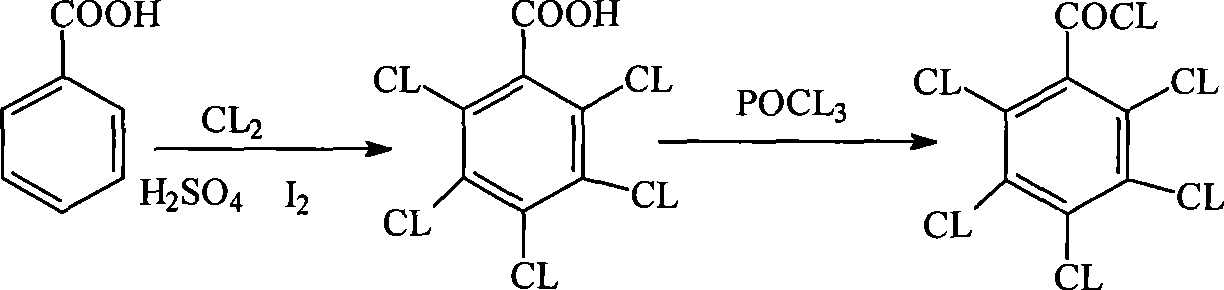
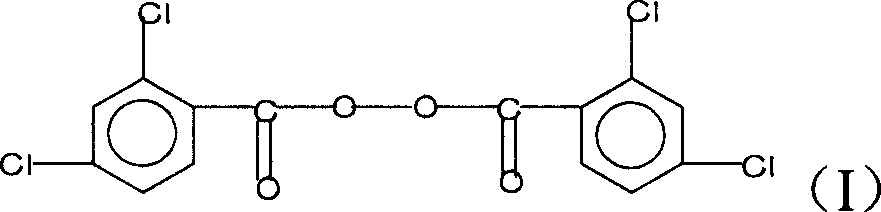
Method for preparing pentachlorobenzoyl chloride
ActiveCN101417946AReasonable workmanshipSimple and safe operationOrganic compound preparationCarboxylic compound preparationChlorosulfuric acidSolvent
The invention relates to a preparation method of pentachlorobenzoyl chloride, which is characterized in that, benzoyl chloride or polychlorobenzoyl chloride is used as the raw material; chlorosulfuric acid, carbon tetrachloride and thionyl chloride, or chlorosulfuric acid, carbon tetrachloride and sulfuryl chloride are used as the mixed solvent; iodine is the catalyst, and chlorine is the chlorination agent. Compared with prior art, the preparation method has the advantages of reasonable process, simple and safe operation, a high productivity as much as 96 percent, recyclable solvent, less three wastes, environmental protection and energy saving, and adaptability for industrial production.
Owner:SHANGHAI HETENG FINE CHEM +1
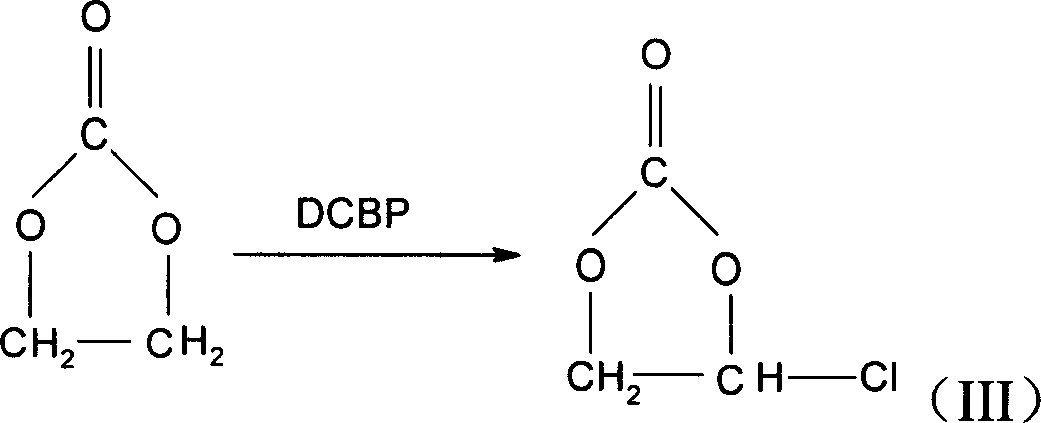
Method for preparing monochloroethylene carbonate
An ethylene carbonate chloride preparation method is that ethylene carbonate and sulfuryl chloride are contacted and reacted with the existing of an initiator of 2, 4, 2, 4-tetrachlorobenzoyl peroxide. The yield of ethylene carbonate chloride provided by the invention is as high as 93 percent or over, and the product is easily separated and purified, with the purity rate being 98 percent. Besides, as the toxicity of suffuryl chloride is much lower than the toxicity of carbon tetrachloride, the requirement to equipment is comparatively lower, and the operation is safer, thus being suitable for the requirements of industrial mass production.
Owner:BYD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com