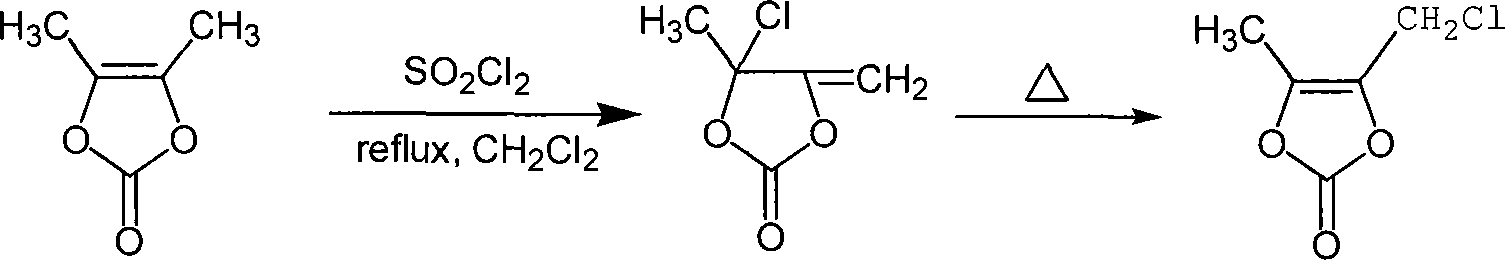
Chemosynthesis method of 4-chloromethyl-5-methyl-1,3-dioxy heterocyclic pentene-2-ketone
A technology of dioxolene and chloromethyl, which is applied in the field of chemical synthesis of 4-chloromethyl-5-methyl-1,3-dioxol-2-one, can solve industrial Problems such as low application value, high toxicity of chlorine gas, and low reaction yield, etc., to achieve high implementation value and social and economic benefits, less three wastes, and high total reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The molar ratio of the feed material is: DMDO: sulfuryl chloride is 1: 1.05. The organic solvent is dichloromethane, the amount of which is 7 times the mass of DMDO; the rearrangement temperature is 90°C.
[0017] Add 300ml of dichloromethane and 60g of DMDO to a 500ml four-neck flask equipped with a mechanical stirrer, a constant pressure dropping funnel, a reflux condenser and a thermometer and a tail gas absorption device, and start to heat up to reflux. Then 74.6 g of sulfuryl chloride was added dropwise for about 2 hours. After dripping, keep it warm for 2 hours under the same conditions, then remove the solvent by rotary evaporation, and rearrange the product after removal of the solvent at 90°C for 2 hours to obtain a crude reaction product, which is separated by rectification to obtain 58.2 g of the product. The yield is 74.5%, and the purity is 98.5%.
[0018] Structure Characterization:
[0019] IR: v max / cm -1 : 1820.4 (C=O), 1733.8 (C=O).
[0020] 1 ...
Embodiment 2
[0022] The molar ratio of the feed material is: DMDO: sulfuryl chloride is 1: 1.05. The organic solvent is dichloromethane, the amount of which is 7 times the mass of DMDO; the rearrangement temperature is 90°C.
[0023] Add 300ml of dichloromethane and 60g of DMDO to a 500ml four-neck flask equipped with a mechanical stirrer, a constant pressure dropping funnel, a reflux condenser and a thermometer and a tail gas absorption device, and start to heat up to reflux. Then 74.6 g of sulfuryl chloride was added dropwise for about 3 hours. After dripping, the reaction was incubated under the same conditions for 1 hour, and then the solvent was removed by rotary evaporation, and the solvent-removed product was rearranged at 90° C. for 4 hours to obtain a crude reaction product. Separation by rectification gave 54.5 g of a colorless product, 4-chloromethyl-5-methyl-1,3-dioxol-2-one, with a yield of 69.7% and a purity of 96.8%.
Embodiment 3
[0025] The molar ratio of the feed material is: DMDO: sulfuryl chloride is 1: 1.05. The organic solvent is dichloromethane, the amount of which is 7 times the mass of DMDO; the rearrangement temperature is 90°C.
[0026] Add 300ml methylene chloride and 60g DMDO to a 500ml four-neck flask equipped with a constant pressure dropping funnel, a reflux condenser and a thermometer and a tail gas absorption device, and start to heat up to reflux. Then 74.6 g of sulfuryl chloride was added dropwise for about 1 hour. After dripping, the reaction was incubated under the same conditions for 3 hours, then the solvent was removed by rotary evaporation, and the solvent-removed product was rearranged at 90° C. for 2 hours to obtain a crude reaction product. Separation by rectification gave 55.3 g of light yellow product 4-chloromethyl-5-methyl-1,3-dioxol-2-one with a yield of 70.8% and a purity of 97.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com