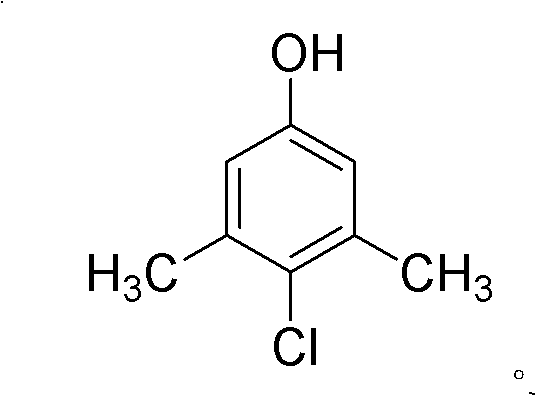
Green industrialized preparation method for 1-hydroxy-3,5-dimethyl-chlorobenzene
A technology of dimethylbenzene and dimethyl, which is applied in the field of 1-hydroxyl-3, can solve the problems of high cost and environmental pollution, and can not solve the problems of catalyst recovery and application, so as to avoid the use and production of organic solvents and catalysts. Low cost and the effect of reducing the discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]Add 122g of 3,5-dimethylphenol (1.0mol) into a 500ml three-necked flask, then add 366g of tap water, start stirring, and keep warm at 15-25°C. Slowly add 108g of sulfuryl chloride (0.8mol) dropwise at this temperature for 4 hours; after the dropwise addition, raise the temperature to 85°C and add 27g of sulfuryl chloride (0.2mol) dropwise after the reactants are melted. After the reaction, the lower organic phase was removed and analyzed by GC. The results were MX: 0.45%; PCMX 84.57%; OCMX (ortho-chlorinated product): 11.56%; DCMX (di-chlorinated product): 3.42%. After the reaction material was suction-filtered and dried, 125 g of a white crystalline product was obtained. The GC analysis results were: MX: 0.25%; PCMX: 99.00%; OCMX: 0.25%; DCMX: 0.50%. The yield was 79.9% (molar yield based on MX).
Embodiment 2
[0035] Add 122g of 3,5-dimethylphenol (1.0mol) into a 500ml three-necked flask, then add 366g of tap water, start stirring, and keep warm at 25-35°C. Slowly add 81g of sulfuryl chloride (0.6mol) dropwise at this temperature, and the dropping time is 4 hours; mol); after the dropwise addition, the temperature was raised to 85°C and then cooled to 25°C, and then 13.5g of sulfuryl (0.1mol) was added dropwise at 25-35°C. After the reaction, the lower organic phase was removed and analyzed by GC. The results were MX: 0.25%; PCMX 89.68%; OCMX (ortho-chlorinated product): 8.19%; DCMX (di-chlorinated product): 1.88%. After the reaction material was filtered and dried by suction, 135 g of a white crystalline product was obtained. The GC analysis results were: MX: 0.2%; PCMX: 99.23%; OCMX: 0.15%; DCMX: 0.42%. The yield was 86.3% (molar yield based on MX).
Embodiment 3
[0037] The experimental process is the same as in Example 1, except that the dripping of sulfuryl chloride is changed into chlorine gas, and after the reaction is finished, the sample analysis is as follows: MX: 0.46%; PCMX: 86.48%; OCMX: 10.65%; DCMX: 2.41%. After the reaction material was suction-filtered and dried, 130 g of a white crystalline product was obtained. The GC analysis results were: MX: 0.25%; PCMX: 99.07%; OCMX: 0.2%; DCMX: 0.48%. The yield was 83% (molar yield based on MX).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com