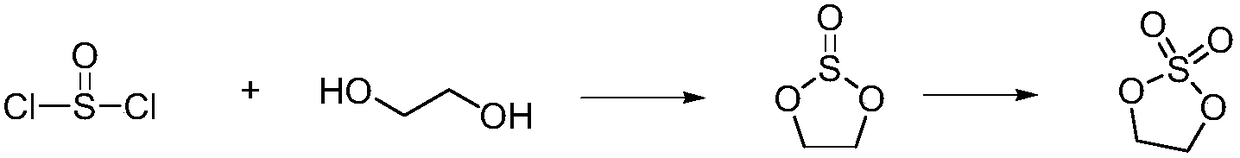
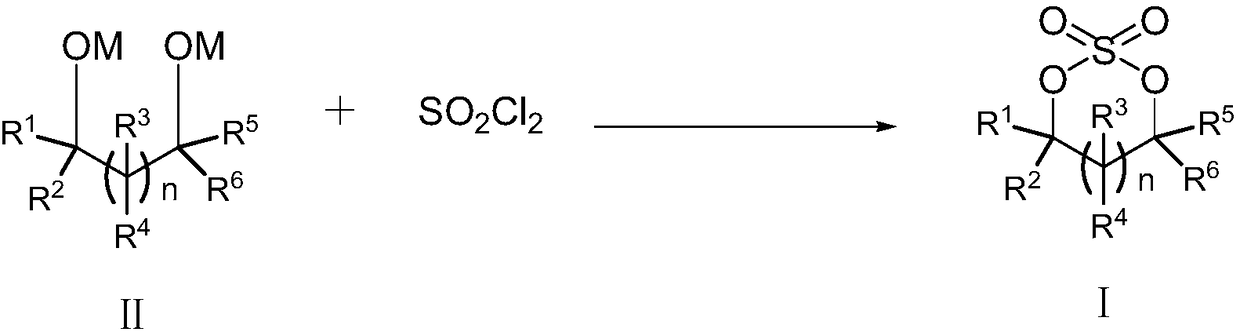Cyclic sulphate preparation method
A technology of sulfate ester and sulfuryl chloride, applied in directions such as organic chemistry, can solve the problems such as restricting the popularization and application of additives, high production cost of oxidation process, unfavorable environmental protection and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention provides a kind of preparation method of sulfuric acid ester on the one hand, the chemical structural formula of described sulfuric acid ester is shown in formula I, and the preparation method of described sulfuric acid ester can comprise: formula II compound is mixed with sulfur under the condition that reaction solvent exists Acyl chloride reaction, preparation obtains formula I compound, and reaction equation is as follows:
[0049]
[0050] Among them, n can be selected from 0, 1, 2, 3 or 4; M can be selected from Li, Na, K; R1, R2, R3, R4, R5, R6 can be independently selected from H, C1~C4 alkyl , C6-C18 aryl, C1-C4 alkoxy, chlorine or bromine. The C1-C4 alkyl group may be, for example, methyl, ethyl, propyl, n-propyl, isopropyl, butyl, n-butyl, isobutyl, sec-butyl, tert-butyl and the like. The C6-C18 aryl group may be, for example, phenyl, naphthyl, fluoranthenyl, fluorenyl, tetrahydronaphthyl, indanyl, anthracenyl and the like. The C1~C...
Embodiment 1
[0070] Preparation of vinyl sulfate:
[0071]Put 100ml of toluene, 41.1g of ethylene glycol and 54g of sodium methoxide into the reaction bottle, stir and raise the temperature to reflux, continuously separate and remove methanol, evaporate to dryness after 12 hours, transfer the residue into an anhydrous 1000ml reaction bottle under nitrogen protection, add 400ml of di Chloromethane, lower the temperature to -10-10°C, slowly add a solution of 84.8g of sulfuryl chloride and 100ml of dichloromethane dropwise, the addition is completed in about 1 hour, and the reaction is kept for 2 hours. After the reaction, add 100ml of water, stir and separate layers, and the organic phase is precipitated at about 50°C until solids are precipitated. Add 250 ml of n-hexane and 0.5 g of 15-crown-5, stir at room temperature for 1 h, filter and dry to obtain 37.5 g of a light brown powdery solid with a yield of 60.4% and a GC content of 99.8%. (Ion content: sodium ion 4ppm)
Embodiment 2
[0073] Preparation of vinyl sulfate:
[0074] Put 100ml of xylene, 40.5g of ethylene glycol and 55.3g of potassium hydroxide into the reaction bottle, stir until the reflux state is about 130-140°C, evaporate the solvent after 15 hours, add 200ml of anhydrous dichloromethane, and stir to form a uniform Suspension. After nitrogen replacement, continuously add the suspension into the mixed solution made of 86g sulfuryl chloride and 200ml dichloromethane, keep the temperature of the reaction system at -10-10°C, complete the addition in about 1 hour, and keep the temperature for 1 hour. After the reaction, add 100ml of water, stir and separate the layers, precipitate the organic phase under reduced pressure at 40°C until the solid precipitates, add 200ml of toluene and 0.6g of 18-crown-6 and stir at room temperature for 1 hour, filter and dry to obtain 37.9g of off-white powder; yield 61.0%; GC content>99.5%, potassium ion 7ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com