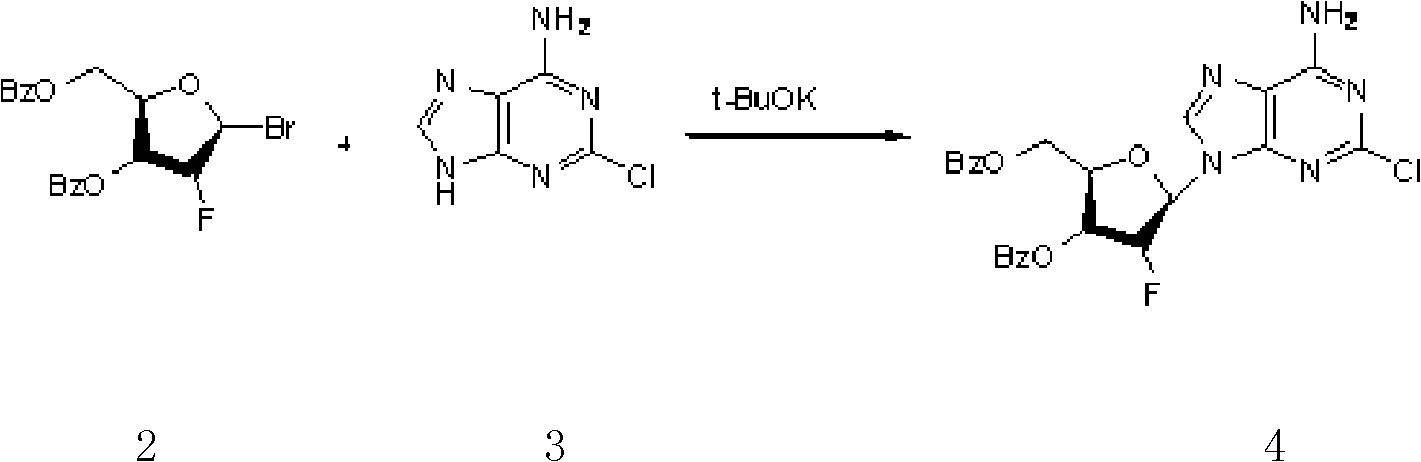Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Clofarabine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
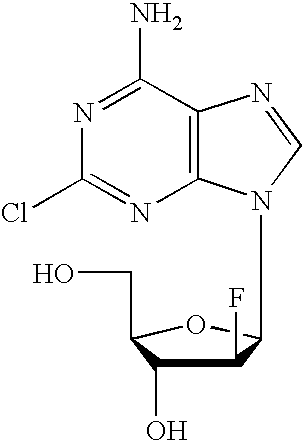
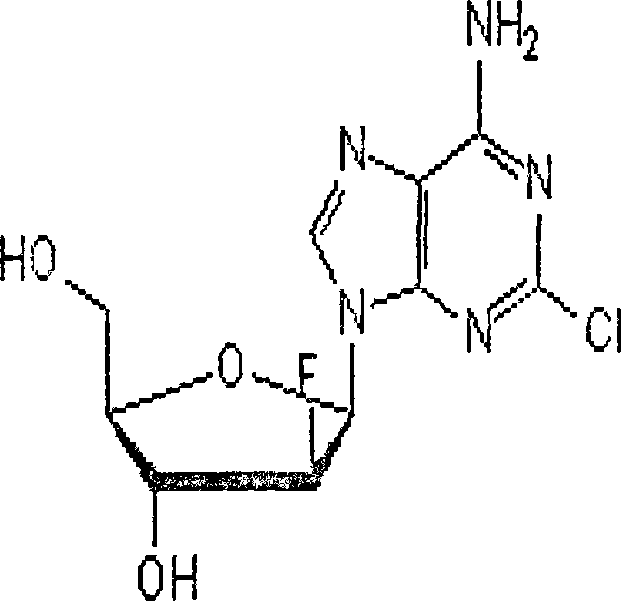
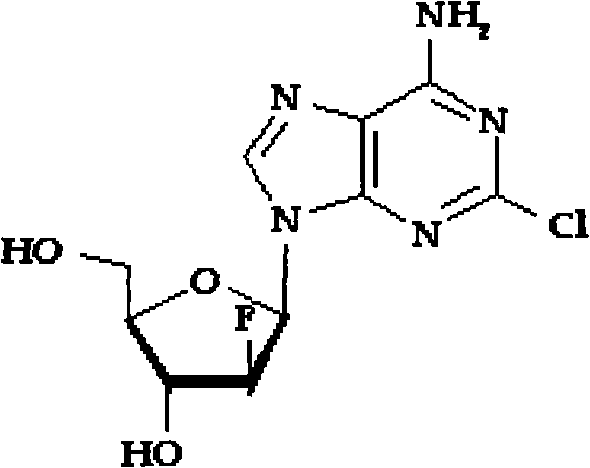
Clofarabine is used to treat a certain type of cancer (acute lymphoblastic leukemia-ALL) in children and young adults ages 1 to 21 whose cancer has not been successfully treated by at least 2 other treatments.
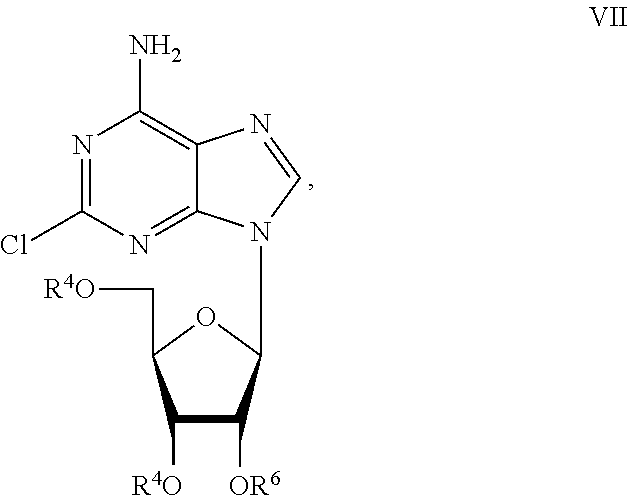
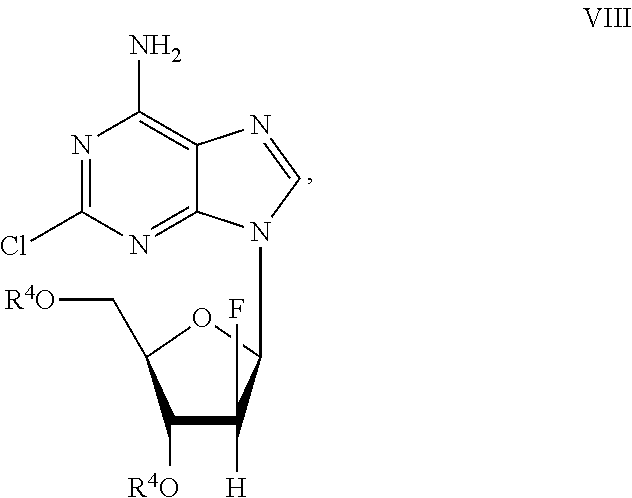
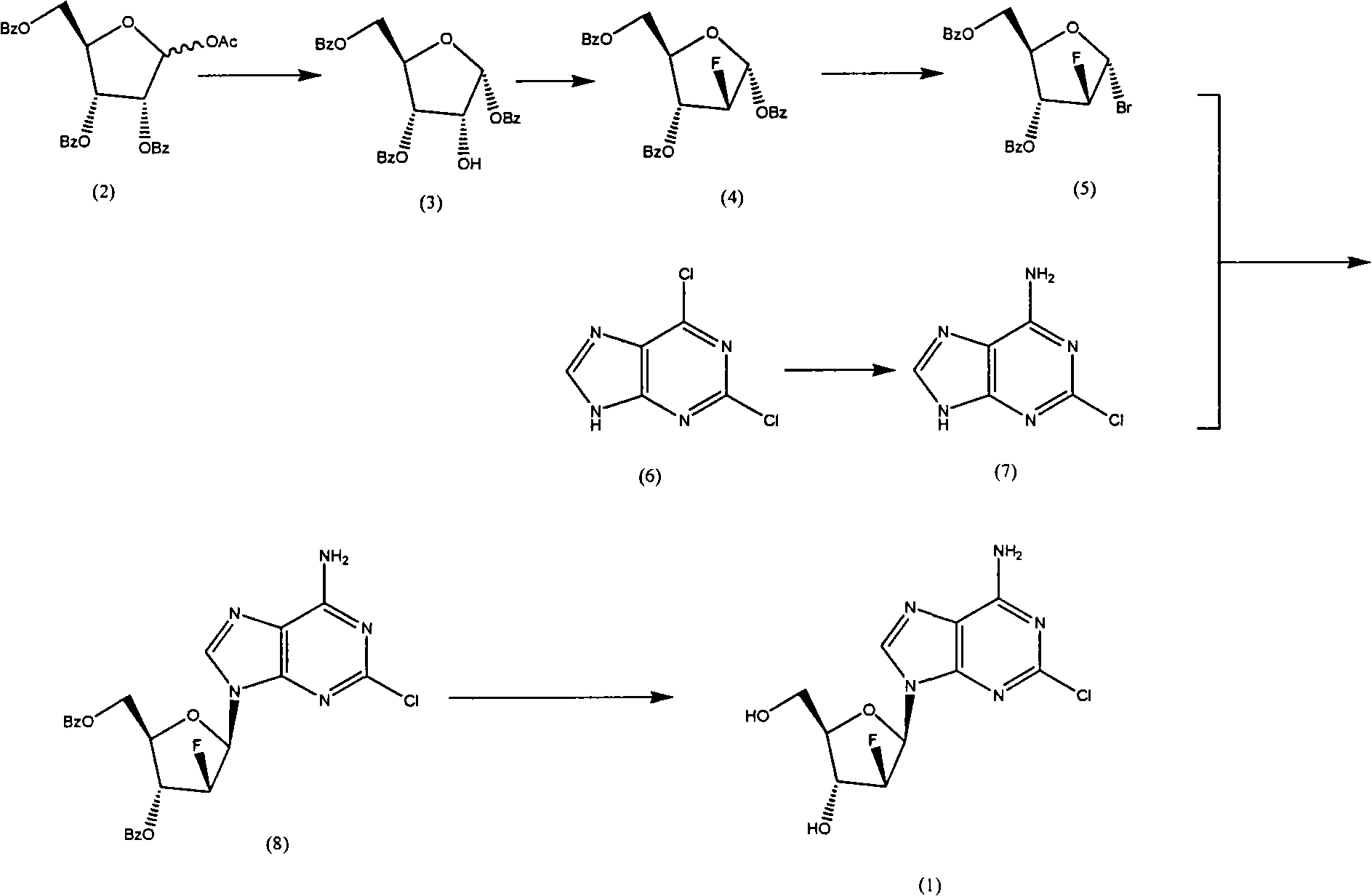
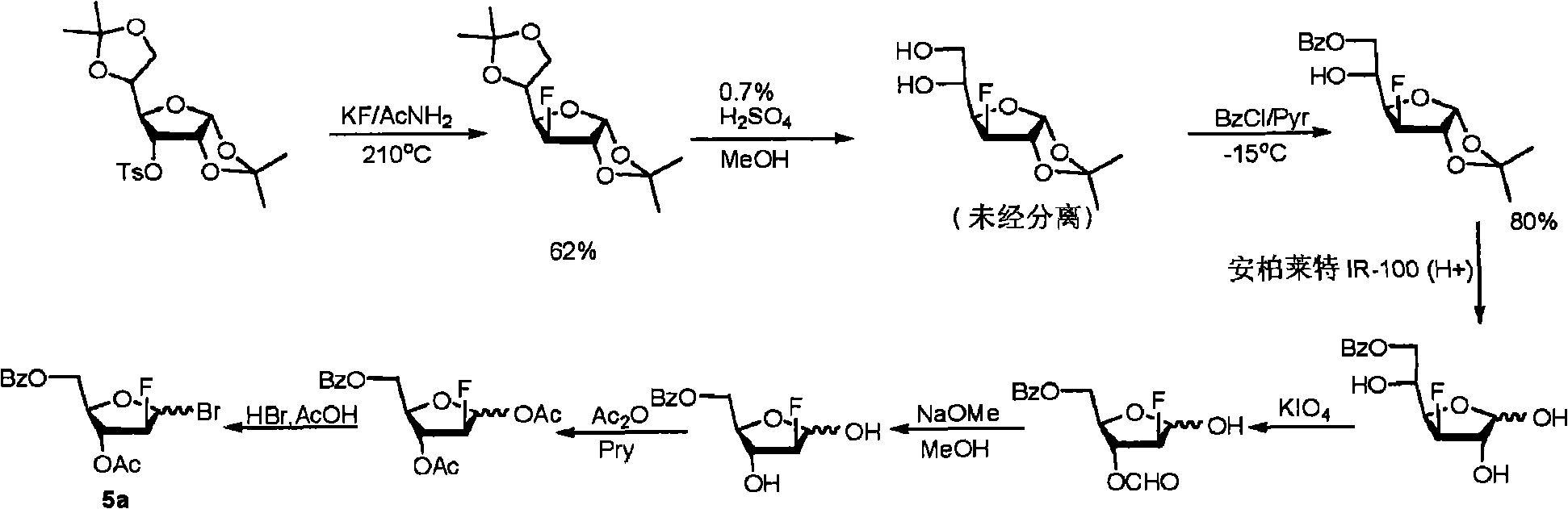
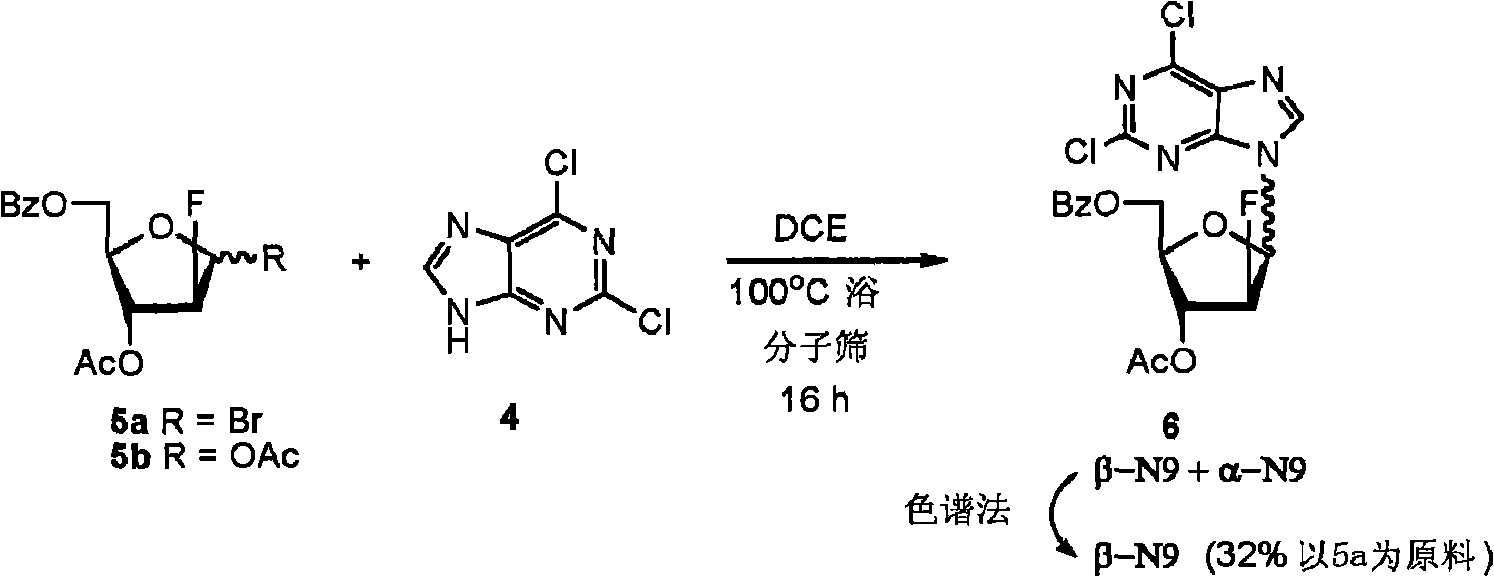
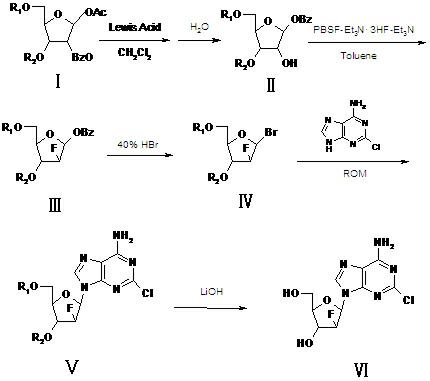
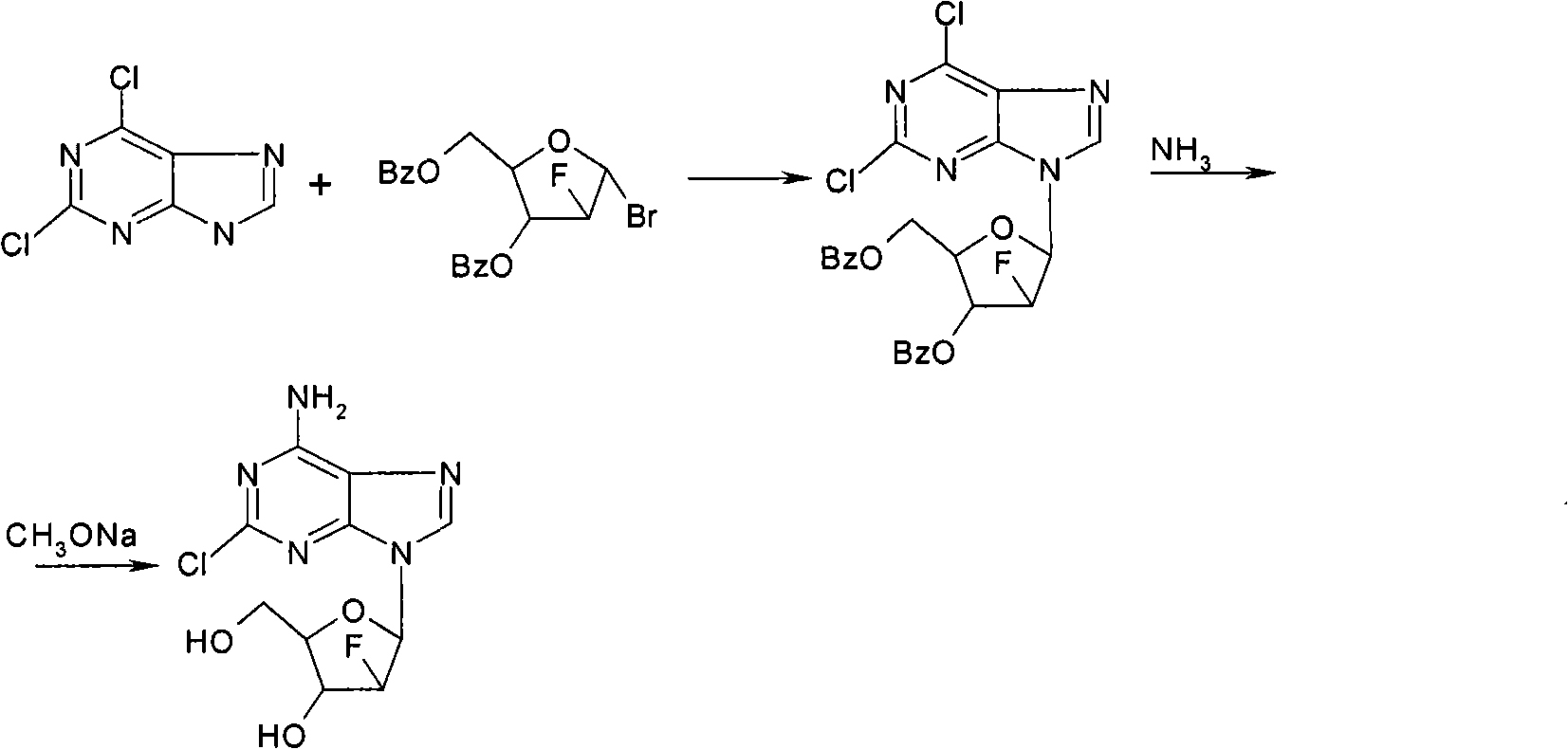
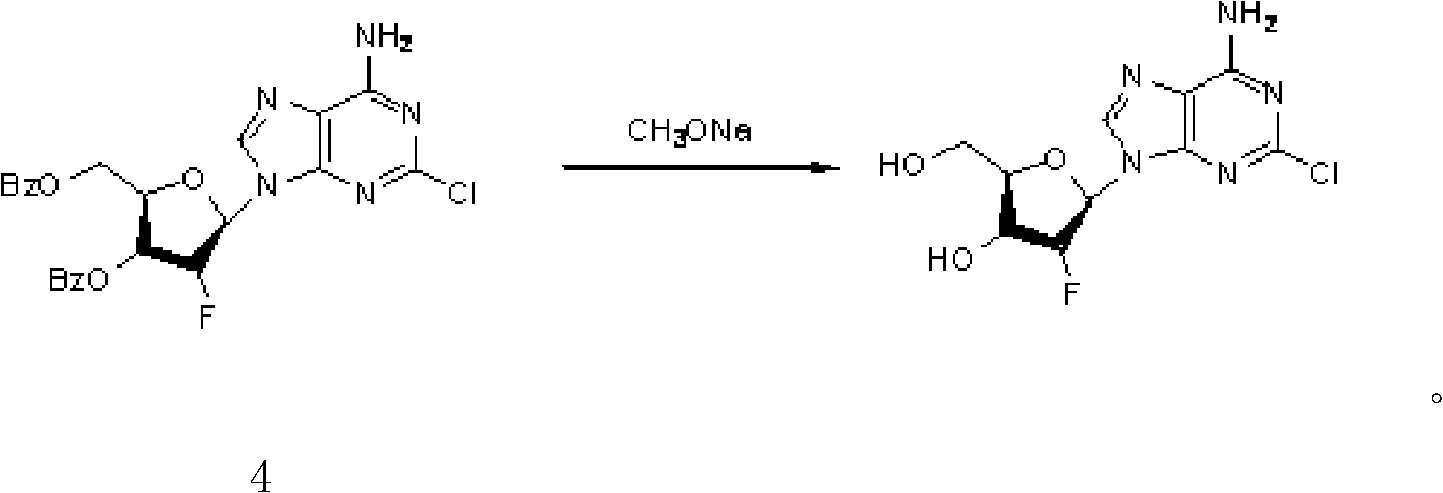
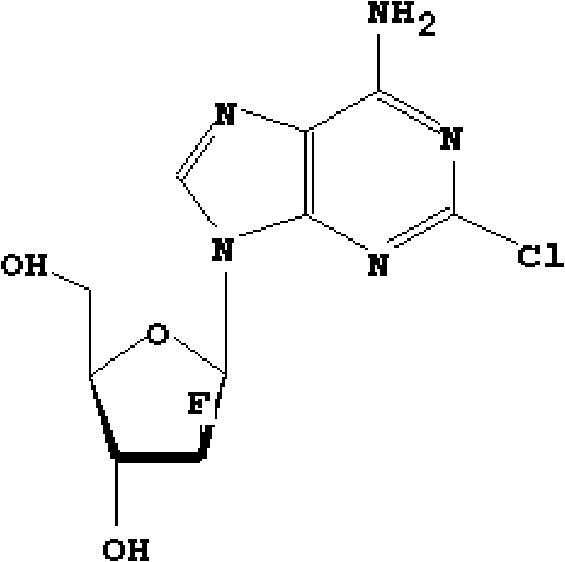
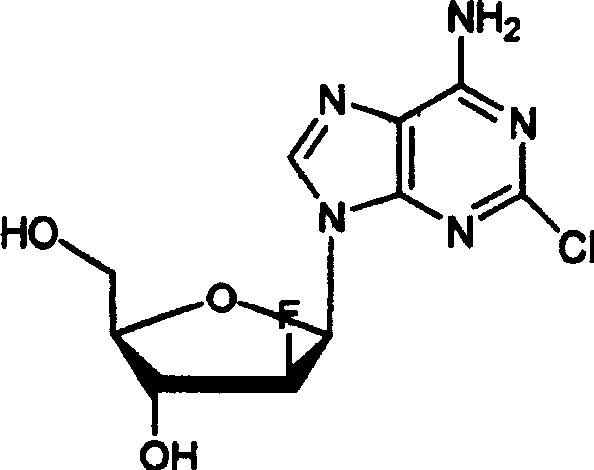
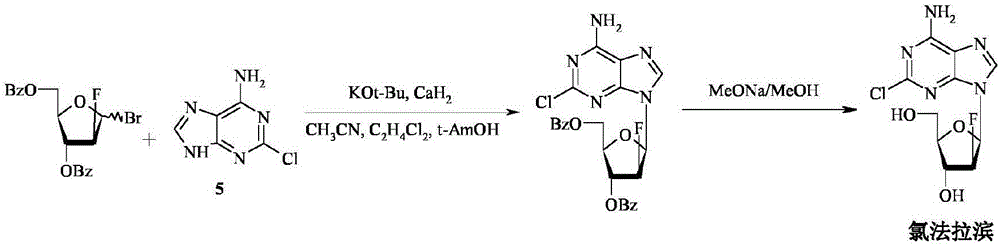
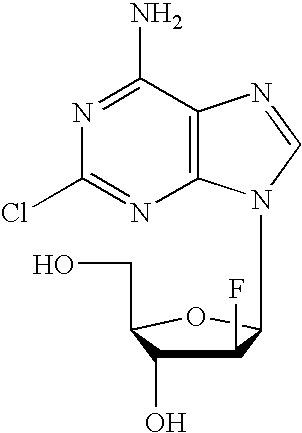
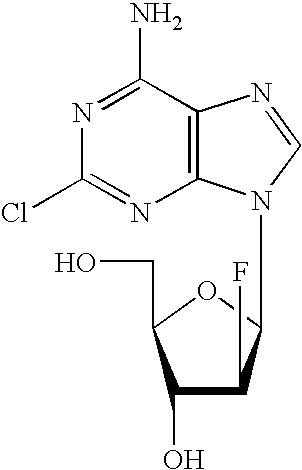
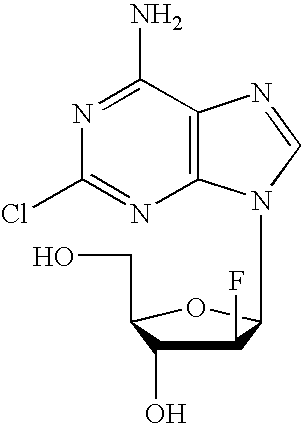
Preparation of 2-chloro-9-(2'-deoxy-2'-fluoro-Beta-D-arabinofuranosyl)-adenine
A process for making clofarabine comprising: fluorinating a compound of formula VIIwherein each R4 is independently a hydroxy protecting group, OR6 is a leaving group, with a fluorinating agent in the presence of guanidine carbonate to give a compound of formula VIII:wherein R4 is as defined above; and deprotecting the compound of formula VIII to give the clofarabine.
Owner:SCINOPHARM TAIWAN LTD
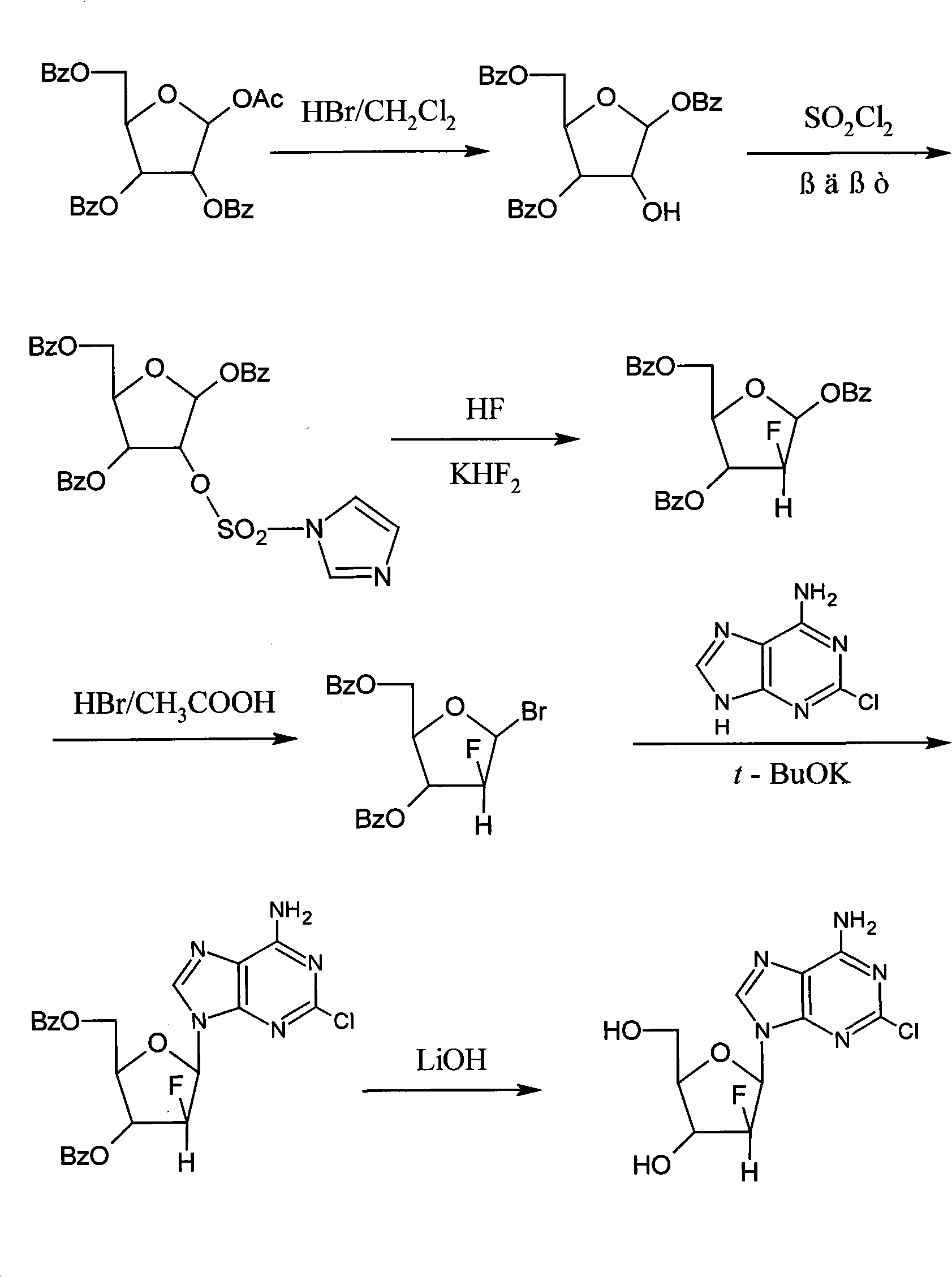
Method for synthesizing clofarabine
InactiveCN101265284AHigh selectivityHigh yieldSugar derivativesAntineoplastic agentsHydrobromidePhenacyl
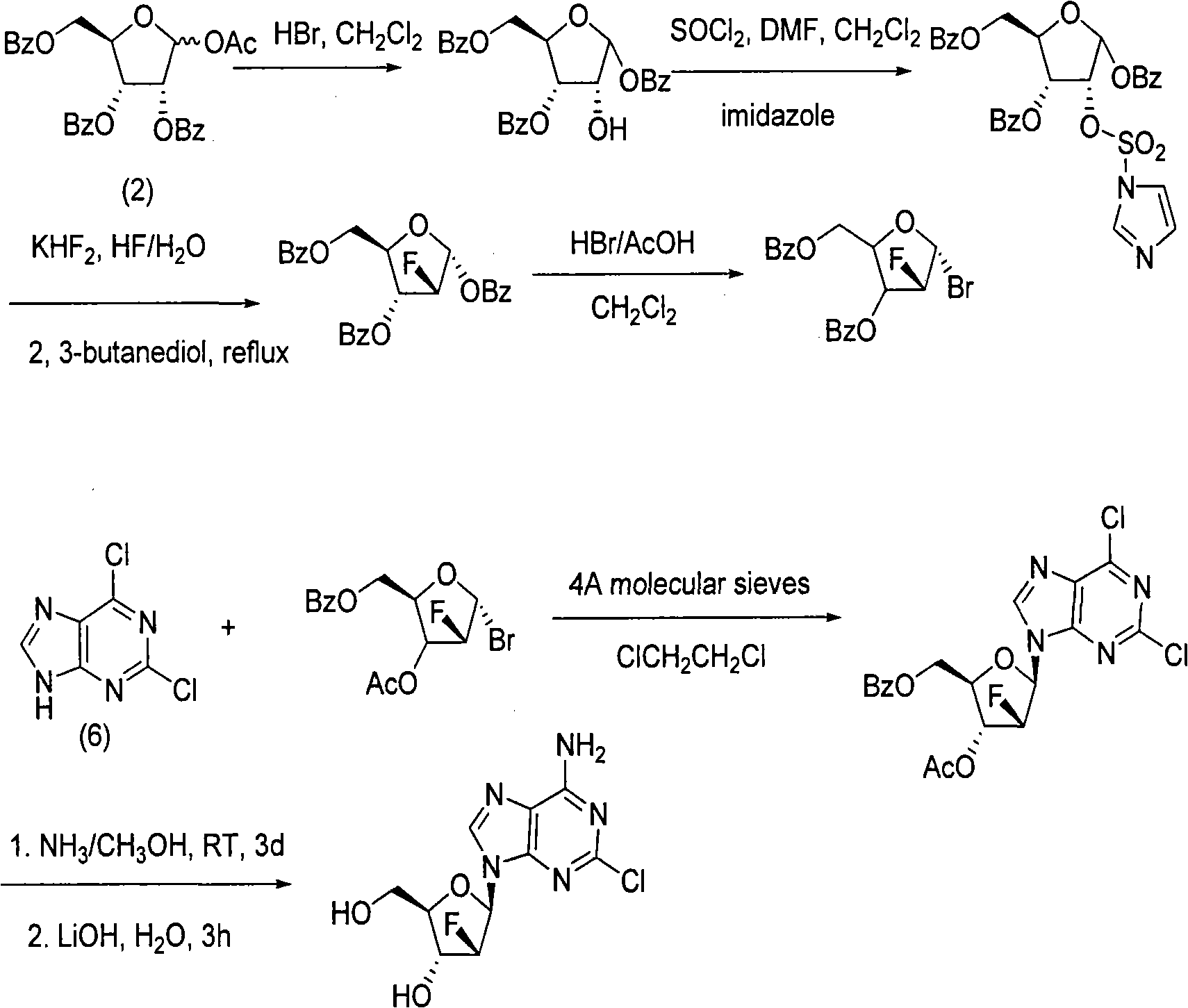
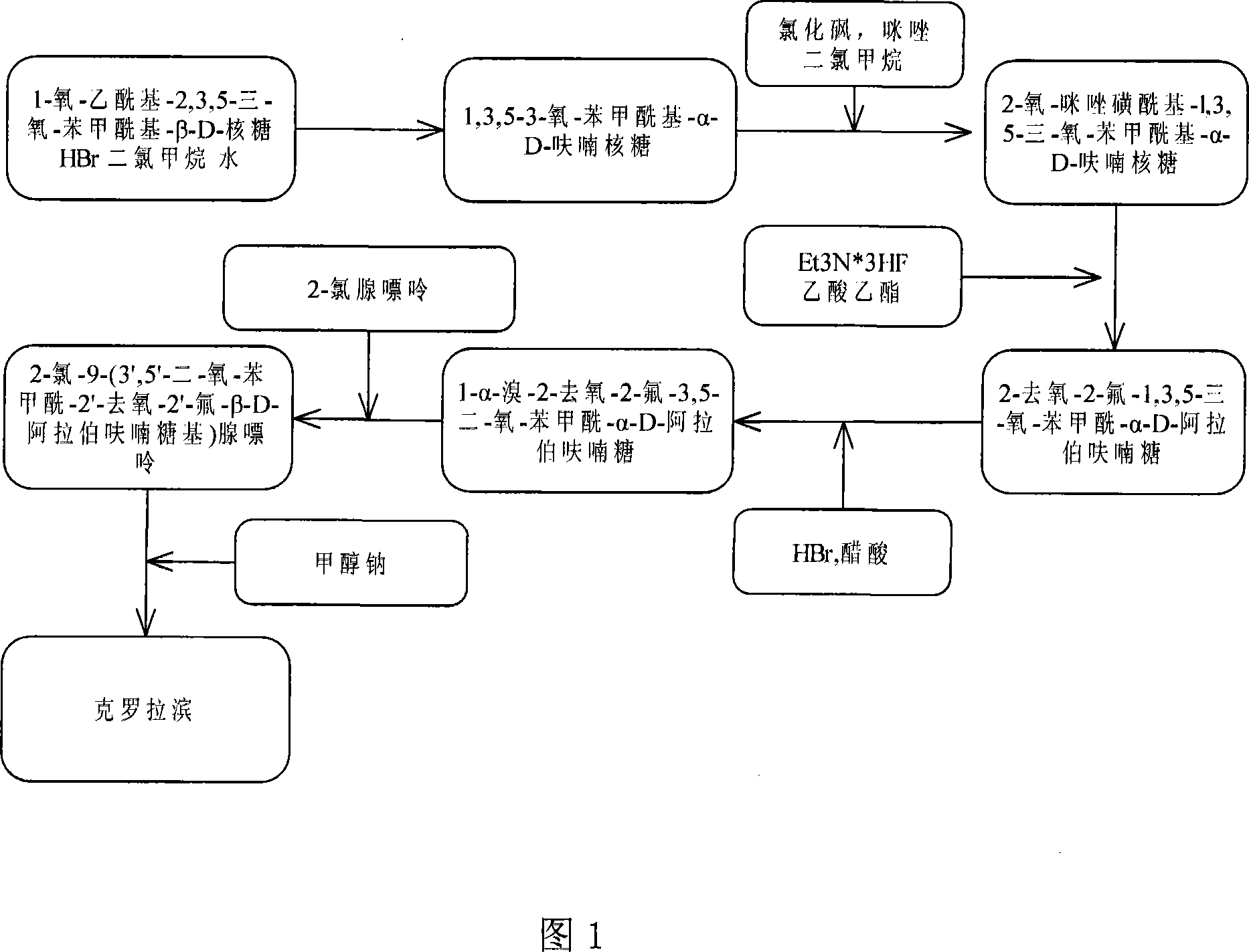
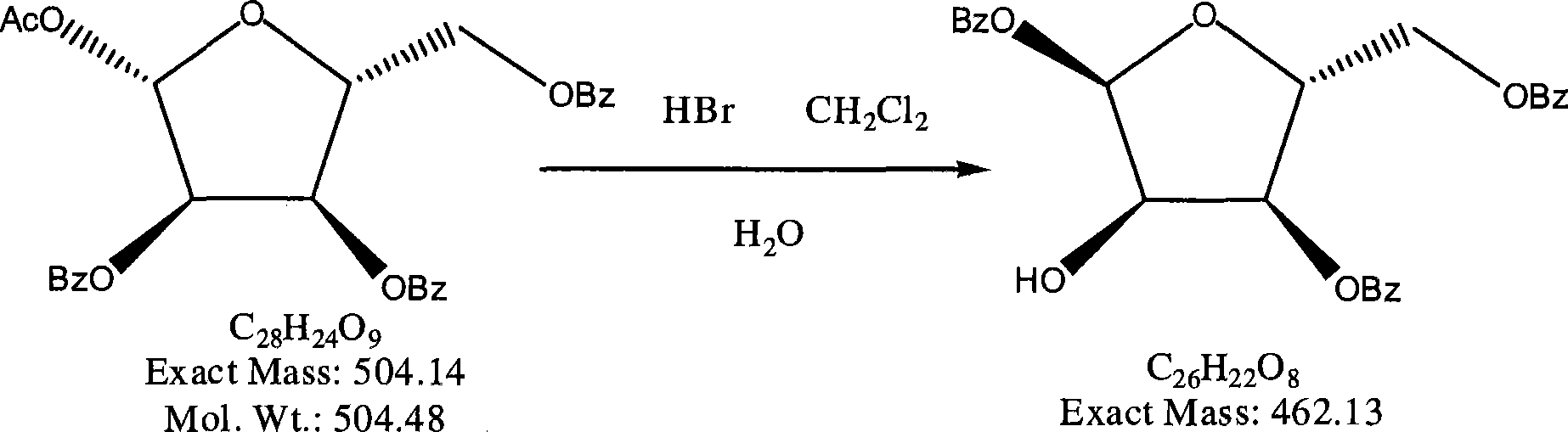
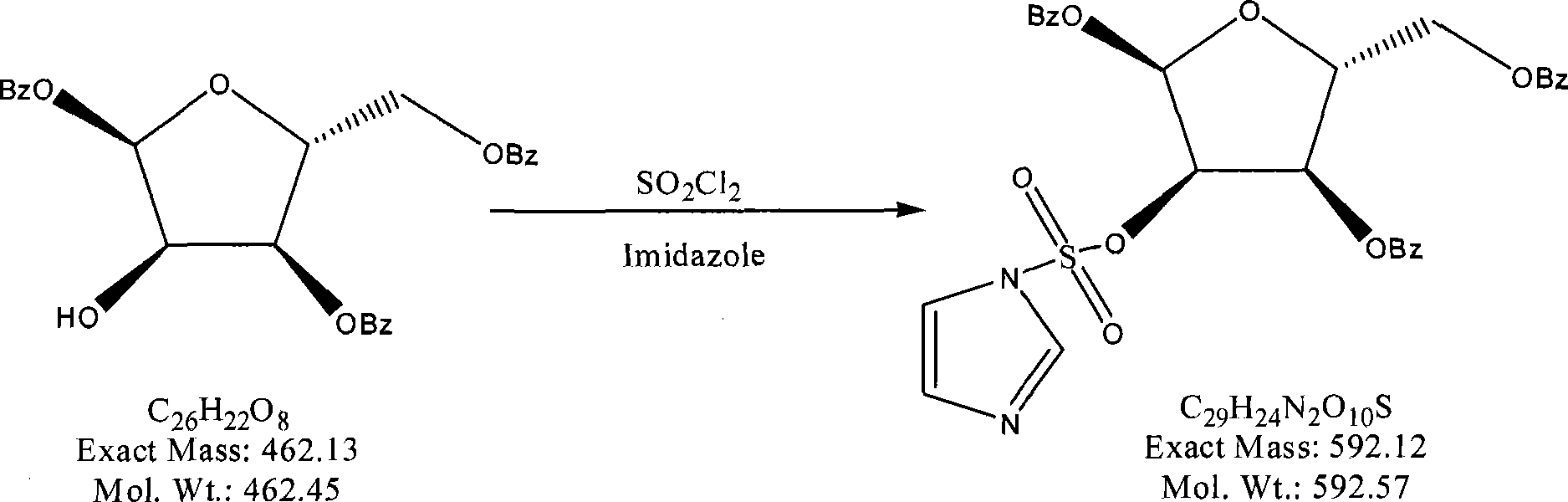
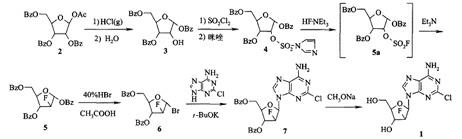
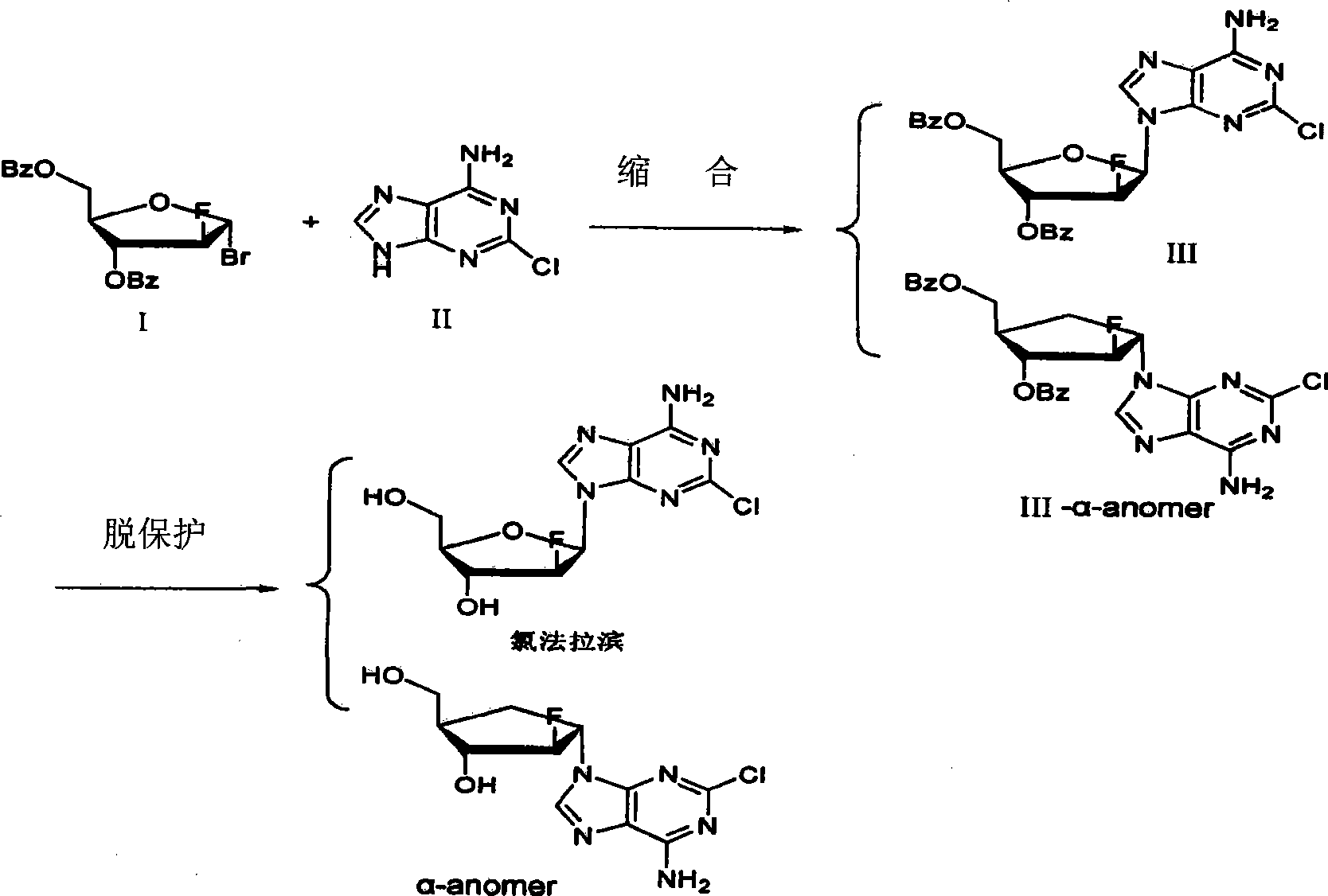
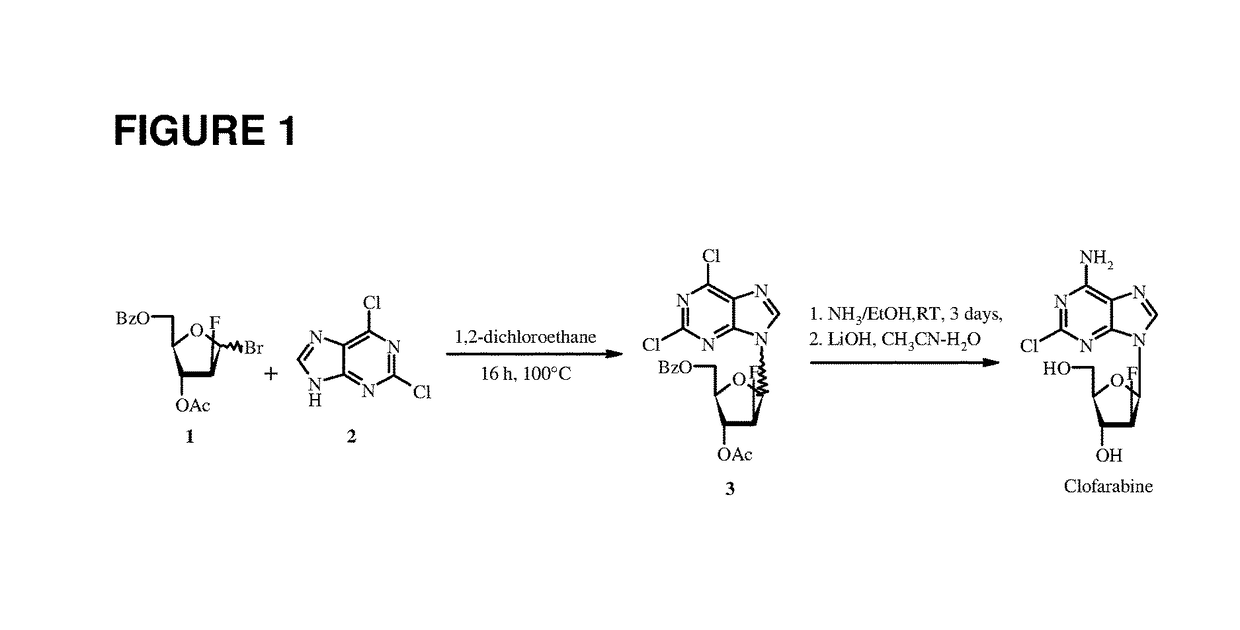
The invention provides a preparation method of clofarabine, which includes allowing 1-acetyl-2,3,5-tri-o-benzoyl-Beta-D-ribofuranose as the initial raw material and dichloromethane solution of hydrobromide to perform the rearrangement reaction, reacting with sulfuryl chloride and imidazole, performing the fluoridation reaction in the presence of hydrogen fluoride aqueous solution and potassium hydrogen fluoride, and performing bromination reaction in acetic acid solution of hydrogen bromide, condensing with 2-chloro adenosine in alkaline condition, and removing benzoyl in the presence of lithium hydroxide to obtain clofarabine. Compared with prior art, the method has the advantages of high yield of each step, higher total yield, and easily realized industrialized production.
Owner:深圳万乐药业有限公司
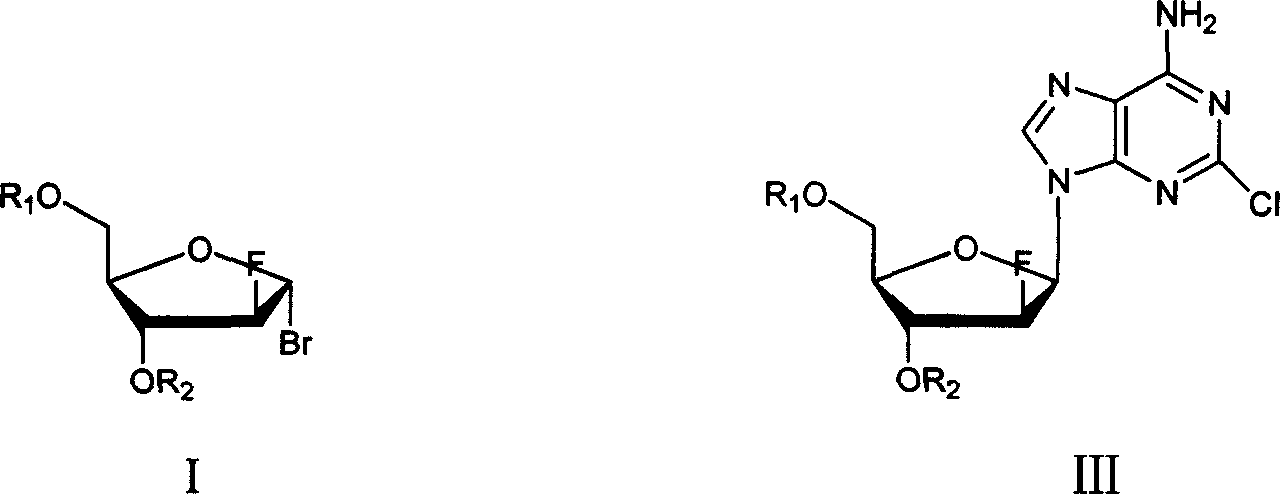
Method of producing high purity clofarabine
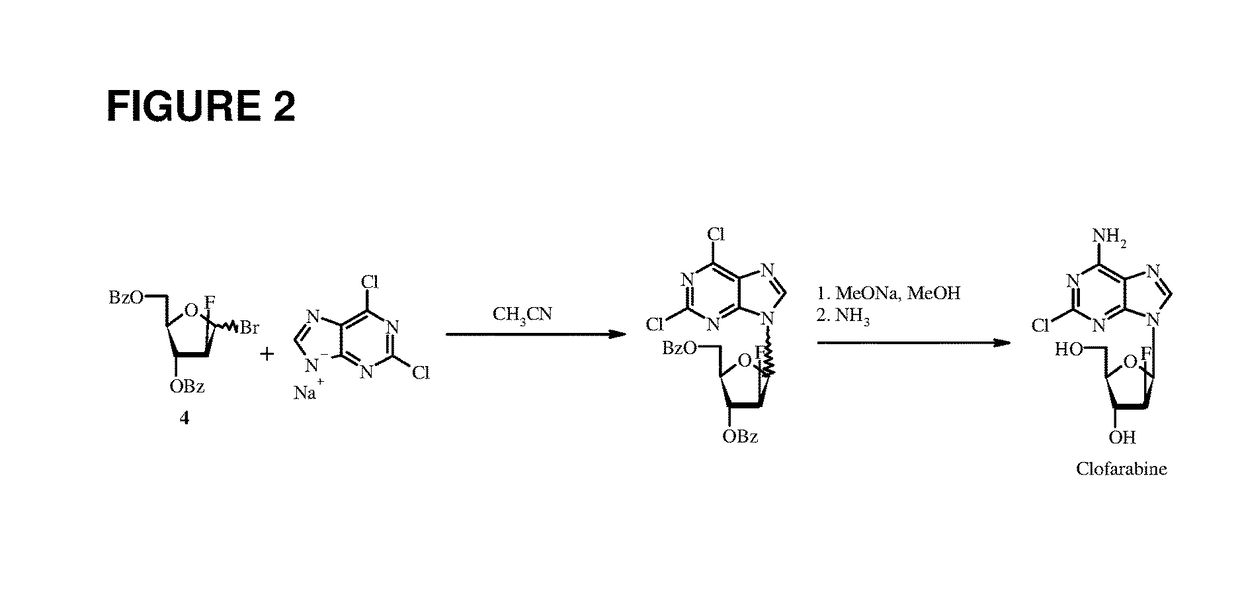
The present invention relates to a preparation method of clofarabine, comprising mixture of 2-adenine chlorine and the compound of formula I. the reaction is done under mild conditions and an appropriate temperature; the basically pure compound of the formula III can be prepared after separation. The high-purity clofarabine can be got through hydrolysis under alkaline conditions.
Owner:BEIJING D VENTUREPHARM TECH DEV
Synthesis method of clofarabine of nucleoside analogues
InactiveCN101555267AMild conditionsEasy to operateSugar derivativesAntineoplastic agentsSynthesis methodsPurine
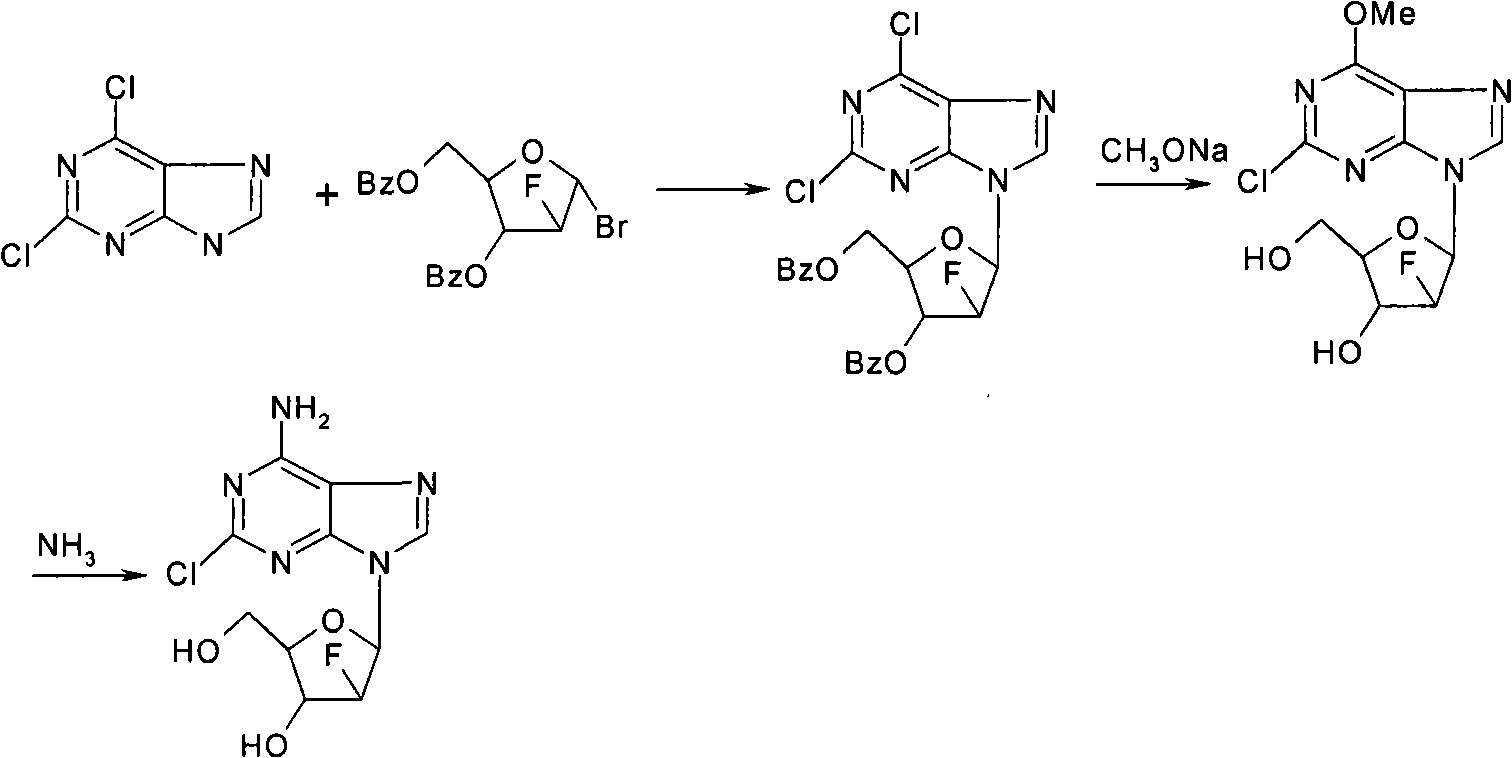
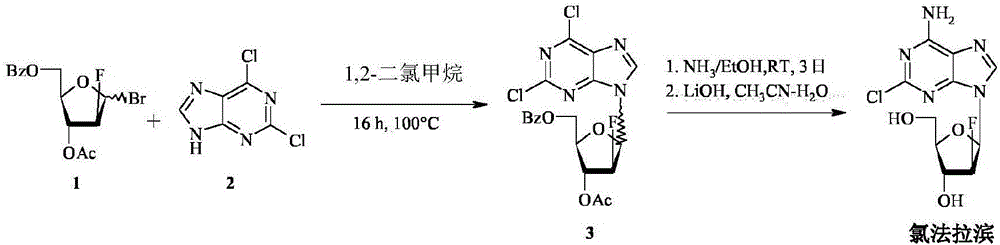
The invention provides a novel synthesis method of clofarabine , that is 6-amino-2-Cl-9-(2-deoxidation-2-Fl-Beta-D-ribofuranose)-9H-purine for treating leukemia, which comprises the following steps of crystallization, fluoridation, bromination reaction, ammonia reaction, selection reaction and methanol reaction. The invention has simple synthesis procedure, mild condition, easy operation, low cost, high yield and easy amplification, and adapts to the industrial production.
Owner:杭州容立医药科技有限公司
Methods and Compositions for the Treatment of Autoimmune Disorders Using Clofarabine
This invention relates to methods of treating or preventing an autoimmune disorder comprising the administration of clofarabine or a pharmaceutically acceptable salt, hydrate, solvate, clathrate, prodrug or metabolite thereof to a patient in need of such treatment. The invention further relates to methods of treating or preventing an autoimmune disorder comprising the administration of clofarabine or a pharmaceutically acceptable salt, hydrate, solvate, clathrate, prodrug or metabolite thereof and an additional therapeutic agent to a patient in need of such treatment.
Owner:GENZYME CORP
Method for preparing clofarabine raw material medicament
InactiveCN101240003AConducive to large-scale industrial productionReduce manufacturing costSugar derivativesInosinePurine
The invention discloses a preparing process of clorfarabine raw medicine, specifically provides steps of the process, material ratio and reaction condition of each step. The process is easy to implement, low in cost. The main material 2-chloro inosine is easy to get. The inventive process is beneficial to extensive industrial production of clorfarabine.
Owner:JILIN PAIGAO BIOLOGICAL PHARMA
Preparation of 2-chloro-9-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-adenine
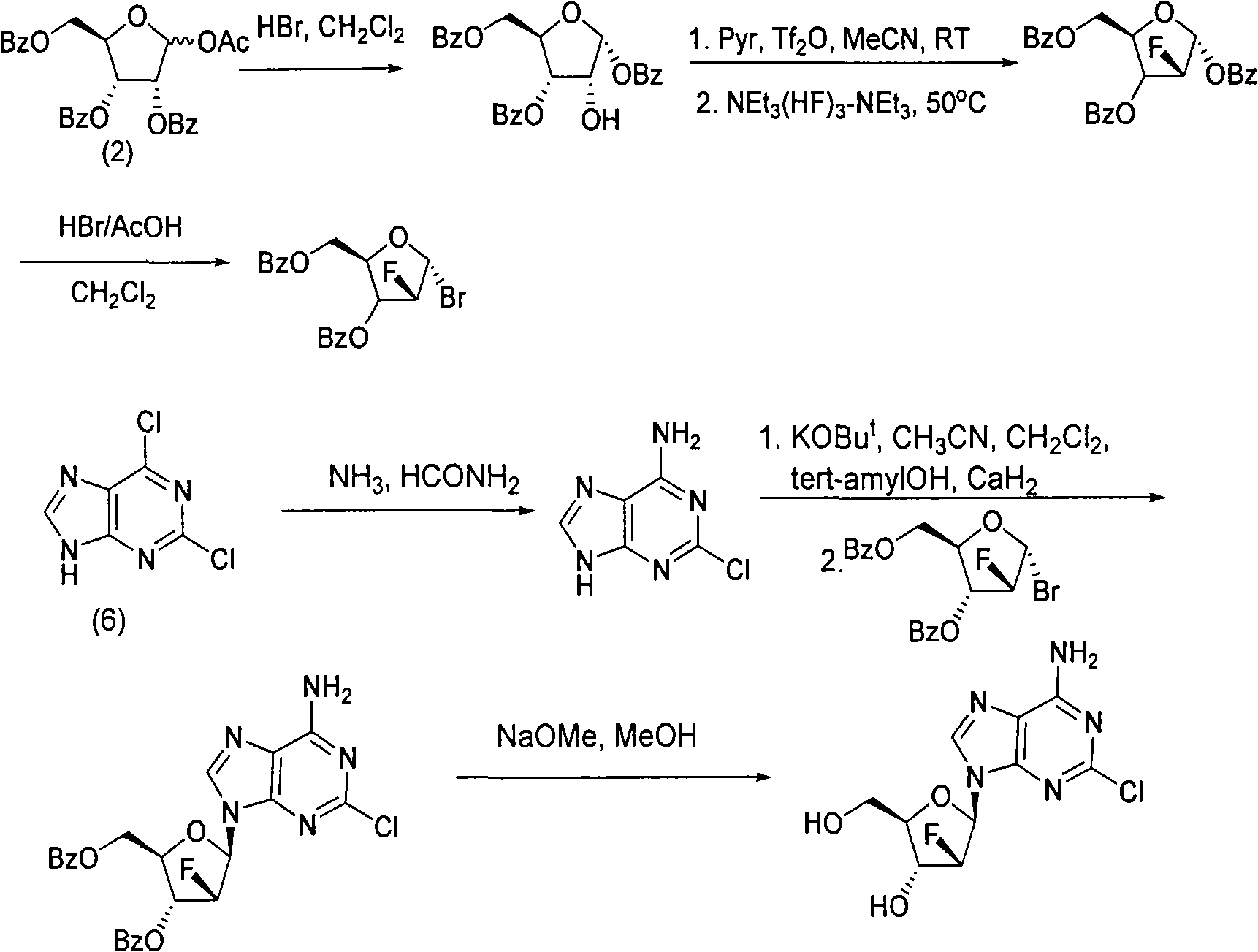
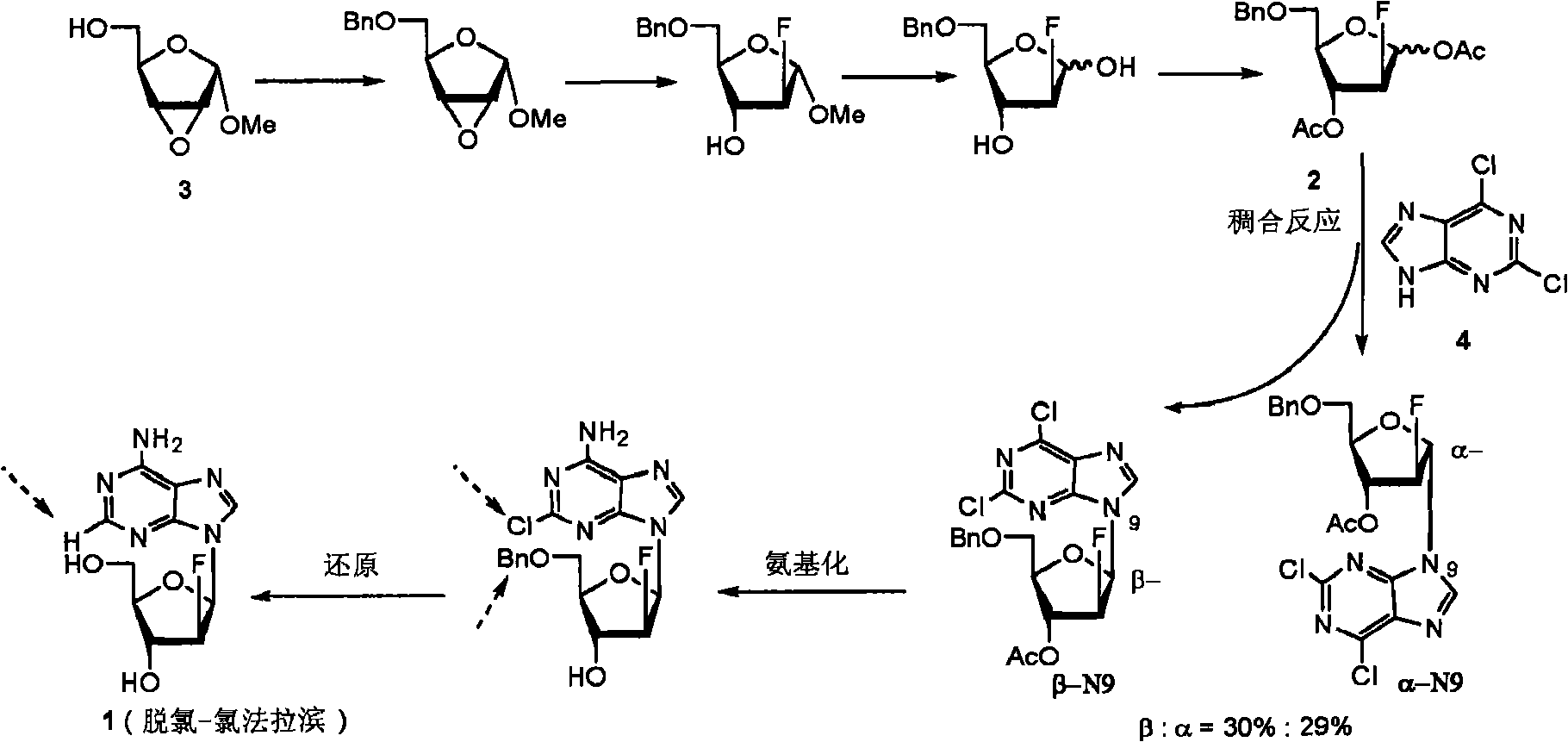
The invention discloses a novel synthesis method for clofarabine (also called 2-chloro-9-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-adenine), and relates to direct coupling of 1-O-acetyl-2,3,5-tri-O-benzoyl-ribofuranose (also called protected sugar) and bis-siliconized 2-chloroadenine (also called siliconized nucleobase), selective removal of a single benzoyl group, formation of sulphonate (sulfonylation), fluorination and removal of a protective group (scheme 8).
Owner:SCINOPHARM KUNSHAN BIOCHEM TECH
Freezing-dried clofarabine powder injection and its preparation method
InactiveCN101120925ALow content of related substancesEasy to storeOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFiltration
The present invention relates to a Clofarabine freeze drying powder and the preparation method, which comprises Clofarabine and at least one biological acceptable excipient. Wight ratio of the Clofarabine and the excipient is from one to 10 till one to 50. The excipient is selected from one kind of mannitol and lactose. The optional choice is the mannitol. The preparation method is as following. The Clofarabine is melted with the injection water and the excipient is added. Followed by well mixing , filtration, packaging, adding stopper and loading in the plate. The freeze dryer is opened in advance. The platelayer is cooled with the heat conduction oil until temperature of the platelayer increases till minus 40 DEG Cto minus 50 DEG C. The Clofarabine solution packaged in the sterile silin bottle can be sent to the freeze dryer quickly.The box door is shut and the plate temperature is kept at minus 40 DEG Cto minus 50 DEG C and the box temperature is decreased. When the sample temperature reaches minus 40 DEG C, The plate cooling is stopped and the condenser is opened. At the same time the product temperature is maintaineed by cooling for 3 hours. When the temperature of the condenser reaches minus 40 DEG, the vacuum system is opened. After temperature holding, four steps of heating, sublimation, drying, plugging, box pressing and opening sealing can be started.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
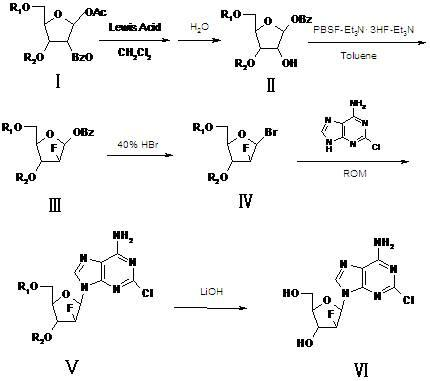
Synthesis method of clofarabine, midbody thereof and preparation method of midbody
ActiveCN102167716ASlightly acidicLow priceEsterified saccharide compoundsSugar derivativesTolueneClofarabine
The invention relates to a synthesis method of clofarabine, a midbody thereof and a preparation method of the midbody. The synthesis method comprises the steps of: 1) a compound I is reacted with strong lewis acid and water in dichloromethane respectively to generate a compound II; 2) the compound II is jointly reacted with PBSF and Et3N.3HF in methylbenzene and triethylamine to be taken as a fluorination agent, and is fluoridized by adding Et3N with proper amount to generate a compound III; 3) the compound III is reacted with hydrogen bromide to generate a compound IV; 4) the compound IV is condensed with 2-arprinocide and ROM to generate a compound V; and 5) the compound V is reacted with lithium hydroxide to prepare the clofarabine. After the synthesis method is used, the strong lewis acid which can be dissolved in the methylene dichloride is taken as a rearrangement agent, so that the complex step of feeding HBr or Hcl gas is eliminated; and as raw materials for the fluoridation are low in price and are relatively stable to water, the strict water-free operation does not need, and equipment can not be corroded, so that the synthesis method is safe to use. The preparation totalyield is improved to 18.3%.
Owner:FUZHOU NEPTUNUS FUYAO PHARMA
Synthesizing process of antineoplastic agent clofarabine
ActiveCN101830955AStarting materials are cheap and readily availableMild conditionsSugar derivativesSugar derivatives preparationChemical synthesisClofarabine
The invention belongs to a chemical synthesis drug process, particularly relating to a new synthesizing process of antineoplastic agent clofarabine, which has cheap, easily obtained original raw materials, moderate condition in the reaction and no device specially required, and can be applied to industrial production. In the method, a new synthesizing intermediate is adopted to carry out systemization, therefore, the invention also relates to a novel intermediate in use of the method.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Clofarabine pharmaceutical composition freeze-dried powder injection and preparation method thereof
ActiveCN101584672AImprove stabilityGood resolubilityPowder deliveryOrganic active ingredientsSodium bicarbonateMANNITOL/SORBITOL
The invention provides a clofarabine pharmaceutical composition freeze-dried powder injection which is prepared by freeze-drying 10-30 parts by weight of clofarabine, 100-300 parts by weight of mannitol, 20-60 parts by weight of sodium bicarbonate, pH regulator and water for injection. The clofarabine pharmaceutical composition freeze-dried powder injection is a white or almost white loose mass or powder with good stability and good product redissolution property. The invention also provides a preparation method of the clofarabine pharmaceutical composition freeze-dried powder injection with simple method and strong operability.
Owner:山东罗欣乐康制药有限公司 +1
Synthesis method of clofarabine
InactiveCN103665075AEasy to operateEasy to controlSugar derivativesSugar derivatives preparationSodium methoxideSynthesis methods
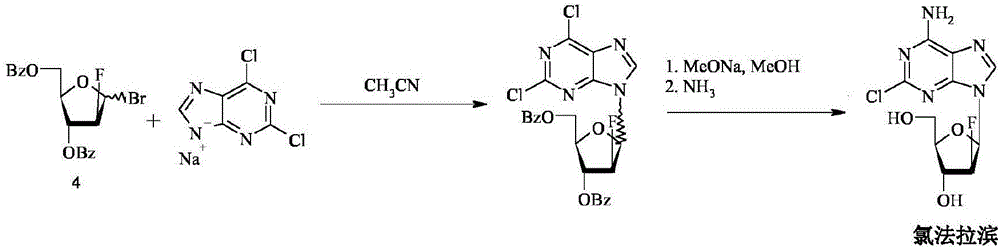
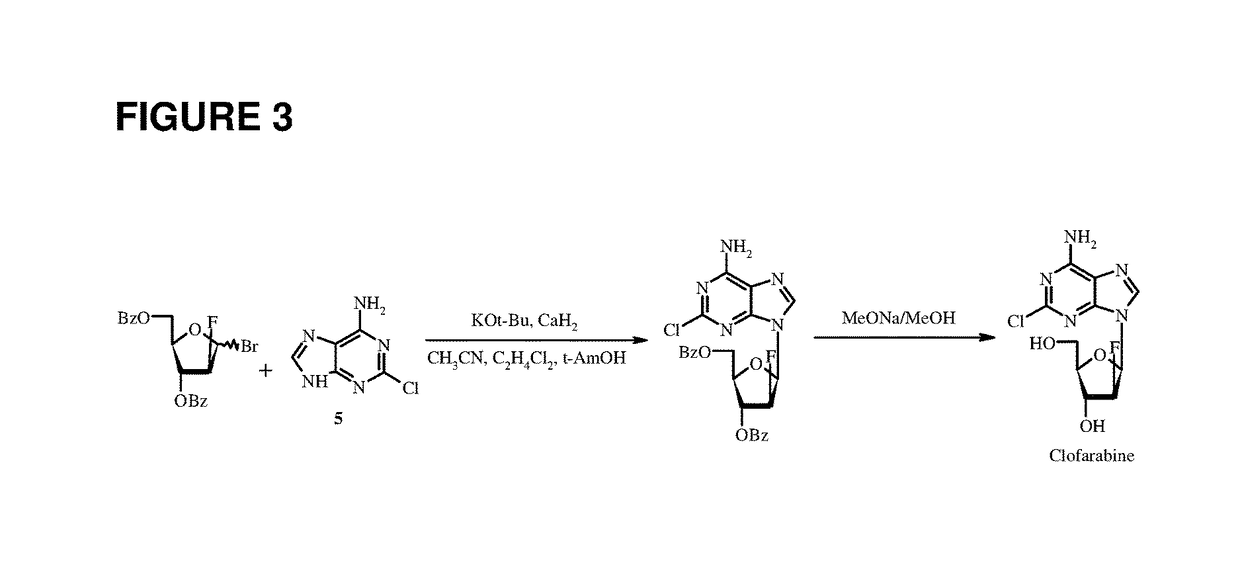
The invention discloses a synthesis method of clofarabine, which comprises the following steps of: 1) dissolving a chemical compound shown as Formula (I) in dichloromethane, adding a hydrogen bromide acetic acid solution for reaction, washing, separating an organic phase, drying, filtering, leaving a filtrate for future use, dissolving 2-chlorine adenine in acetonitrile, adding potassium tert-butoxide, calcium hydride and the above filtrate for reaction, filtering, washing with dichloromethane, reducing pressure, condensing, evaporating to dryness, adding butyl acetate, stirring, dropwise adding methyl tertiary butyl ether, stirring for crystallization, filtering, washing a filter cake with methyl tertiary butyl ether, drying to obtain a yellow solid, and 2) adding the yellow solid into a mixed solution of methanol and sodium methoxide, regulating pH (potential of hydrogen) to 6-6.5, filtering, washing a filter cake with methanol, obtaining a clofarabine crude product, adding absolute ethyl alcohol, stirring, heating to backflow, dissolving, filtering, washing a filter cake with alcohol, performing vacuum drying, and obtaining a target product. The method is simple in reaction operation, easy to control, high in yield and suitable for industrial production.
Owner:南通康鑫药业有限公司
Method for purifying clofarabine by using chromatographic column
InactiveCN101475621AEasy to operateNon-toxicSugar derivativesAntineoplastic agentsPurification methodsElution
The invention discloses a method for purifying clofarabine by a chromatographic column. The method adopts an inverse macroporous adsorption resin as a chromatographic column packing material to prepare the chromatographic column, adds a crude product water solution of the clofarabine with a concentration of between 0.001 and 0.025 weight percent into the chromatographic column, then performs the elution by pure water first, then orderly uses pure water / organic solution mixed liquids with the gradually reduced volume ratios as eluents for gradient elution, and collects and concentrates the eluents with qualified purity. The purification method ensures that the purity of a clofarabine product reaches more than 99.8 percent and the yield reaches more than 90 percent, has simple operation, and is suitable for industrial mass production.
Owner:深圳万乐药业有限公司
Method for the synthesis of clofarabine
Method for the production of the anticancer nucleoside clofarabine in high yield, the method comprising the preparation of 2- chloroadenosine by enzymatic transglycosylation between 2- chloroadenine and nucleosides, benzoylation, isomerization, sulfonate ester formation, fluorination, and deprotection.
Owner:SYNBIAS PHARMA
A sustained release anticancer agent carrying angiogenesis inhibitor and clorfarabine
Disclosed is an anticancer slow release injection carrying both anti-angiogenesis agent and clofarabine, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents, slow release auxiliary materials, and specific dissolvent containing suspension adjuvant. The anti-angiogenesis agent is selected from Marimastat, Fumagillin, gefinitib, erlotinib, lapatinib, lapatinib, endothelium chalone, imatinib, Imatinib, Gasanib, Avastin, Cananib, sorafenib, sunitinib, oersteda or panitoma, the slow release auxiliary materials are selected from Polifeprosan, sebacylic acid copolymer, EVAc, polylactic acid and copolymer, The viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
Method for the synthesis of clofarabine
The present invention relates to a method for the high yield production of the anticancer nucleoside clofarabine, the method comprising the preparation of 2-chloroadenosine by enzymatic transglycosylation between 2-chloroadenine and nucleosides, benzoylation, isomerization, sulfonate ester formation, fluorination, and deprotection.
Owner:SYNBIAS PHARMA
Methods and compositions for the treatment of autoimmune disorders using clofarabine
InactiveUS20060100167A1Minimizing spreadMinimizing worseningBiocideCarbohydrate active ingredientsDiseaseMetabolite
This invention relates to methods of treating or preventing an autoimmune disorder comprising the administration of clofarabine or a pharmaceutically acceptable salt, hydrate, solvate, clathrate, prodrug or metabolite thereof to a patient in need of such treatment. The invention further relates to methods of treating or preventing an autoimmune disorder comprising the administration of clofarabine or a pharmaceutically acceptable salt, hydrate, solvate, clathrate, prodrug or metabolite thereof and an additional therapeutic agent to a patient in need of such treatment.
Owner:WOOD CHRISTOPHER B +1
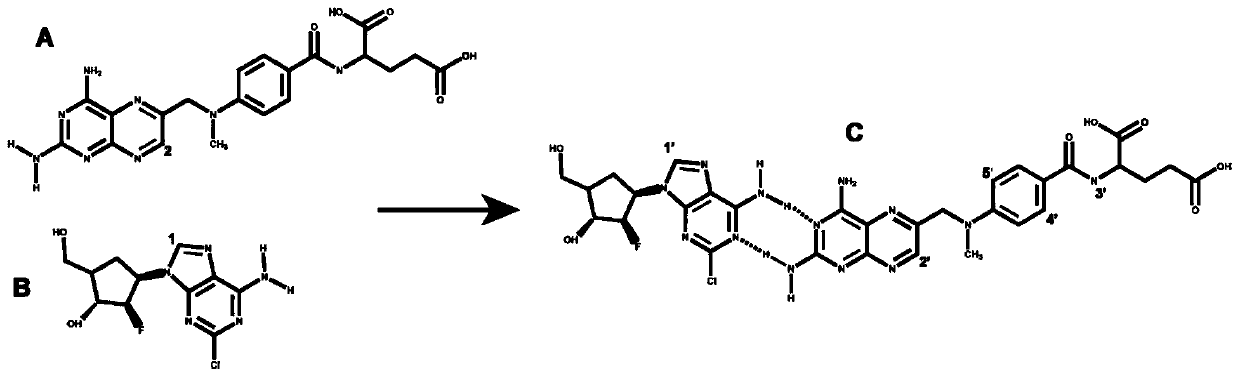
Clofarabine and methotrexate double-medicine preparation and preparation method thereof
InactiveCN110063962AImprove stabilityLow toxicityOrganic active ingredientsPowder deliveryMedicineFreeze-drying
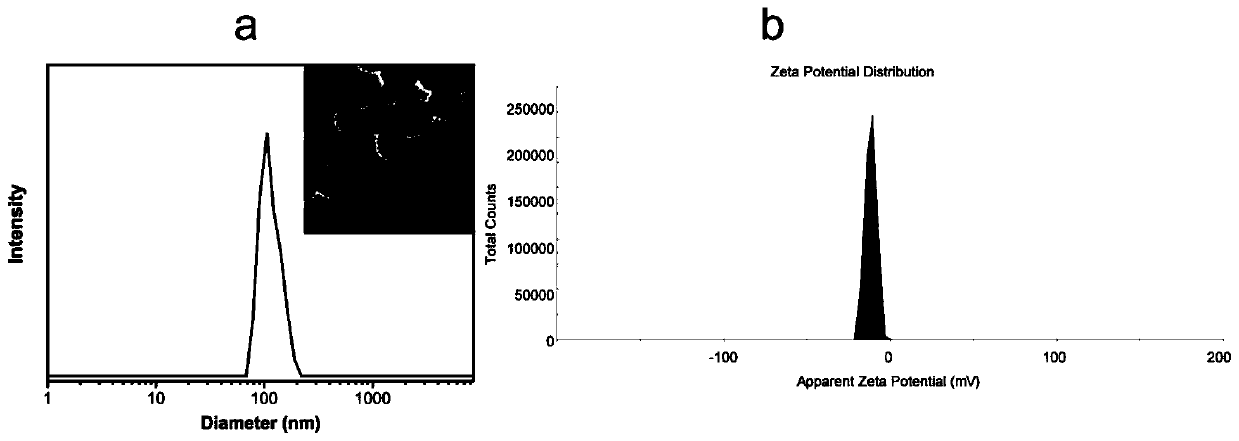
The invention discloses a clofarabine and methotrexate double-medicine preparation and a preparation method thereof. The clofarabine and methotrexate double-medicine preparation is prepared from the effective components of clofarabine and methotrexate according to the molar ratio of (0.8-1.2):(0.8-1.2), wherein the clofarabine serves as main medicine, the methotrexate serves as auxiliary medicineand targeting molecules, the methotrexate and the clofarabine are connected through the hydrogen-bond effect to form micelle, and the micelle is freeze-dried under vacuum. The grain diameter of the clofarabine and methotrexate double-medicine nano-micelle is 100-200 nm, the stability of the preparation obtained after freeze-drying is good, a good responsiveness release characteristic is achieved after the preparation is injected into the human body, it is proved through the cytotoxicity test that compared with pure double-medicine use of the clofarabine and the methotrexate, a higher tumor suppression effect is achieved, and the preparation is suitable for serving as an intravenous injection preparation, and has the advantages of being high in targeting performance, long in circulation andlow in toxicity and achieving synergistic treatment.
Owner:XIAMEN UNIV
An anticancer sustained release injection carrying clorfarabine and its synergist
InactiveCN101254166AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientPolyethylene glycol
A slow release anticancer injection carrying clofarabine and a synergist thereof comprises slow release microspheres and a solvent, wherein the slow release microspheres comprise effective anticancer ingredients and slow release adjuvants, and the solvent is a special solvent containing a suspending agent. The effective anticancer ingredients comprise clofarabine and a clofarabine synergist selected from phosphoinositol-3-kinase inhibitor, pyrimidine analogues and / or DNA repairase inhibitor; the slow release adjuvants comprise biocompatible polymers such as polylactic acid (PLA) and copolymer thereof, polylactic acid-polyethylene glycol (PEG)-COOH copolymers, fatty acid dimmer-sebacic acid copolymers, polys(erucic acid dimmer-sebacic acid), polys(fumaric acid-sebacic acid), polifeprosan, polylactic acid and EVAc; the suspending agent has viscosity of 100-3,000cp (at 20-30 DEG C) and is selected from sodium carboxymethyl cellulose, etc. The effective anticancer ingredients and the slow release microspheres can also be made into slow release implant, and the slow release injection and implant can be injected into tumor or around tumor to effectively inhibit tumor growth and remarkably strengthen the effect of non-surgery treatments as chemotherapy or the like.
Owner:JINAN KANGQUAN PHARMA TECH
Methods and pharmaceutical compositions for inhibiting influenza viruses replication
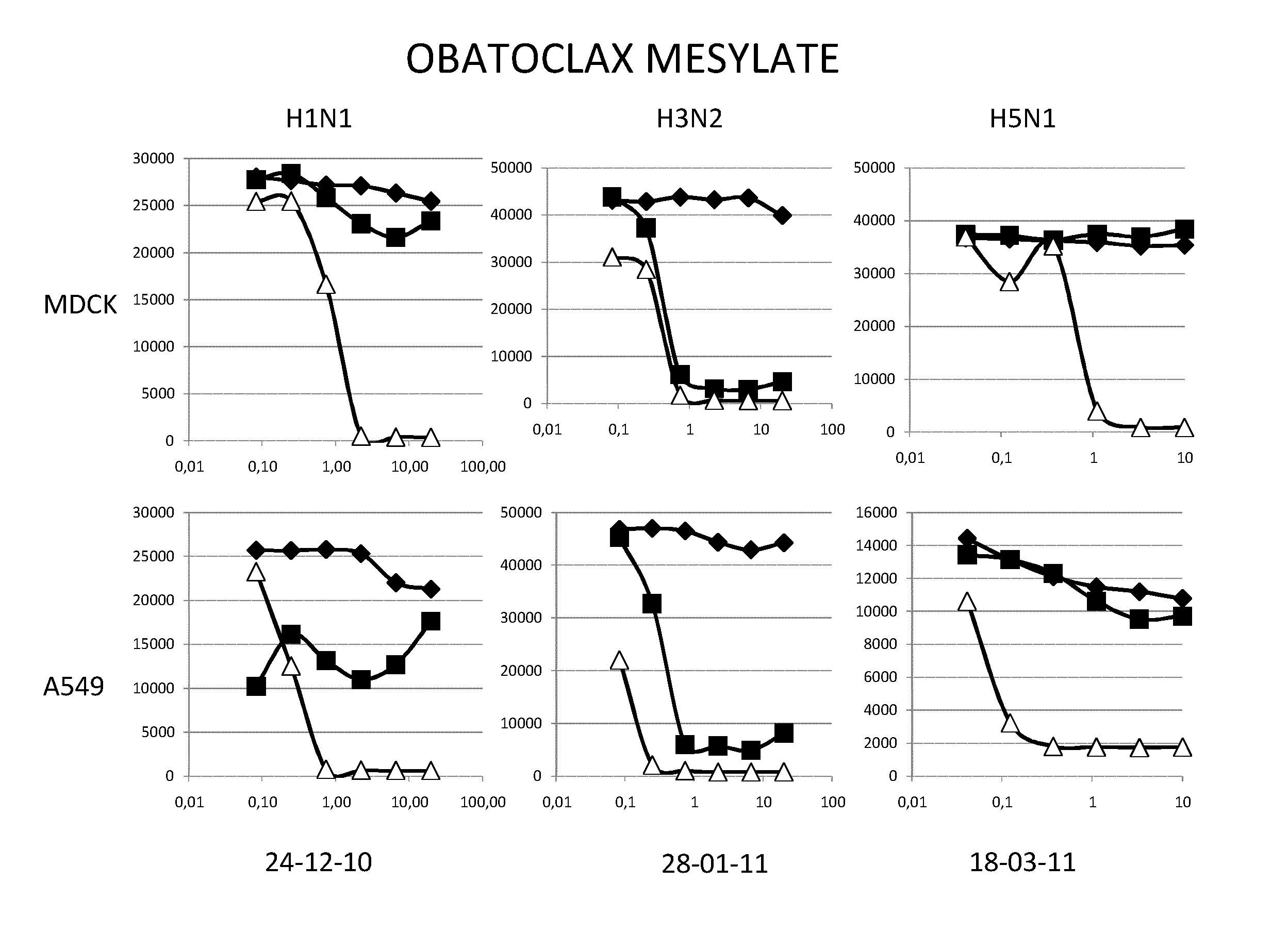
InactiveUS9168236B2Reduction and amelioration of progression and severity and durationReduce severityTripeptide ingredientsAntiviralsGSK3B geneDocetaxel
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Compound anticancer sustained-release agent containing Clofarabine
InactiveCN101273969AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismMicrospherePolyethylene glycol
A compound anti-cancer sustained-release injection containing clofarabine is composed of sustained-release microspheres and a solvent, wherein, the sustained-release microspheres comprise anti-cancer effective components and sustained-release excipients, the anti-cancer effective components are composed of the clofarabine and a clofarabine synergist which is selected from anti-tumor antibiotics and / or anti-metabolism drugs, the solvent is the special solvent containing a suspending agent; the sustained-release excipients are selected from polylactic acid / glycolic acid copolymer, monomethyl polyethylene glycol / polylactic acid, polyethylene glycol / polylactic acid, terminal carboxyl polylactic acid, terminal carboxyl polylactic acid / glycolic acid copolymer, EVAc, copolymer of fatty acid and sebacic acid and so on; the viscosity of the suspending agent is 80cp to 3000cp ( at 20 DEG C to 30 DEG C ), and the suspending agent is selected from carboxymethyl cellulose and so on; the sustained-release microspheres can further be made into a sustained-release implant which is injected or arranged in a tumor or the tumor periphery, and the local drug release can approximately last 30 to 50 days. The independent application of the sustained-release injection and the sustained-release implant can effectively inhibit the tumor growth, and the combined application with chemotherapy drugs and / or radiotherapy and other non-surgical therapies, and also can significantly enhance the efficacy.
Owner:JINAN KANGQUAN PHARMA TECH
Universal cart/tcrt cells with chemotherapeutic drug resistance and construction method thereof
ActiveCN107746831BPreserve targetingAvoid exclusionImmunoglobulin superfamilyTransferasesAntigenAntigen receptors
The invention belongs to the fields of genetic engineering and synthetic biology, and particularly relates to a universal CART / TCRT cell with chemotherapeutic drug resistance and a construction methodof the universal CART / TCRT cell. The universal CART / TCRT cell is an allogeneic T cell with chimeric antigen receptors and T cell receptors, wherein the alpha and beta chains of the T cell receptors and deoxycytidine kinase molecules are knocked out; and the knocking-out technology for the alpha and beta chains of the T cell receptors and the deoxycytidine kinase molecules is a CRISPR technology;for the universal CART / TCRT cell, the targeting property of the tumor specific antigen is reserved, the problems of GvHD and rejection are also eliminated, meanwhile, the sensitivity for the chemotherapeutic medicines Fludarabine and Clofarabine is weakened, and the universal CART / TCRT cell can be used as a universal simple, low-cost and high-activity CART / TCRT cell preparation.
Owner:NANJING BIOHENG BIOTECH CO LTD
Methods and Compositions for the Treatment of Lupus Using Clofarabine
InactiveUS20090297478A1Good curative effectMinimize delayBiocidePeptide/protein ingredientsSystemic lupus erythematosusClofarabine
This invention relates to methods of treating or preventing lupus comprising the administration of clofarabine or a pharmaceutically acceptable salt, hydrate, solvate or clathrate thereof to a patient in need of such treatment. The invention further relates to methods of treating or preventing lupus comprising the administration of clofarabine or a pharmaceutically acceptable salt, hydrate, solvate or clathrate thereof and an additional therapeutic agent to a patient in need of such treatment.
Owner:WOOD CHRISTOPHER B +1
Methods and compositions for the treatment of lupus using clofarabine
InactiveUS20060135463A1Minimizing spreadMinimizing worseningBiocideCarbohydrate active ingredientsClofarabineSystemic lupus erythematosus
This invention relates to methods of treating or preventing lupus comprising the administration of clofarabine or a pharmaceutically acceptable salt, hydrate, solvate or clathrate thereof to a patient in need of such treatment. The invention further relates to methods of treating or preventing lupus comprising the administration of clofarabine or a pharmaceutically acceptable salt, hydrate, solvate or clathrate thereof and an additional therapeutic agent to a patient in need of such treatment.
Owner:WOOD CHRISTOPHER B +1
A sustained release anticancer agent containing clorfarabine and cytotoxic drug
InactiveCN1875935AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryAdjuvantMicrosphere
Disclosed is an anticancer slow release injection containing clofarabine and cytotoxic drugs, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents include clofarabine and cytotoxic drugs, the cytotoxic drugs are selected from anti-taxone, alkylating agent, Topo enzyme inhibitor and / or plant alkaloid, The slow release auxiliary materials are selected from polylactic acid and glycollic acid copolymer, polyethylene glycol and polylactic acid copolymer, PLA-COOH copolymer, EVAc, aliphatic acid and sebacylic acid copolymer, The viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C),and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
An anticancer sustained release injection carrying clorfarabine and its synergist
InactiveCN1875934AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryAdjuvantMicrosphere
Disclosed is an anticancer slow release injection carrying both clofarabine and its synergistic agent, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer effective ingredients include clofarabine and its synergistic agents selected from phosphoinositide-3-enzyme inhibitor, pyrimidine analog and / or DNA restoring enzyme inhibitor, the slow release auxiliary materials are selected from polylactic acid and copolymer, polyethylene glycol, PLA-COOH copolymer, di-aliphatic acid and sebacylic acid copolymer, poly(erucic aciddipolymer-sebacylic acid), poly(fumaric acid-sebacylic acid), Polifeprosan, poly(lactic acid) and EVAc, the viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
A sustained release injection carrying clorfarabine and cytotoxic drug
PendingCN1875937AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryAdjuvantMicrosphere
Disclosed is an anticancer slow release injection carrying both clofarabine and cytotoxic drugs, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents include clofarabine and cytotoxic drugs selected from adriamycin, pidorubicin, Melphalan, 4-hydroperoxycyclophosphamide, actinomycin D, Vinorelbine, Tamoxifen, Tallimustine, Atrimustine, Semustine and ranimustine, the slow release auxiliary materials are selected from polylactic acid and copolymer, monomethyl polyethylene glycol, polyethylene glycol and polylactic acid copolymer, EVAc, aliphatic acid and sebacylic acid copolymer, The viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
Compound anticancer sustained-release injection containing clofarabine
InactiveCN101352413AEasy injectionIncrease drug concentrationOrganic active ingredientsPharmaceutical delivery mechanismMicrosphereAdditive ingredient
The invention discloses a clofarabine compound anticancer slow-release injection which is an anticancer slow-release injection and consists of slow-release microspheres and solvent; wherein, the slow-release microspheres comprise anticancer effective ingredients and slow-release auxiliary materials; the solvent is a special solvent containing a suspending agent; the anticancer effective ingredients are combination of the clofarabine and a clofarabine synergist which is selected from antitumor antibiotics and / or antimetabolic drugs; the slow-release auxiliary materials are selected from one or combination of copolymer of racemic polyactic acid and copolymer thereof, copolymer of monomethyl polyethylene glycol or polyethylene glycol or carboxyl-terminated polyactic acid and polyactic acid, copolymer of bi-fatty acid and sebacic acid, poly-(erucic acid dimmer-sebacic acid), poly-(fumaric acid-sebacic acid), polifeprosan and EVAc; the suspending agent has 80cp-3000cp of viscosity; the slow-release microspheres also can be made into a slow-release implant, is injected or placed in tumor or around the tumor, and release drugs for about 40 days; the slow-release injection and the slow-implant can be applied separately so as to suppress the tumor growth or is applied with the non-operative treatment of chemoradiotherapy, and the like, in a combining way, so as to enhance the curative effect thereof significantly.
Owner:JINAN KANGQUAN PHARMA TECH
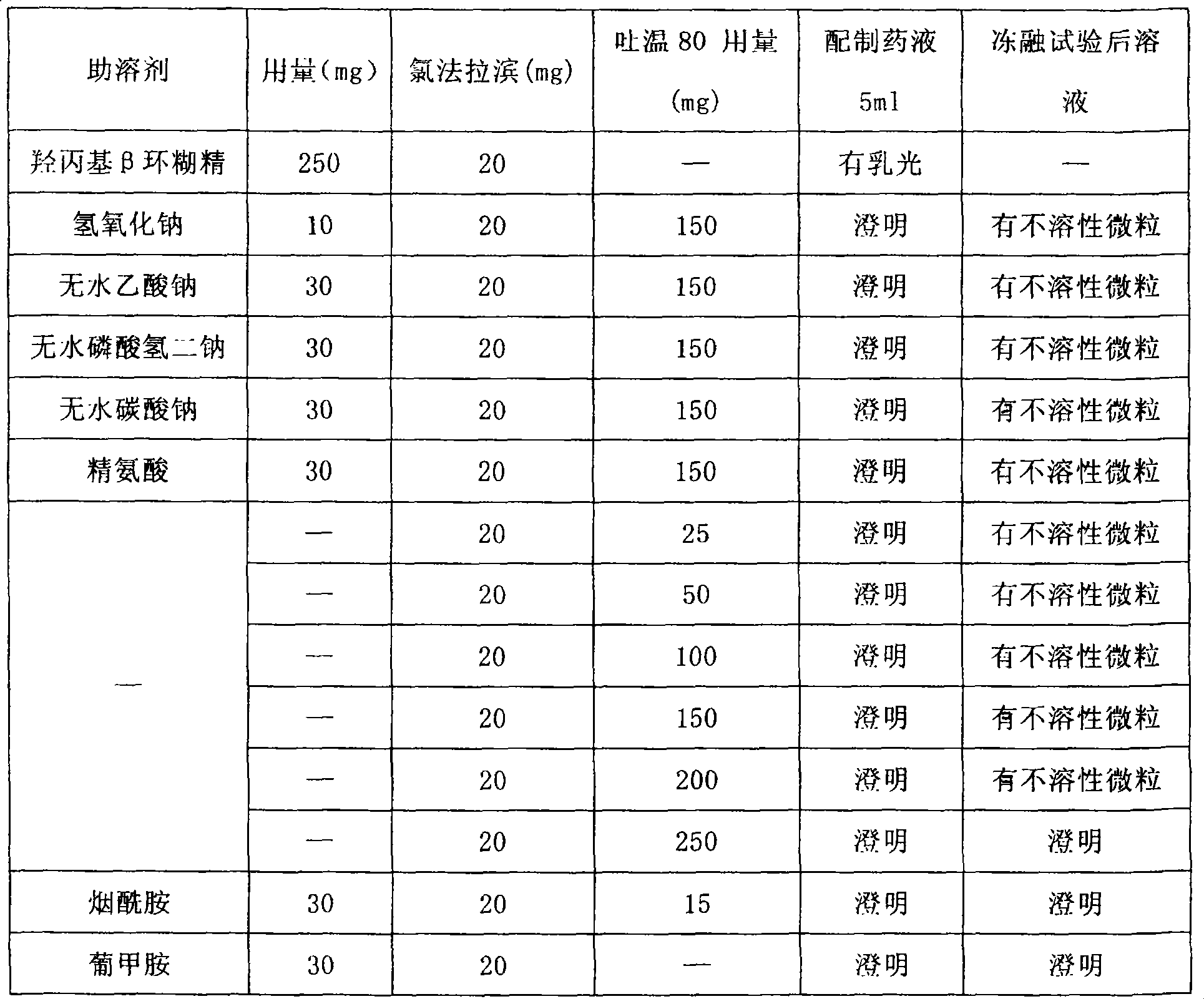
Clorfarabine composition
ActiveCN100493516CImprove solubilityImprove stabilityOrganic active ingredientsPowder deliveryMedicineNiacinamide
The present invention relates to medicine composition containing clofarabine, and is especially one kind of non-gastrointestinal tract administrated clofarabine composition. The clofarabine composition contains clinically effective clofarabine and pharmaceutically acceptable cosolvent niacinamide or meglumine in the weight ratio of 1 to 0.25-40. The clofarabine composition has high disslubility, high stability and other advantages.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Method for separating and purifying Clofarabine
The invention belongs to pharmaceutical technical field, and provides a method for separating and purifying Clofarabine, the method comprises the following steps: a step of dissolving and filtering, a step of separating and purifying by high performance preparative liquid chromatography, a step of recovering products and the like, wherein the step of separating and purifying employs inverse high performance preparative liquid chromatography, the mobile phase is an acetonitrile solution, and an UV detector carries out on-line detection. The method of the invention has the advantages of simple operation, high separation efficiency, stable technology and low cost, and separation with high purify of mass Clofarabine can be realized, the purity is more than 99.8% and the yield is more than 98%.
Owner:SHANDONG NEWTIME PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com