Method for preparing clofarabine raw material medicament
A raw material drug, the technology of arabinofuranose, applied in the field of medicine, can solve the problems of difficulty, high price, complex synthesis route, etc., and achieve the effect of low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
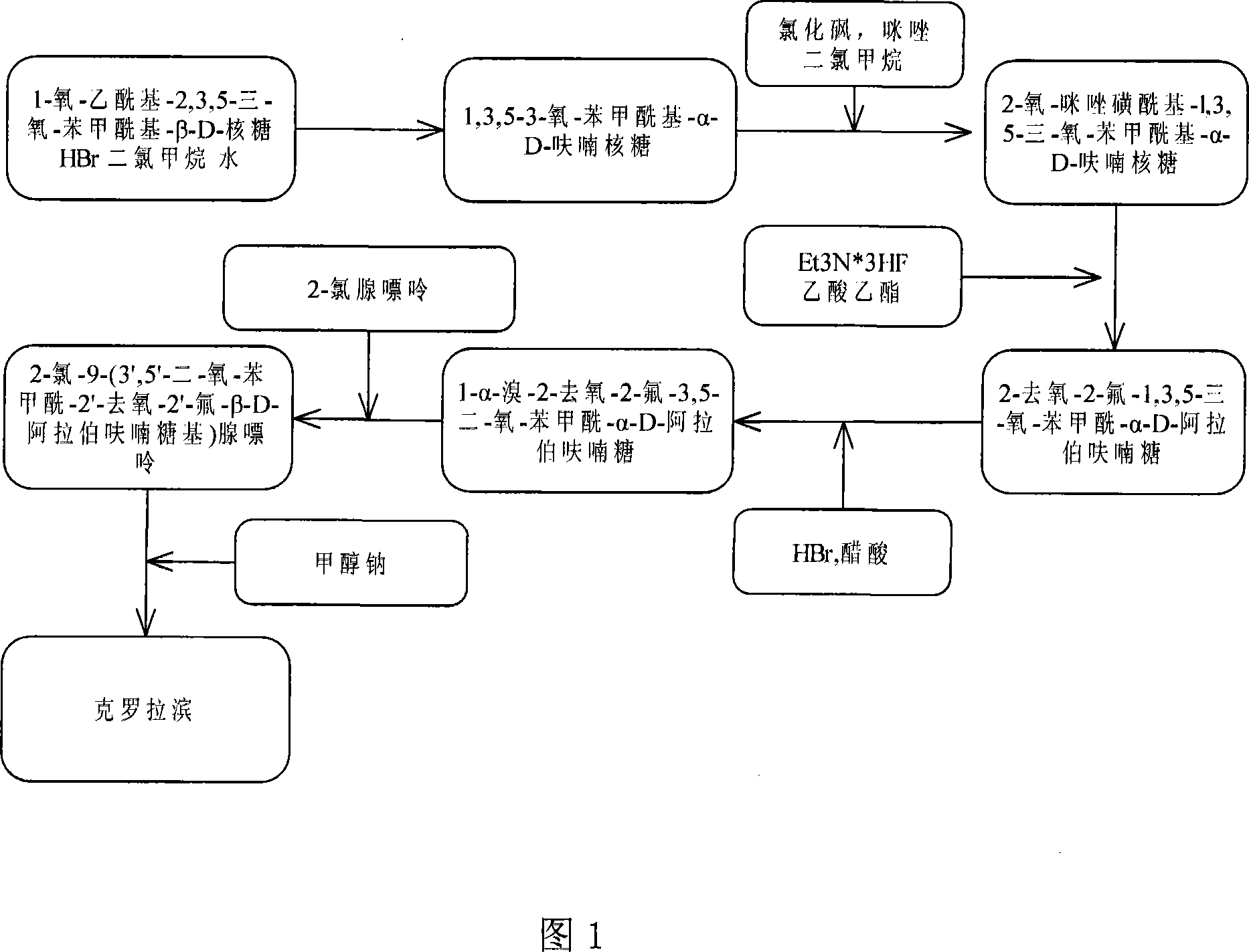
[0027] Example 1 Preparation of Cronabine crude drug, and the synthetic route is shown in Figure 1.
[0028] 1. The instruments used are shown in Table 1:
[0029] Table 1
[0030] Preface
[0031] 7
[0032] 2. Reagents used
[0033] The main raw material specifications and manufacturers are shown in Table 2:
[0034] Table 2:
[0035] raw material name
[0036] Anhydrous magnesium sulfate
[0037] Follow the steps below to prepare Cronabine API:
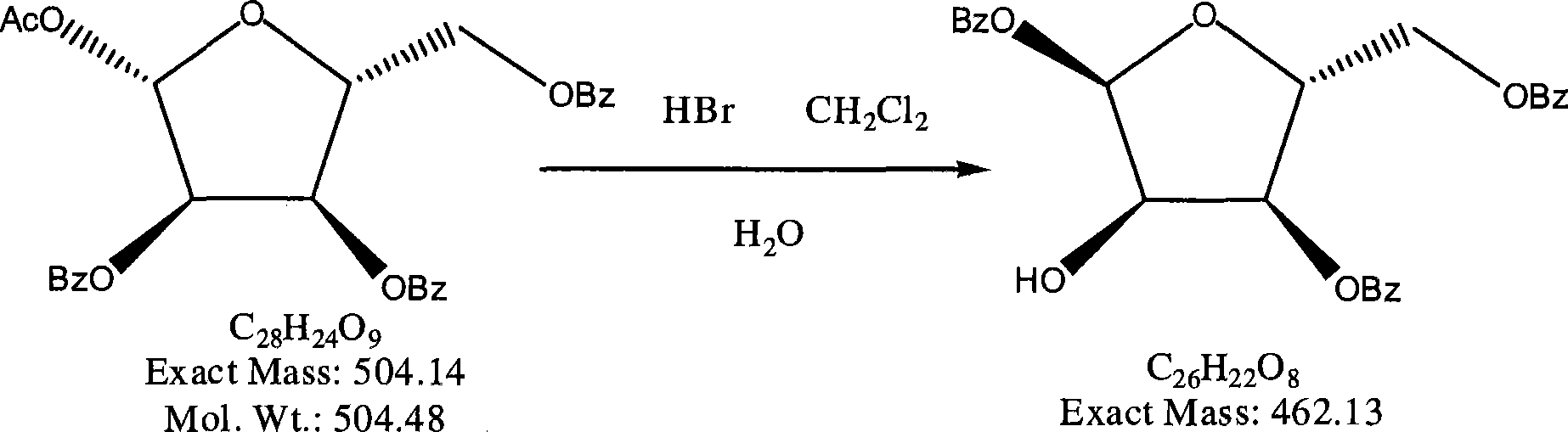
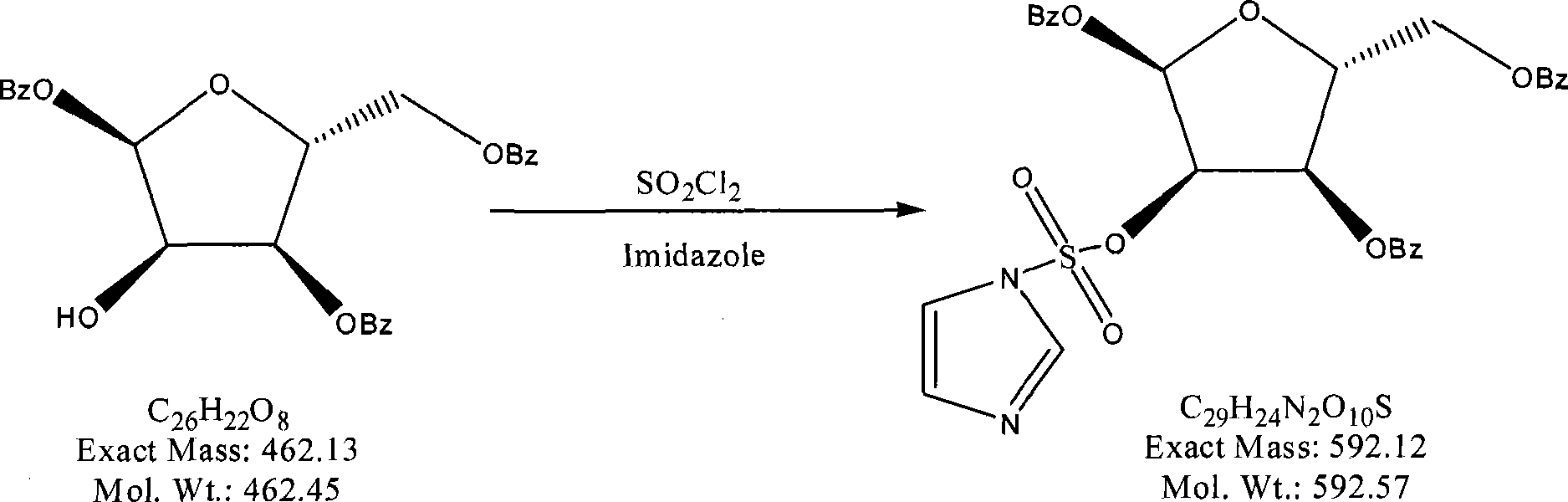
[0038] (1) Preparation of 1,3,5-3-oxo-benzoyl-α-D-ribofuranose
[0039] Reaction formula:
[0040]
[0041] For ingredients, the weight ratio of 1-oxy-acetyl-2,3,5-tri-oxy-benzoyl-β-D-ribose, dichloromethane, hydrogen bromide, n-hexane and anhydrous magnesium sulfate is 10: 50:1:6:10; or according to the ratio shown in Table 3:
[0042] table 3
[0043] 1-oxy-acetyl-2,3,5-tri-oxy-benzoyl
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com