Clorfarabine composition
A technology for clofarabine and its composition, which is applied in the field of pharmaceutical compositions containing clofarabine, can solve problems such as inconvenience in clinical application, and achieve the effects of simple and feasible production process, good solubility, and industrialized production
Active Publication Date: 2009-06-03
SHANDONG BESTCOMM PHARMA CO LTD
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This brings great inconvenience to clinical application
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0021] Clofarabine 20mg
[0022] Niacinamide 5mg
[0023] Tween 80 150mg
[0024] Mannitol 100mg
[0025] Add water for injection to 10ml
Embodiment 2
[0027] Clofarabine 20mg
[0028] Niacinamide 50mg
[0029] Tween 80 15mg
[0030] Mannitol 250mg
[0031] Add water for injection to 5ml
Embodiment 3
[0033] Clofarabine 20mg
[0034] Niacinamide 100mg
[0035] Tween 80 50mg
[0036] Dextran 40 250mg
[0037] Add water for injection to 5ml
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The present invention relates to medicine composition containing clofarabine, and is especially one kind of non-gastrointestinal tract administrated clofarabine composition. The clofarabine composition contains clinically effective clofarabine and pharmaceutically acceptable cosolvent niacinamide or meglumine in the weight ratio of 1 to 0.25-40. The clofarabine composition has high disslubility, high stability and other advantages.
Description
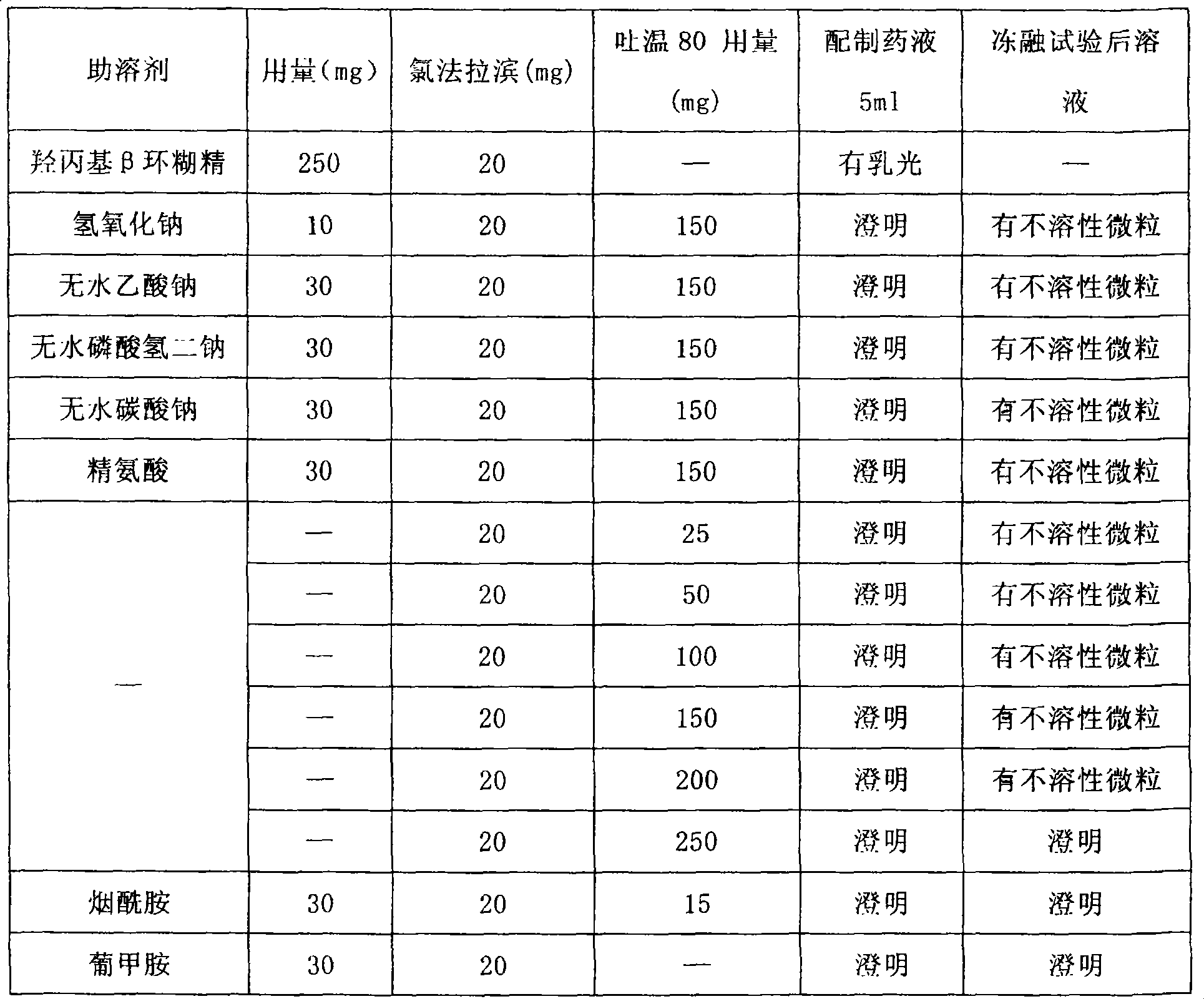
technical field [0001] The invention relates to a pharmaceutical composition containing clofarabine, in particular to a parenteral administration composition containing clofarabine. technical background [0002] The chemical name of clofarabine is 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-9H-purin-6-amine, and the molecular formula is C 10 h 11 cFN 5 o 3 , the molecular weight is 303.68. It is the second generation of purine nucleoside derivatives. After being phosphorylated into triphosphate by deoxycytidine kinase, it can first effectively inhibit nucleotide reductase and terminate DNA synthesis; it can also inhibit DNA polymerase a to make The DNA chain is no longer extended. Clofarabine is cytotoxic to a variety of solid tumors and is particularly effective against acute leukemia. On December 29, 2004, the US FDA approved clofarabine for the treatment of refractory or relapsed acute lymphoblastic leukemia (ALL) in children. [0003] Clofarabine is slightl...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K31/7076A61K47/26A61K9/19A61P35/02
Inventor 牛传芹陈芹利
Owner SHANDONG BESTCOMM PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com