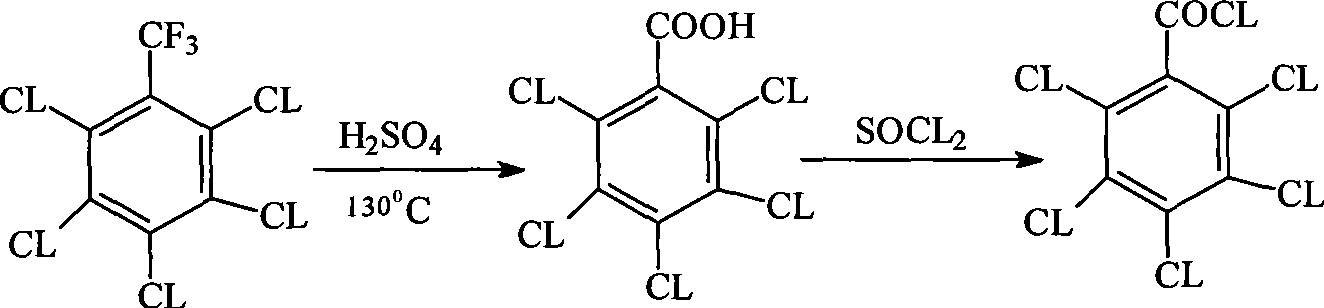
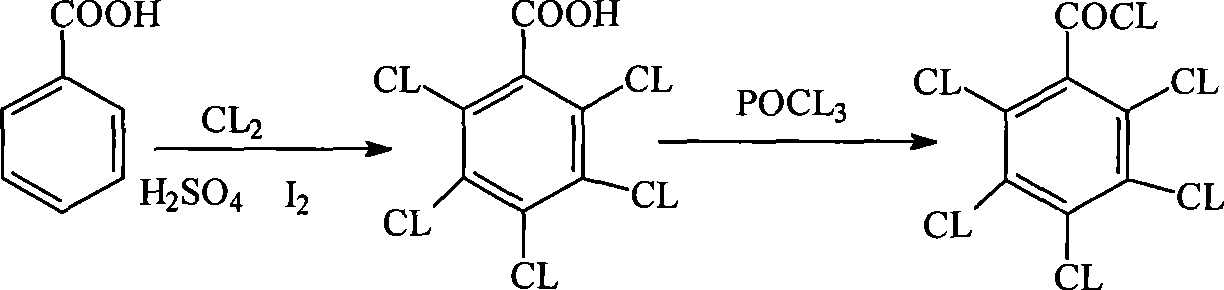
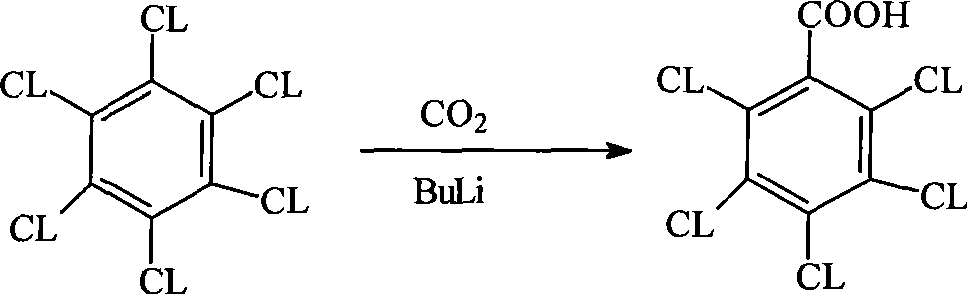Method for preparing pentachlorobenzoyl chloride
A technology of pentachlorobenzoyl chloride and polychlorobenzoyl chloride, which is applied in the field of preparation of pentachlorobenzoyl chloride, can solve the problems of difficult preparation, incomplete reaction, and high equipment requirements, and achieves less three wastes, simple and safe operation, and high process efficiency. reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 1200 milliliters (1460 grams) of benzoyl chloride, 1500 milliliters of chlorosulfonic acid, 1500 milliliters of carbon tetrachloride, 500 milliliters of thionyl chloride and 6 grams of iodine into the reaction flask, cool with ice water, and pass chlorine gas at 10 to 20 degrees to Reaction exothermic slows down, temperature is raised to 45~50 degrees slowly and keeps logical chlorine gas, GC is tracked to stop logical chlorine gas when the conversion ratio of pentachlorobenzoyl chloride to benzoyl chloride or polychlorobenzoyl chloride reaches 98%, and then After reacting at 55-60°C for one hour, keep the temperature below 10°C and pour the reaction mixture into a mixture of ice and carbon tetrachloride, then extract, separate layers, and concentrate the carbon tetrachloride layer to obtain the finished pentachlorobenzyl Acid chloride 3052 g, yield: 93.9%.
Embodiment 2
[0030] Add 200 milliliters (243 grams) of benzoyl chloride, 500 milliliters of chlorosulfonic acid, 500 milliliters of carbon tetrachloride, 10 milliliters of thionyl chloride and 1 gram of iodine into the reaction flask, cool with ice water at 10-20 degrees, and pass chlorine gas to react Exothermic slowing down, temperature is raised to 50~55 degrees slowly and keeps logical chlorine gas, GC is tracked to stop logical chlorine gas when the conversion ratio of pentachlorobenzoyl chloride to benzoyl chloride or polychlorobenzoyl chloride reaches 98%, and then After reacting at 50-55°C for one hour, distill the reaction mixture to an internal temperature of 90°C to recover carbon tetrachloride and thionyl chloride, and then distill under reduced pressure (25mmHg) to recover chlorosulfonic acid to obtain the finished pentachlorobenzyl Acid chloride 520 g, yield: 96%.
[0031] The recovered solvent can be directly used in the preparation of the next batch of pentachlorobenzoyl ch...
Embodiment 3
[0033] Add 200 milliliters (299 grams) of 2,4-dichlorobenzoyl chloride, 300 milliliters of chlorosulfonic acid, 350 milliliters of thionyl chloride and 1 gram of iodine into the reaction flask, cool with ice water at 30-35 degrees, and pass chlorine gas until the reaction releases. Heat slows down, and temperature is raised to 50~55 degrees slowly and keeps logical chlorine gas, and GC tracks to stop logical chlorine gas when the conversion rate of pentachlorobenzoyl chloride to benzoyl chloride or polychlorobenzoyl chloride reaches 98%, and then at 50 ~55 degrees of reaction for one hour, the reaction mixture was first atmospherically distilled to an internal temperature of 90 degrees to reclaim thionyl chloride, and then reduced pressure (25mmHg) to distill and reclaim chlorosulfonic acid to obtain 421 grams of finished product pentachlorobenzoyl chloride. : 94.3%.
[0034] The recovered solvent can be directly used in the preparation of the next batch of pentachlorobenzoyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com