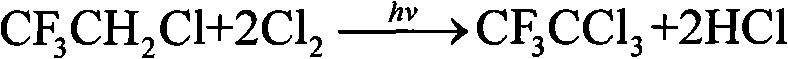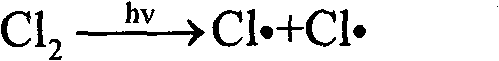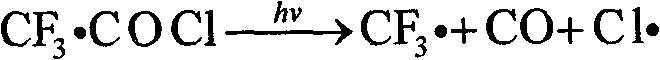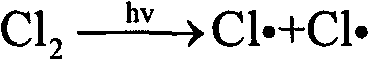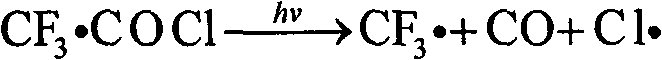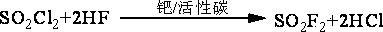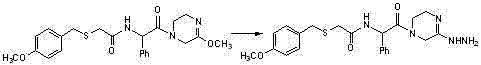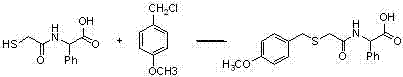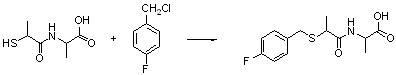Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Trifluoroacetyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
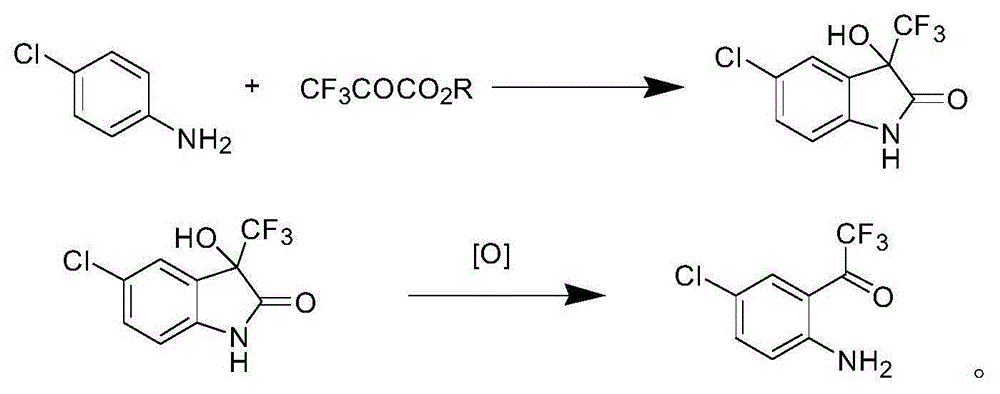
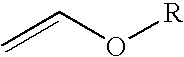
Trifluoroacetyl chloride (also known as TFAC) is a gaseous chemical compound with the chemical formula C₂ClF₃O. It is usually shipped as a liquid under high pressure, however. The compound is a toxic gas.
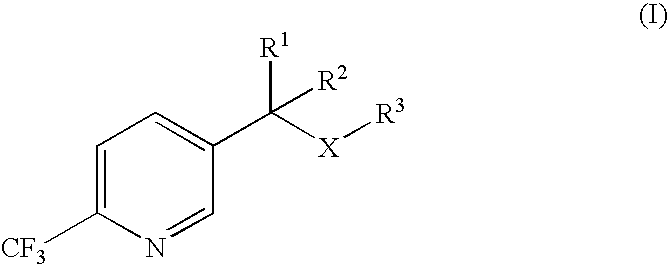
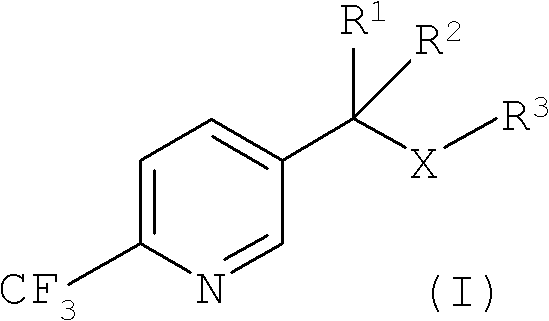
Process for the preparation of 2-trifluoromethyl-5-(1-substituted)alkylpyridines
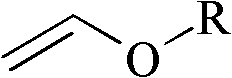
InactiveUS20100004457A1Reduce usageEliminating the transportation and storage issues associatedBiocideOrganic chemistryVinyl etherMedicinal chemistry
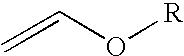
2-Trifluoromethyl-5-(1-substituted)alkylpyridines are produced efficiently and in high yield from an alkyl vinyl ether and trifluoroacetyl chloride by cyclization.
Owner:DOW AGROSCIENCES LLC
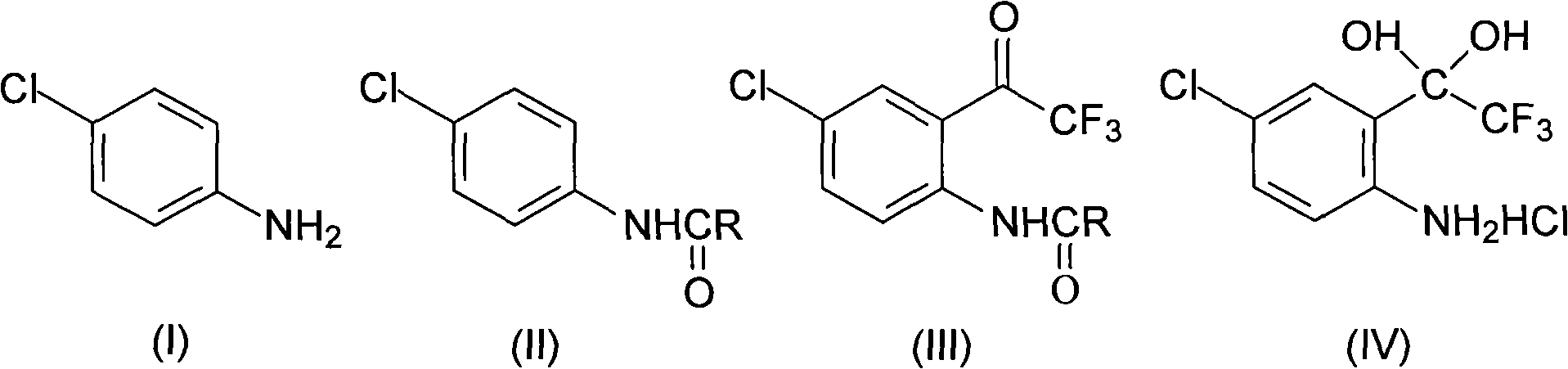
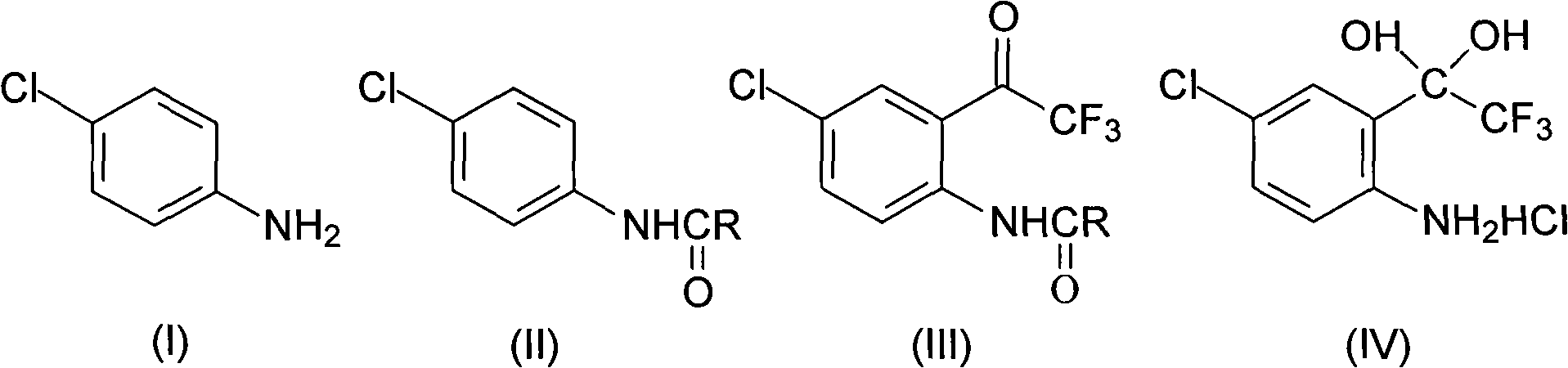
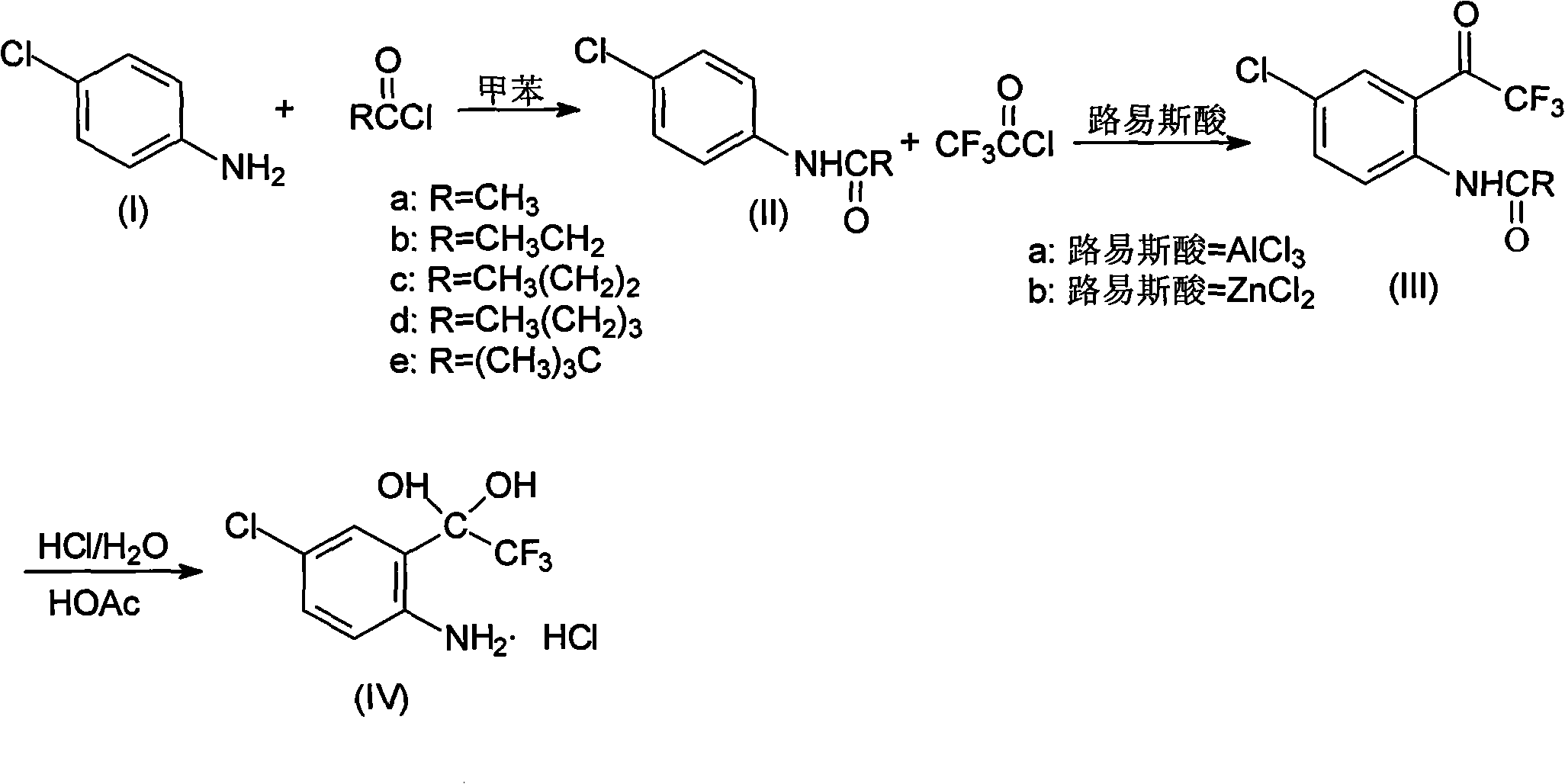
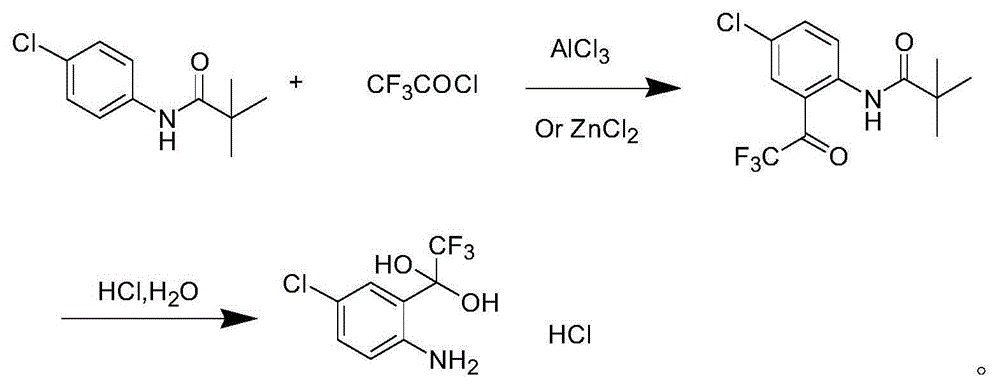
Method for synthesizing 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate intermediate
ActiveCN101844990AGood reaction safetyLow costOrganic chemistryOrganic compound preparationAcetyl chlorideOrganic solvent
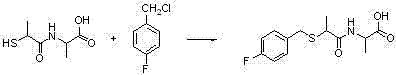
The invention discloses a method for synthesizing a 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate intermediate, which belongs to the technical field of compound preparation methods. The method comprises the following steps of: 1) reacting parachloroaniline with acyl chloride in the presence of a methylbenzene solvent to obtain an intermediate (II); 2) dissolving the intermediate (II) in organic solvent to obtain solution, introducing trifluoro-acetyl chloride into a flask which is filled with the mixed liquor of the organic solvent and lewis acid and slowly filling the solution ofthe intermediate (II) into the flask dropwise to perform a Friedel-Crafts reaction to obtain an acylate intermediate (III), wherein a charging molar ratio of the trifluoro-acetyl chloride to the intermediate (II) is 1: 1; and 3) hydrolyzing the intermediate (II) to remove protective groups to form a salt under acid condition to obtain the 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate intermediate(IV). The method has the advantages of high safety, low cost, simple operation, less pollution, total yield of over 78.1 percent and content of more than or equal to 99.0 percent.
Owner:浙江沙星科技股份有限公司
Method for preparation of trifluoro acetyl chloride with trifluoroethane chlorinated mixture
ActiveCN101747176AReduce consumptionThe synthetic route is simpleCarboxylic acid halides preparationAcetyl chlorideGas phase
The invention discloses a method for preparation of trifluoro acetyl chloride with trifluoroethane chlorinated mixture, which comprises the following steps: 1) adding trifluoroethane chlorinated mixture into a reactor, continuously introducing oxygen and chlorine under stirring, irradiating with a mercury lamp for photochemical oxidation, continuously discharging reactants in the form of gas phase from the upper part of the reactor, and continuously supplementing the trifluoroethane chlorinated mixture to maintain the level in the reactor constant; and 2) condensing the gasified trifluoroethane chlorinated mixture in the reactor through a condensation and separation tower and making the condensed trifluoroethane chlorinated mixture return to the reactor to continuously react, discharging the product trifluoro acetyl chloride from the top of the condensation and separation tower and collecting the trifluoro acetyl chloride after compression and condensation. Compared with the existing preparation method, the invention has the advantages that: the trifluoroethane chlorinated mixture can be directly used for preparation of the trifluoro acetyl chloride without separation, the processis advanced and reasonable, the synthesis route is simplified, the time is reduced, the production efficiency is greatly enhanced, and the equipment investment and energy consumption are reduced.
Owner:福建舜跃科技股份有限公司
Method for preparing trifluoroacetyl chloride
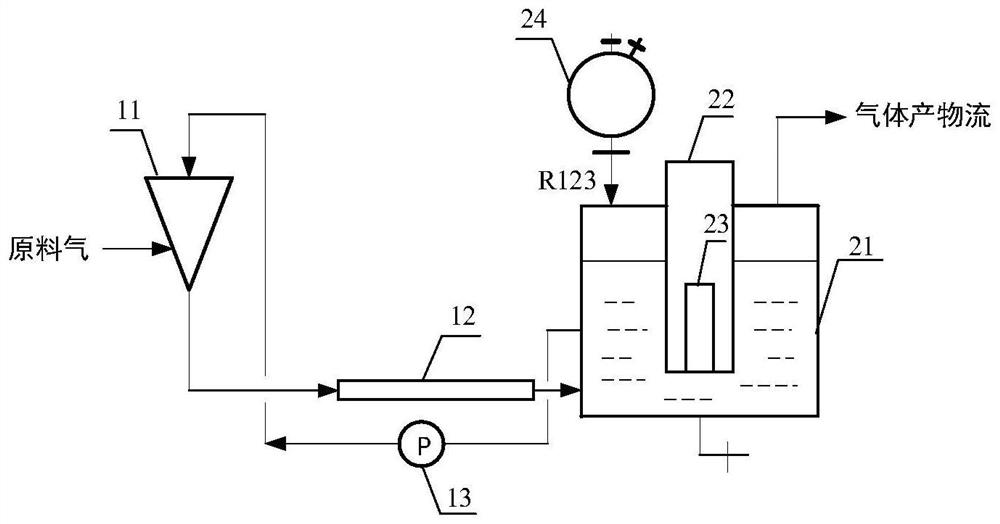
ActiveCN101735034AAvoid decompositionSolving Corrosion ProblemsCarboxylic acid halides preparationGas phaseDecomposition
The invention discloses a method for preparing trifluoroacetyl chloride (TFAC), comprising the following steps: 1) HCFC-123 is added into a reactor, oxygen and chlorine are continuously introduced while stirring, photochemical oxidation reaction is carried out through mercury lamp radiation, the reactant is continuously exhausted from the upper part of the reactor in the form of gas phase, and the liquid level in the reactor keeps constant by continuously supplementing HCFC-123; and 2) the vaporized HCFC-123 in the reaction is condensed by a condenser installed above the reactor and flows back into the reactor to continue participating in the reaction, the product TFAC is exhausted from the top of the condenser and is collected after compression and condensation, the by-product hydrogen chloride is absorbed by water washing and the unreacted chlorine is processed by alkali washing absorption. In the method, the reactor is required to have gas phase space, a shading component is arranged below the gas-liquid phase interface and the mercury lamp is totally immersed below the shading component, thus effectively preventing the generated TFAC from radiolytic decomposition by the mercury lamp in the gas phase space and avoiding the decomposition products from corroding the glass reactor and the lamp tube.
Owner:福建舜跃科技股份有限公司
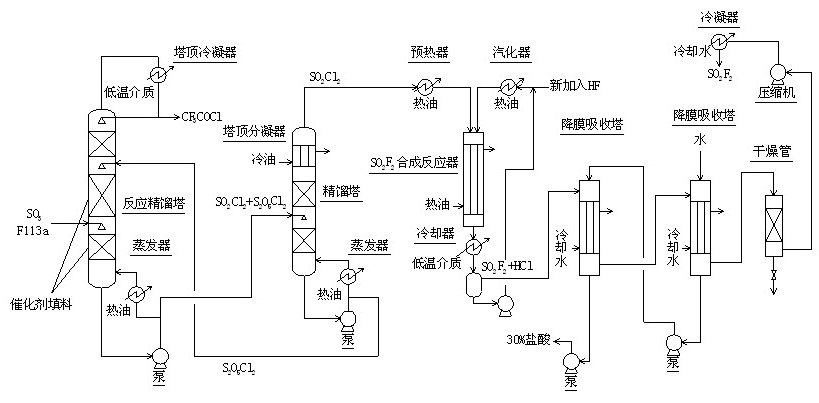
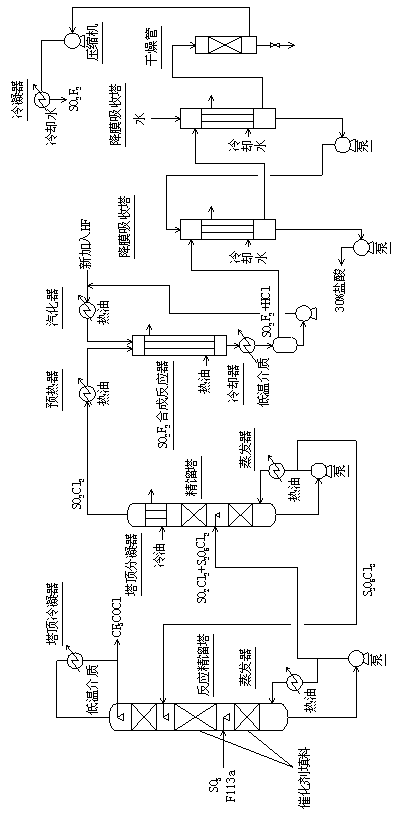
Method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride
InactiveCN102351681AHigh reaction yieldThree wastes lessSulfur-halogen-hydrogen-oxygen compoundsCarboxylic acid halides preparationReaction temperatureTrifluoroacetyl chloride
The invention relates to a method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride, which comprises the following process: continuously introducing sulfur trioxide and trifluorotrichloroethane (F113a) into a reaction rectifying tower containing a catalyst and a filler thorough the lower part in the tower according to a mol ratio of 1:1, wherein the temperature of the tower is controlled to range between 120 DEG C and 130 DEG C, and the reflux ratio at the top of the tower is 2.5-3; introducing tower bottoms into a sulfuryl chloride separating tower for rectification and separation, wherein the tower temperature is 145-150 DEG C, and the reflux ratio is 0.5-1.0; returning pyrosulfuryl chloride in the separating tower to the middle upper part of the reaction rectifying tower, heating the sulfuryl chloride rectified off from the top of the tower to 150 DEG C, and then introducing the rectified sulfuryl chloride and preheated recycled and newly-added hydrogen fluoride gas into a reactor containing a palladium / charcoal catalyst, wherein the reaction temperature is controlled to range between 150 DEG C and 160 DEG C; separating out unreacted hydrogen fluoride from the reaction product through a cold trap, and returning to the reactor for further use; and then absorbing and separating hydrogen chloride through a falling film, drying, compressing, and condensing to obtain the sulfuryl fluoride. The invention has the advantage of easy acquisition of raw materials, and the sulfuryl chloride byproduct can be directly used for the synthesis of sulfuryl fluoride.
Owner:ZHEJIANG UNIV +1
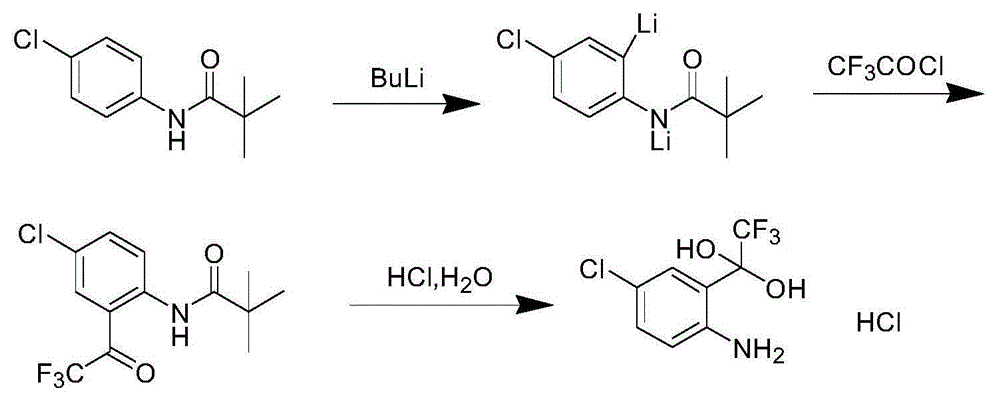
Synthetic method of 4-chlorine-2-trifluoroacetyl aniline aquo-complex hydrochloride
ActiveCN105001101AReduce processing difficultyEasy to recycleOrganic compound preparationAmino-hyroxy compound preparationLithium chlorideAniline
The invention relates to an efavirenz intermediate synthesis method, in particular to a synthetic method of 4-chlorine-2-trifluoroacetyl aniline aquo-complex hydrochloride, and belongs to the technical field of medicine intermediate synthesis. The synthesis method comprises the following steps that, at the low temperature, an n-butyllithium solution is added into a 4-chlorine-N-pivalic acyl aniline solution in a dropwise mode, and a 4-chlorine-N-pivalic acyl aniline bi-lithium salt solution is obtained after a reaction is finished; trifluoroacetyl chloride gas is led to the solution at the low temperature to obtain 4-chlorine-2-trifluoroacetyl-N-pivalic acyl aniline; finally, the 4-chlorine-2-trifluoroacetyl-N-pivalic acyl aniline is hydrolyzed on the acidic condition to obtain the product 4-chlorine-2-trifluoroacetyl aniline aquo-complex hydrochloride. According to the method, the n-butyllithium serves as alkali and is basically converted into a lithium chloride solution with the high economic value after being neutralized through hydrochloric acid, recycling is facilitated, and the waste water treatment difficulty can be lowered; the low-price trifluoro-acetylchloride serves as the raw material, and the production cost can be reduced.
Owner:LEPING RUISHENG PHARMA CO LTD
Method for preparing trifluoroacetyl chloride from 2,2-dichloro-1,1,1-trifluoroethane
InactiveCN101735033AConvenient and quick escapeProtection against radiolysisCarboxylic acid halides preparationGas phaseDecomposition
The invention discloses a method for preparing trifluoroacetyl chloride (TFAC) from 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123), comprising the following steps: 1) HCFC-123 is added into a reactor, oxygen and chlorine are continuously introduced while stirring, photochemical oxidation reaction is carried out through mercury lamp radiation, the reactant is continuously exhausted from the upper part of the reactor in the form of gas phase, and the liquid level in the reactor keeps constant by continuously supplementing HCFC-123; and 2) the vaporized HCFC-123 in the reaction is condensed by a condenser or a condensation and separation tower and flows back into the reactor to continue participating in the reaction, the product TFAC is exhausted from the top of the condenser or the condensation and separation tower and is collected after compression and condensation, the by-product hydrogen chloride is absorbed by water washing and the unreacted chlorine is absorbed by alkali washing. In the invention, reaction is required to be carried out at or above the bubble point temperature of the materials in the reactor, thus being beneficial to ensuring the product TFAC to rapidly escape from the materials in the reactor and preventing TFAC from radiation decomposition by the mercury lamp; meanwhile, plentiful reaction heat can be transferred through phase change of the materials in the reactor, thus solving the problem of safely transferring the reaction heat.
Owner:杭州原正工程技术装备有限公司
Nalpha-fluorenylmethyloxycarbonyl-Nepsilon-trifluoroacetyl-lysine preparation method
ActiveCN103333088AAvoid irritationAvoid hard to washCarbamic acid derivatives preparationOrganic compound preparationAcetyl chlorideBiochemical engineering
The invention relates to a Nalpha-fluorenylmethyloxycarbonyl-Nepsilon-trifluoroacetyl-lysine preparation method, wherein a purpose of the present invention is mainly to solve a technical problem of large production operation difficulty in the existing synthesis method. The technical scheme comprises that the Nalpha-fluorenylmethyloxycarbonyl-Nepsilon-trifluoroacetyl-lysine preparation method comprises the following steps: adopting Nalpha-fluorenylmethyloxycarbonyl-Nepsilon-tertbutyloxycarbonyl-lysine as a starting material, carrying out tertbutyloxycarbonyl removal, purifying Nalpha-fluorenylmethyloxycarbonyl-lysine, carrying out a reaction of the purified Nalpha-fluorenylmethyloxycarbonyl-lysine and trifluoroacetyl chloride to obtain the Nalpha-fluorenylmethyloxycarbonyl-Nepsilon-trifluoroacetyl-lysine, and carrying out a purification treatment to obtain the product, wherein the product is used in the field of polypeptide synthesis.
Owner:GL BIOCHEM SHANGHAI +2
Improved process for the preparation of 2-trifluoromethyl-5-(1-substituted)alkylpyridines
2-Trifluoromethyl-5-(1-substituted)alkylpyridines are produced efficiently and in high yield from an alkyl vinyl ether and trifluoroacetyl chloride by cyclization.
Owner:CORTEVA AGRISCIENCE LLC
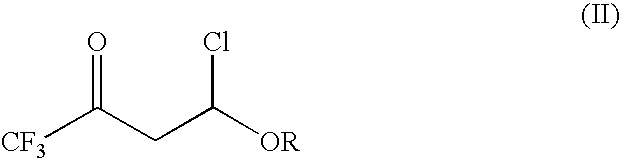
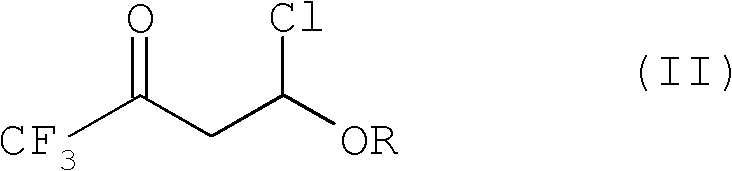
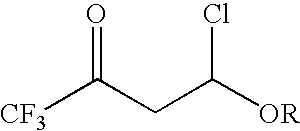
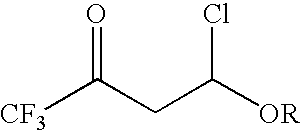
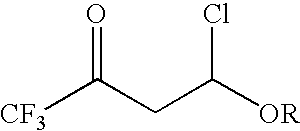
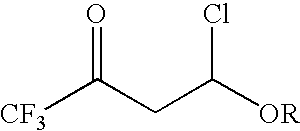
4-chloro-4-alkoxy-1,1,1-trifluoro-2-butanones, their preparation and their use in preparing 4-alkoxy-1,1,1-trifluoro-3-buten-2-ones
ActiveUS20090005603A1Organic compound preparationPreparation by hydrogenolysisVinyl etherAlkoxy group
4-Chloro-4-alkoxy-1,1,1-trifluoro-2-butanones, prepared by reacting alkyl vinyl ethers with trifluoroacetyl chloride, are useful for preparing 4-alkoxy-1,1,1-trifluoro-3-buten-2-ones.
Owner:CORTEVA AGRISCIENCE LLC
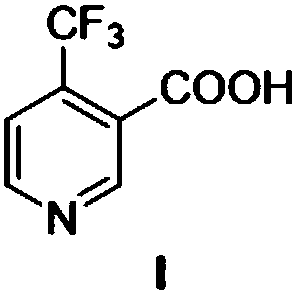
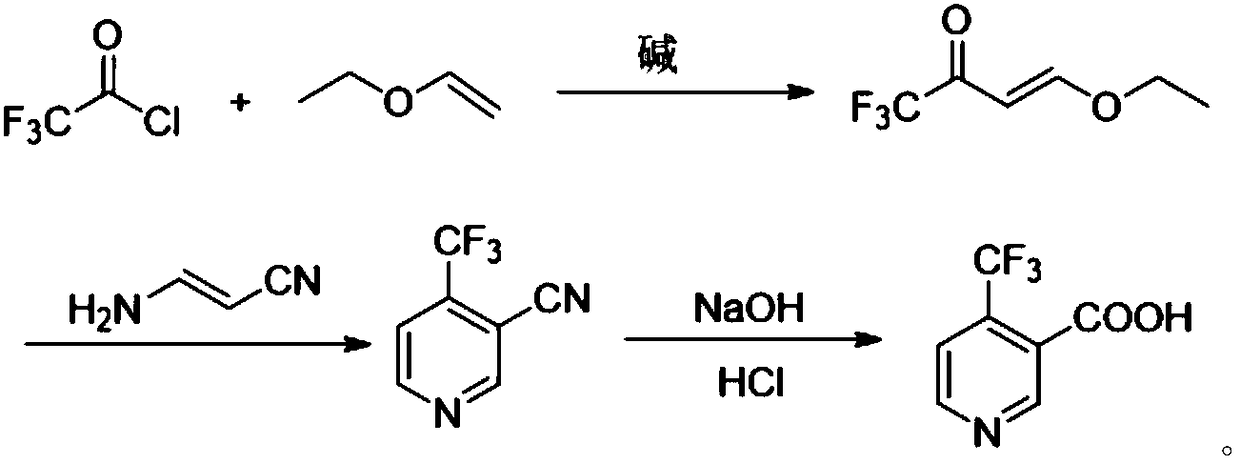
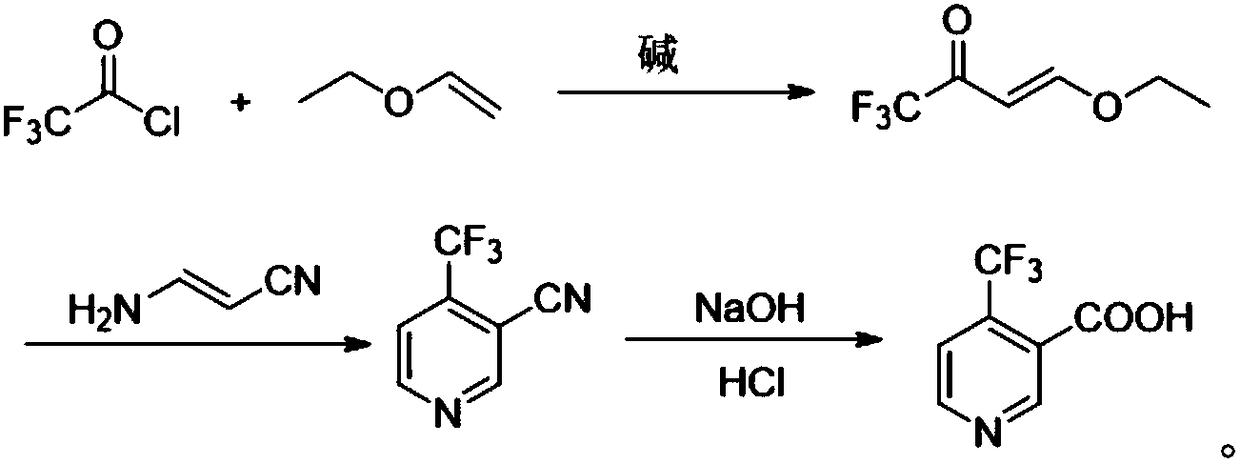
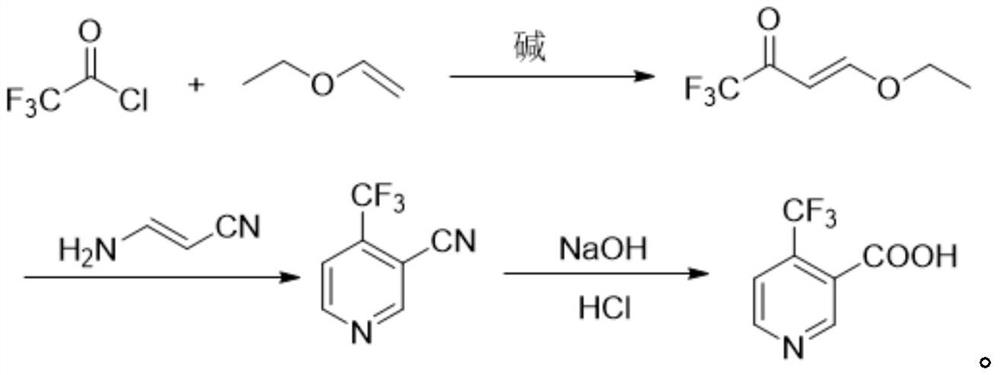
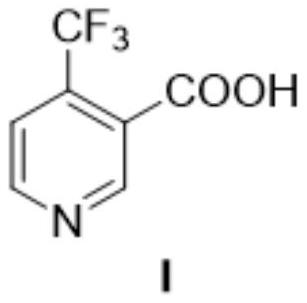
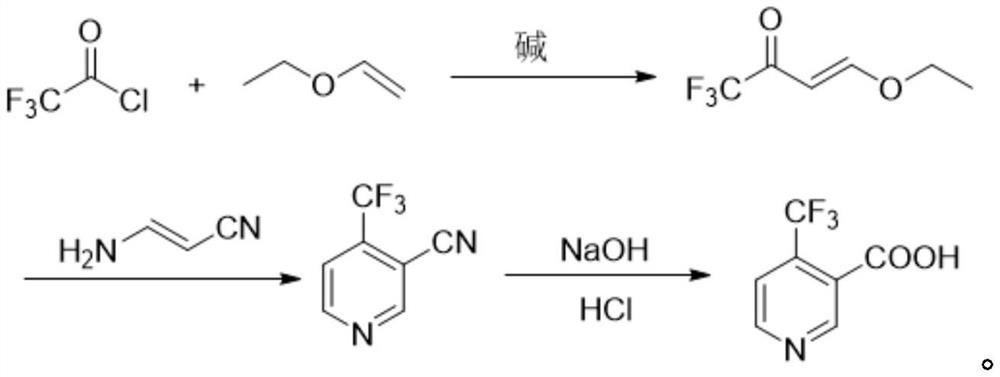
Method for preparing 4-(trifluoromethyl)nicotinic acid
ActiveCN109467532AEasy to separate and purifySimple and fast operationOrganic chemistryHydrolysisMedicinal chemistry
The invention discloses a method for preparing 4-(trifluoromethyl)nicotinic acid, and belongs to the technical field of methods for preparing chemical pharmaceutical intermediates. The method comprises steps of using trifluoroacetyl chloride, vinyl ethyl ether and 3-aminoacrylonitrile as raw materials to prepare the 4-(trifluoromethyl)nicotinic acid through acylation, cyclization and hydrolysis reactions. The method provided by the invention is relatively cheap and easily available in adopted raw material, simple and convenient to operate, easy in separation and purification of products in each step, high in yield, and more suitable for industrial production.
Owner:浙江工业大学上虞研究院有限公司
Method for preparing trifluoro-acetic chloride from tetrachloroethylene
InactiveCN108558650AReduce pollutionSimple processPreparation by halogen replacementCarboxylic acid halides preparationAluminium chlorideHydrogen fluoride
The invention relates to a preparation method of a compound, in particular to a method for preparing trifluoro-acetic chloride from tetrachloroethylene. The method comprises the following steps: (1) preparation of dichlorotrifluoroethane: preheating hydrogen fluoride through a hydrogen fluoride preheater to a temperature which is higher than a saturation temperature by 5-80 DEG C, preheating tetrachloroethylene to the temperature being 25-70 through a tetrachloroethylene preheater, then separately feeding the hydrogen fluoride and the tetrachloroethylene into a reactor under the condition thatthe molar ratio of the hydrogen fluoride to the tetrachloroethylene is 6-8: 1, controlling the reaction temperature to be 25-180 DEG C, reacting 10-20 min under the pressure being 0.5-1.5 MPa and under the effect of a catalyst, feeding a material reacted by a reactor into a refluxing tower with the number of theoretical plates being 20-100, and carrying out primary separation to obtain a mixtureof dichlorotrifluoroethane, chlorotetrafluoroethane and hydrogen fluoride on the bottom of the tower, wherein the catalyst is one or more of antimony chloride, zinc chloride, aluminium chloride and bismuth chloride; and (2) preparation of trifluoro-acetic chloride. The preparation method is simple in process, low in cost, high in reaction conversion rate and small in environmental pollution.
Owner:JIANGSU BLUESTAR GREEN TECH
Process method for reducing content of organic matters in trichloroacetyl chloride byproduct hydrochloric acid
InactiveCN111333031AEfficient removalUtilize resourceChlorine/hydrogen-chloride purificationActivated carbonAcetyl chloride
The invention relates to the technical field of trichloroacetyl chloride production, and discloses a process method for reducing the content of organic matters in trichloroacetyl chloride byproduct hydrochloric acid. The process method comprises the following steps: S1, preparing an ABS modified fiber ball filter tower for coarse filtration, an activated carbon adsorption reaction tower for further fine filtration and an adsorption resin column for final filtration; S2, pretreating the adsorption resin column, namely adding ethanol having a concentration of 95% or above into an exchange columnor an extractor, with the height of ethanol being 10 cm higher than the height of the resin layer, soaking for 4 h, leaching with distilled water till effluent is not turbid when diluted with water in a test tube, and finally repeatedly washing with water till the ethanol content is smaller than 1%. The process method for reducing the content of the organic matters in the trichloroacetyl chloridebyproduct hydrochloric acid has the advantages that insoluble organic matters and soluble organic matters contained in the trifluoroacetyl chloride byproduct hydrochloric acid can be effectively removed, so that the byproduct hydrochloric acid can be applied to other required fields, resources can be utilized, and the process method is energy-saving, environment-friendly and good in economical efficiency.
Owner:JIANGSU QIHENG AGRI & CHEM TECHCO
Synthetic method of trifluoroacetyl tripeptide-2
ActiveCN107857796AOvercome costsOvercome efficiencyPeptide preparation methodsTrifluoroacetic acidFreeze-drying
The invention discloses a synthetic method of trifluoroacetyl tripeptide-2. According to the synthetic method, NH2-Val-Tyr(tBu)-Val-Wang Resin resin peptide is synthesized firstly through Wang Resin,then resin peptide is reacted with 6-chlorobenzotriazole-1,1,3,3-tetramethylurea hexafluorophophate, 1-hydroxybenzotriazole, trifluoroacetic acid and N, N'-diisopropylethylamine to synthesize N-(2,2,2-trifluoroacetyl)-Val-Tyr(tBu)-Val-Wang Resin, finally cutting treatment, purification and freeze drying are conducted on N-(2,2,2-trifluoroacetyl)-Val-Tyr(tBu)-Val-Wang Resin, and thus trifluoroacetyl ripeptide-2 is obtained. According to the synthetic method, trifluoroacetic acid which is low in cost and easy to get is adopted to replace trifluoroacetic acid ethyl ester or trifluoro-acetic chloride which is relatively high in cost, meanwhile, the technology that trifluoroacetic acid is activated in firstly and then acidity is neutralized is adopted, the influence of trifluoroacetic acid toNH2-Val-Tyr(bTu)-Val-Wang Resin is overcome, the synthetic method has the advantages of being low in cost, high in efficiency and simple in method, and is an ideal synthetic method of trifluoroacetyltripeptide-2.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Recycling method of waste containing ethyl acetate and ethyl alcohol in production process of ethyl trifluoroacetoacetate
ActiveCN104672091AAtom economy is highProcess environmental protectionChlorine/hydrogen-chloridePreparation from carboxylic acid halidesAcetic acidAlcohol
The invention discloses a recycling method of waste containing ethyl acetate and ethyl alcohol in a production process of ethyl trifluoroacetoacetate. A mixture of ethyl trifluoroacetate and ethyl acetate is obtained by reaction of trifluoroacetyl chloride, ethyl acetate and ethyl alcohol under the operation condition of reactive rectification. The method disclosed by the invention has the advantages of low cost, safety, environmental friendliness, simple technology and the like.
Owner:中化蓝天氟材料有限公司 +2
Method for using trichloroethylene to prepare trifluoro-acetic chloride
InactiveCN108516932AReduce pollutionSimple processHalogenated hydrocarbon preparationCarboxylic acid halides preparationDecompositionGas phase
The invention relates to a preparation method of compounds, in particular to a method for using trichloroethylene to prepare trifluoro-acetic chloride. The preparation method includes the following steps: (1) 1, 1, 1 three fluorine 2, 2 preparation of dichloroethane: mixed gas is obtained by the trichloroethylene through fluorination and thermal chlorination; the mixed gas enters a reaction tank filled with activated carbon for catalytic chlorination; the reaction temperature is 180-240 DEG C; the speed ratio of controlling chlorine and the mixed gas in a reaction catalysis process is 1:10-80;after reaction, the gas is washed by water alkali and fractionated to obtain products; (2) preparation of the trifluoro-acetic chloride. The preparation method of the method for using the trichloroethylene to prepare the trifluoro-acetic chloride has the advantages of being simple in process, low in cost, high in reaction conversion rate and little in environmental pollution. The method for usingthe trichloroethylene to prepare the trifluoro-acetic chloride requires gas phase space in a reactor and shading components is arranged below a gas-liquid interface. Mercury lamps are completely submerged under the shading components, thereby being capable of effectively preventing TFAC from being radiated and decomposed by the mercury lamps in the gas phase space and avoiding a corrosion problemof decomposition products to a glass reactor and lamp tubes.
Owner:JIANGSU BLUESTAR GREEN TECH
Method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride
InactiveCN102351681BHigh reaction yieldThree wastes lessSulfur-halogen-hydrogen-oxygen compoundsCarboxylic acid halides preparationReaction temperatureTrifluoroacetyl chloride
The invention relates to a method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride, which comprises the following process: continuously introducing sulfur trioxide and trifluorotrichloroethane (F113a) into a reaction rectifying tower containing a catalyst and a filler thorough the lower part in the tower according to a mol ratio of 1:1, wherein the temperature of the tower is controlled to range between 120 DEG C and 130 DEG C, and the reflux ratio at the top of the tower is 2.5-3; introducing tower bottoms into a sulfuryl chloride separating tower for rectification and separation, wherein the tower temperature is 145-150 DEG C, and the reflux ratio is 0.5-1.0; returning pyrosulfuryl chloride in the separating tower to the middle upper part of the reaction rectifying tower, heating the sulfuryl chloride rectified off from the top of the tower to 150 DEG C, and then introducing the rectified sulfuryl chloride and preheated recycled and newly-added hydrogen fluoride gas into a reactor containing a palladium / charcoal catalyst, wherein the reaction temperature is controlled to range between 150 DEG C and 160 DEG C; separating out unreacted hydrogen fluoride from the reaction product through a cold trap, and returning to the reactor for further use; and then absorbing and separating hydrogen chloride through a falling film, drying, compressing, and condensing to obtain the sulfuryl fluoride. The invention has the advantage of easy acquisition of raw materials, and the sulfuryl chloride byproduct can be directly used for the synthesis of sulfuryl fluoride.
Owner:ZHEJIANG UNIV +1
4-Chloro-4-alkoxy-1,1,1-trifluoro-2-butanones, their preparation and their use in preparing 4-alkoxy-1,1,1-trifluoro-3-buten-2-ones
4-Chloro-4-alkoxy-1,1,1-trifluoro-2-butanones, prepared by reacting alkyl vinyl ethers with trifluoroacetyl chloride, are useful for preparing 4-alkoxy-1,1,1-trifluoro-3-buten-2-ones.
Owner:CORTEVA AGRISCIENCE LLC
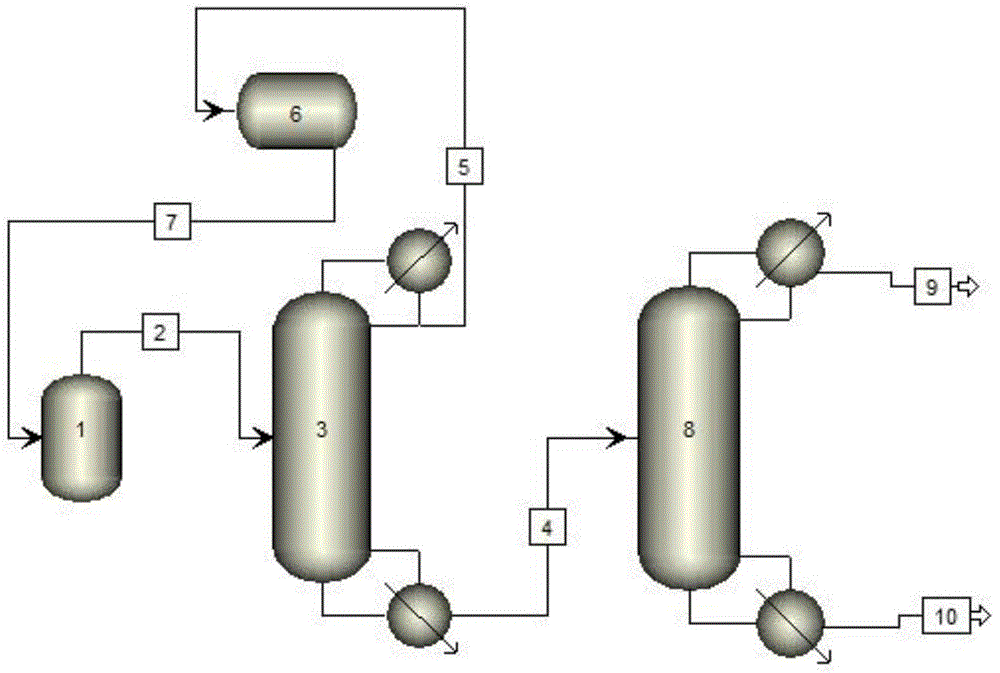
Sulfuric chloride separation and purification technique
InactiveCN104671219AEfficient recyclingReduce manufacturing costSulfur-halogen-hydrogen-oxygen compoundsSulfuryl chlorideAcetyl chlorideChloride
The invention discloses a technique for separating and purifying sulfuric chloride from a trifluoro-acetyl chloride production reaction waste liquid by a double-tower rectification process. The technique can obtain the high-purity sulfuric chloride product, and has the advantage of low production cost. The prepared sulfuric chloride can be used for chlorination of aromatic compounds and the like.
Owner:浙江化工院科技有限公司 +1
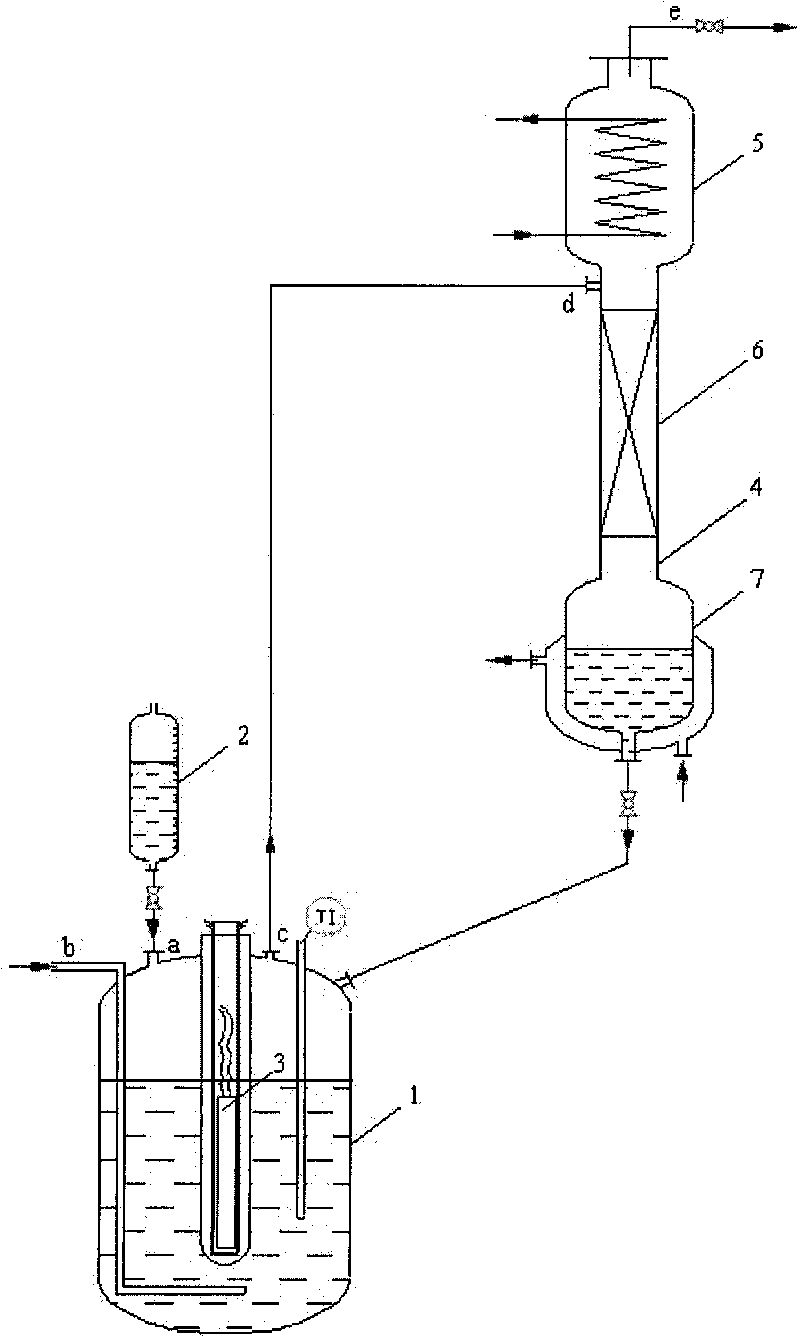
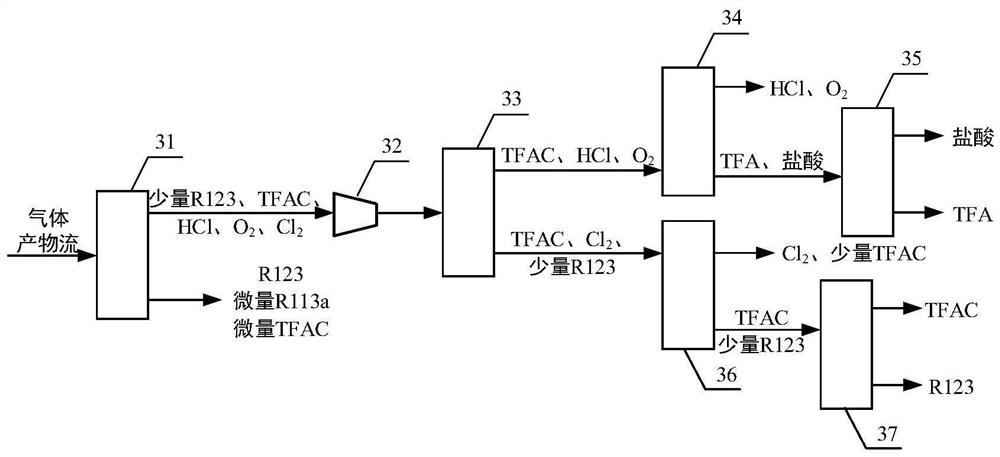
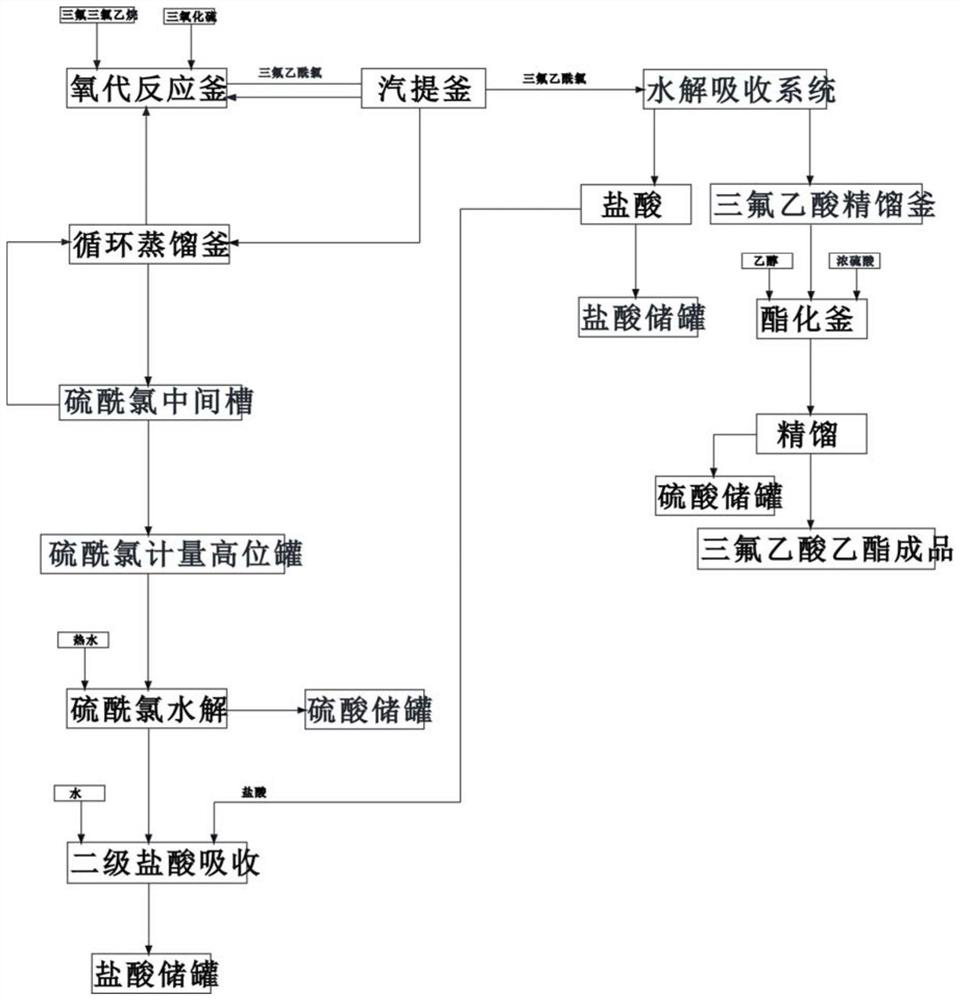
Continuous preparation device and method of trifluoroacetyl chloride
PendingCN114505041ARealize continuous productionImprove mass transfer effectPreparation from carboxylic acid halideOrganic compound preparationAcetyl chloridePhysical chemistry
The invention discloses a continuous preparation device and method of trifluoroacetyl chloride, the continuous preparation device comprises: a side circulation unit comprising a gas injector provided with a liquid inlet, a gas inlet and an injection orifice, a material of a reaction unit and a raw material gas being mixed in the gas injector and then returning to the reaction unit through the injection orifice; the reaction unit comprises a reactor, a cold trap arranged in the reactor and a mercury lamp arranged in the cold trap, and the outer surface of the cold trap is coated with a thermal shrinkage fluororesin material; the separation unit comprises a coarse separation tower, a compressor, a flexible separation tower, a dechlorination tower and a TFAC product tower which are connected in sequence, the tower top of the coarse separation tower is connected with an inlet of the compressor, a tower kettle of the flexible separation tower is connected with the dechlorination tower, a tower kettle of the dechlorination tower is connected with the TFAC product tower, and a trifluoroacetyl chloride product is obtained at the tower top of the TFAC product tower. The method has the advantages of low equipment corrosion, high reaction yield, capability of selectively preparing products, suitability for industrial production and the like.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +2
Synthesis method of 4-ethoxy-1, 1, 1-trifluoro-3-butene-2-ketone
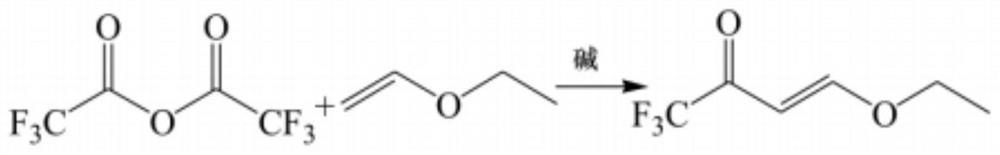
PendingCN114805047AEasy to makeGet goodOrganic compound preparationCarbonyl compound preparation by condensationEthylic acidEthyl group
The invention discloses a synthesis method of 4-ethoxy-1, 1, 1-trifluoro-3-butene-2-one, which comprises the following steps: adding absolute ethyl alcohol, mercury acetate and n-butyl vinyl ether into a reaction flask which is provided with a fractionating column (connected with a condenser and cooled by an ice salt bath for the flask), heating and refluxing, controlling the temperature of the top of the fractionating column to 36-37 DEG C, evaporating out ethyl vinyl ether, and cooling to obtain the 4-ethoxy-1, 1, 1-trifluoro-3-butene-2-one, namely the 4-ethoxy-1, 1, 1-trifluoro-3-butene-2-one. The preparation method comprises the following steps: mixing ethyl vinyl ether and trifluoroacetic anhydride, adding a deacidification agent, and reacting at 50-110 DEG C for 2-4 hours to obtain the 4-ethoxy-1, 1, 1-trifluoro-3-butene-2-ketone. According to the method, trifluoroacetic anhydride is adopted to replace trifluoroacetyl chloride to serve as a reaction raw material, trifluoroacetic anhydride is easy to prepare and convenient to obtain, and large-batch production of 4-ethoxy-1, 1, 1-trifluoro-3-butene-2-ketone is facilitated.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
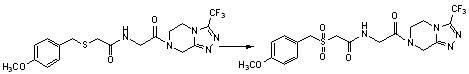
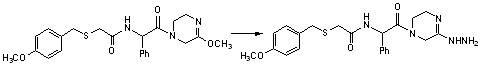
Benzylthio acetamido acetylpyrazine triazole derivative as well as preparation thereof and application thereof
ActiveCN102260268BReasonable designThe synthesis process is reasonableOrganic active ingredientsOrganic chemistryDipeptidyl peptidaseTrimethyloxonium tetrafluoroborate
Owner:HANGZHOU ADAMERCK PHARMLABS INC
A kind of synthetic method of trifluoroacetyl tripeptide-2
ActiveCN107857796BImprove efficiencyLow efficiencyPeptide preparation methodsChlorobenzeneFluoroacetic acid
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Compressor surface treating agent and anti-corrosion treatment method
PendingCN112011201AExtended service lifeGood for high temperature and high pressureAnti-corrosive paintsCarbon nanotubeCarboxylic acid
The invention discloses a compressor surface treating agent and an anti-corrosion treatment method. The surface treating agent is prepared from the following components in percentage by weight: 1%-6%of polytetrafluoroethylene, 6%-18% of dimethyl formamide, 5%-7% of trifluoroacetyl chloride, 2%-6% of triethyl benzene tricarboxylate, 3%-7% of zinc undecylenate, 6%-12% of tetranitrotetrazole blue, 10%-15% of carbon nanotubes, 2%-6% of trifluoroethyl acrylate, 5%-10% of nano diamond, 1%-6% of a magnesium reagent, 3%-6% of dioctyl sebacate, 3%-9% of trifluoromethoxybenzene and 2%-4% of diphenyl phosphoryl chloride. The compressor surface treating agent can be effectively prevented from being corroded, the service life of the compressor is prolonged, the investment cost of an enterprise is reduced, and good application prospects are achieved.
Owner:BENGBU AIPU COMPRESSOR MACHINERY
The preparation method of 4-trifluoromethyl nicotinic acid
The invention discloses a method for preparing 4-(trifluoromethyl)nicotinic acid, and belongs to the technical field of methods for preparing chemical pharmaceutical intermediates. The method comprises steps of using trifluoroacetyl chloride, vinyl ethyl ether and 3-aminoacrylonitrile as raw materials to prepare the 4-(trifluoromethyl)nicotinic acid through acylation, cyclization and hydrolysis reactions. The method provided by the invention is relatively cheap and easily available in adopted raw material, simple and convenient to operate, easy in separation and purification of products in each step, high in yield, and more suitable for industrial production.
Owner:浙江工业大学上虞研究院有限公司
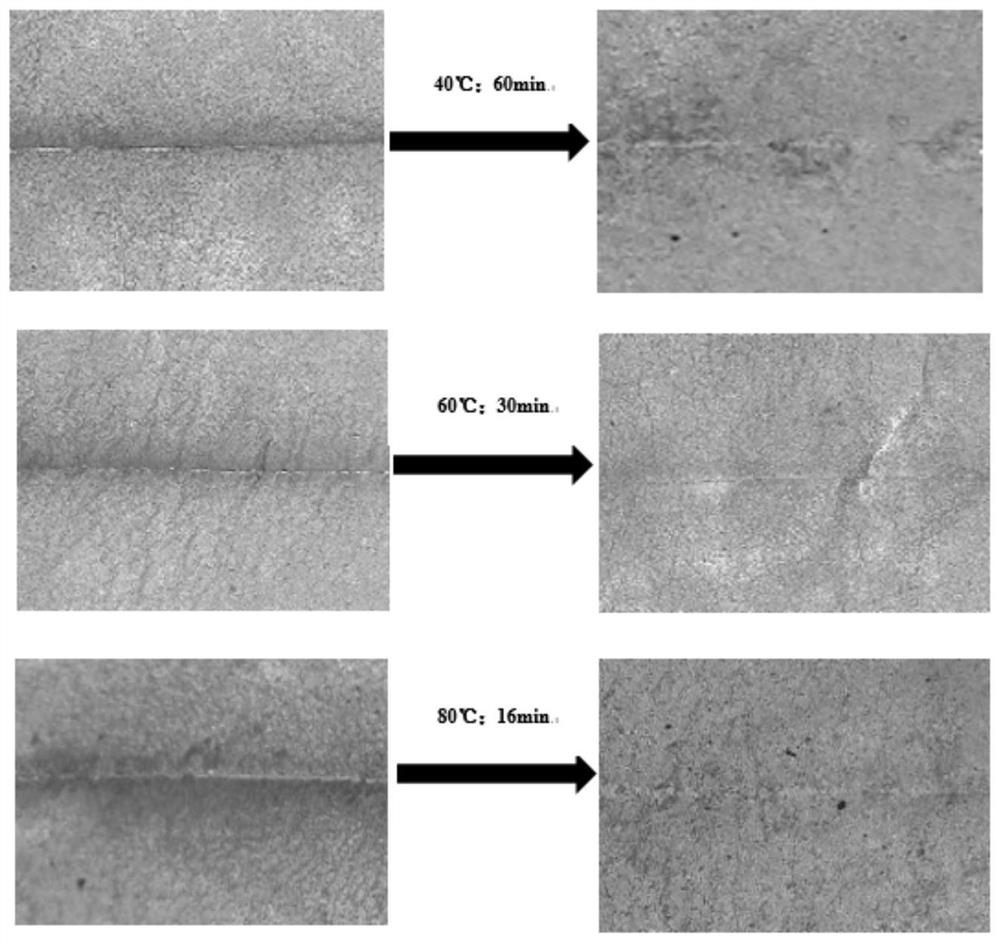
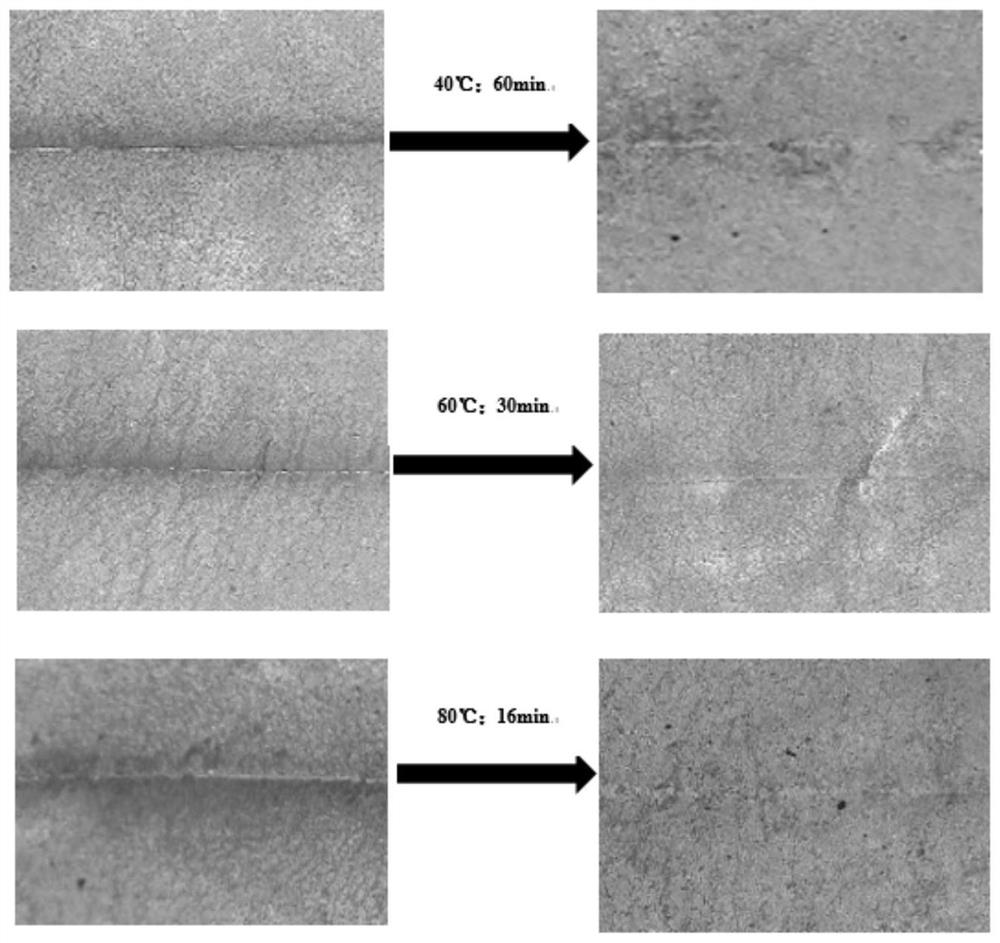
A kind of preparation method of visible light curing self-healing fluorine-containing polyurethane resin
ActiveCN112794983BAchieve partial degradationHigh tensile strengthPreparation from carboxylic acid halidesOrganic compound preparationPhotochemistrySmart material
The invention belongs to the field of light-curing bio-based intelligent materials, and in particular relates to a preparation method of visible light-curing self-repairing fluorine-containing polyurethane resin. First use dichloromethane, dual active functional group photoinitiator, weakly basic acid-binding agent and trifluoroacetyl chloride to react in a nitrogen protection device to prepare a fluorine-containing photoinitiator; Dicarboxylation treatment, under the synergistic action of dihydroxy fluorine-containing monomer, fluorine-containing photoinitiator and diisocyanate, finally prepares self-healing fluorine-containing polyurethane resin cured under visible light conditions. The polyurethane resin of the present invention overcomes the shortcomings of traditional light-curing resins such as high brittleness, easy damage, and poor high temperature resistance. It is cured by visible light that is friendly to the human body, and the material has a self-repairing function, which can prolong the service life of the material and enhance weather resistance and high temperature resistance. properties, the source of the monomer is renewable, and the application prospect is good.
Owner:CHANGZHOU UNIV
Preparation method of visible light curing self-repairing fluorine-containing polyurethane resin
ActiveCN112794983AAchieve partial degradationHigh tensile strengthPreparation from carboxylic acid halidesOrganic compound preparationPolymer scienceHydrolysis
The invention belongs to the field of photocuring bio-based intelligent materials, and particularly relates to a preparation method of visible light curing self-repairing fluorine-containing polyurethane resin. The preparation method comprises the following steps: firstly, reacting dichloromethane, a double-active-functional-group photoinitiator, a weakly alkaline acid-binding agent and trifluoroacetyl chloride in a nitrogen protection device to prepare a fluorine-containing photoinitiator; and subjecting a hydrolysis product eleostearic acid of eleostearic acid triglyceride to dicarboxylation treatment. Under the synergistic effect of a dihydroxyl fluorine-containing monomer, a fluorine-containing photoinitiator and diisocyanate, the self-repairing fluorine-containing polyurethane resin cured under the visible light condition is finally prepared. The polyurethane resin overcomes the defects that traditional light-cured resin is large in brittleness, easy to damage and poor in high temperature resistance, visible light friendly to the human body is used for curing, the material has a self-repairing function, the service life of the material can be prolonged, the weather resistance and the high temperature resistance are enhanced, the monomer source is renewable, and the application prospect is good.
Owner:CHANGZHOU UNIV
Benzylthioacetamidoacetylpyrazinetriazole derivatives and their preparation and application
ActiveCN102260268AReasonable designThe synthesis process is reasonableOrganic active ingredientsOrganic chemistryDipeptidyl peptidaseHydrazine compound
The invention provides a benzylthio acetamido acetylpyrazine triazole derivative. A target compound (I) is obtained by condensing a sulfydryl acetamido acetic acid derivative and a benzyl chloride derivative, condensing the condensed derivatives and 2-piperazinone, reacting the obtained condensed product and trimethyloxonium tetrafluoroborate so as to obtain a methoxy imide compound, and carryingout cyclization on the methoxy imide compound and hydrazine, trifluoroaceticanhydride, acetic anhydride, propionic anhydride, trifluoroacetyl chloride, acetyl chloride or acrylyl chloride. The preparation method provided by the invention has the advantages of reasonable design, stable process, easily obtained raw materials for production, no use of high pressure hydrogenation and good production feasibility. By using the benzylthio acetamido acetylpyrazine triazole derivative provided by the invention, dipeptidyl peptidase-IV (DPP-4) can be selectively inhibited; an effect of reducing blood sugar is obtained; and the derivative can be applied to the preparation of drugs for reducing the blood sugar and has the following structural formula (I).
Owner:HANGZHOU ADAMERCK PHARMLABS INC
Steel pipe surface treating agent and anti-corrosion treatment method
InactiveCN111548658AGood for high temperature and high pressureQuality improvementPretreated surfacesAnti-corrosive paintsCarboxylic acidSebacic acid
The invention discloses a steel pipe surface treating agent and an anti-corrosion treatment method. The surface treating agent is composed of the following components in percentage by weight: 3%-9% ofpolytetrafluoroethylene, 6%-12% of dimethyl formamide, 3%-6% of trifluoroacetyl chloride, 1%-3% of triethyl phenyltricarboxylate, 3%-6% of zinc undecylenate, 6%-8% of carbon nanotubes, 2%-6% of trifluoroethyl acrylate, 5%-8% of nano diamond, 1%-3% of a magnesium reagent, 3%-6% of dioctyl sebacate, 1%-3% of diphenyl phosphoryl chloride, 2%-8% of amino, 6%-12% of alkoxy and 10%-20% of silane. The steel pipe surface treating agent can effectively prevent a steel pipe from being corroded, the service life of the steel pipe is prolonged, the strength of the steel pipe is effectively improved, andapplication prospects are good.
Owner:安徽唐道电子科技有限公司
A kind of preparation method of ethyl trifluoroacetate
ActiveCN109369385BReduce dosageHigh reaction conversion ratePreparation from carboxylic acid halideOrganic compound preparationFluoroacetic acidHydrolysis
The invention discloses a preparation method of ethyl trifluoroacetate, which comprises the following steps: 1) passing sulfur trioxide and trifluorotrichloroethane into an oxygenation reactor twice; 2) trifluoroacetyl chloride and By-products and some raw materials enter the stripping kettle for stripping, condensation and separation; 3) The separated trifluoroacetyl chloride is transported to the hydrolysis absorption system, and part of the raw materials are condensed and refluxed to the oxo reactor; 4) The raw materials after rectification are returned to the oxygen reactor. generation reaction kettle; sulfuryl chloride, pyrothionyl chloride and impurities to be hydrolyzed; 5) obtain crude trifluoroacetic acid, and rectify to obtain trifluoroacetic acid; 6) add trifluoroacetic acid to the esterification kettle; 7) obtain ethyl trifluoroacetic acid finished product . The present invention adds reactants in stages, the reaction conversion rate is higher, and lays a good reaction foundation for subsequent reactions. The reaction conditions are mild, the amount of concentrated sulfuric acid is greatly reduced, and the corrosion to equipment is reduced; adding fibers in frozen brine, Separation of trifluoroacetyl chloride is more efficient.
Owner:江山鑫隆化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com