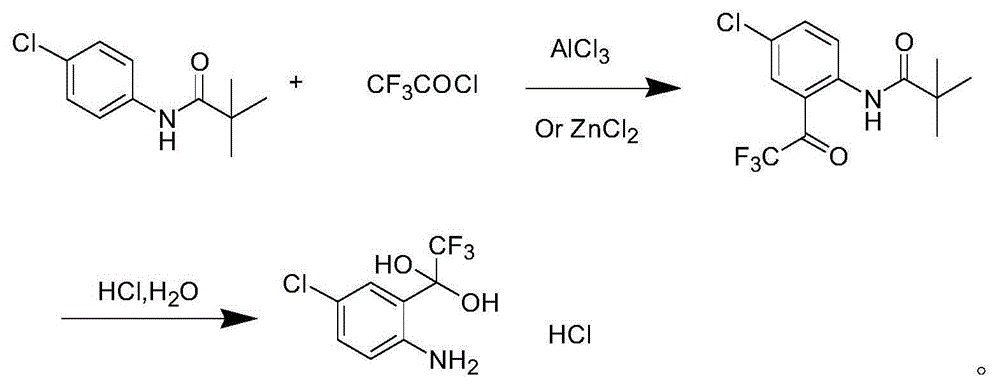
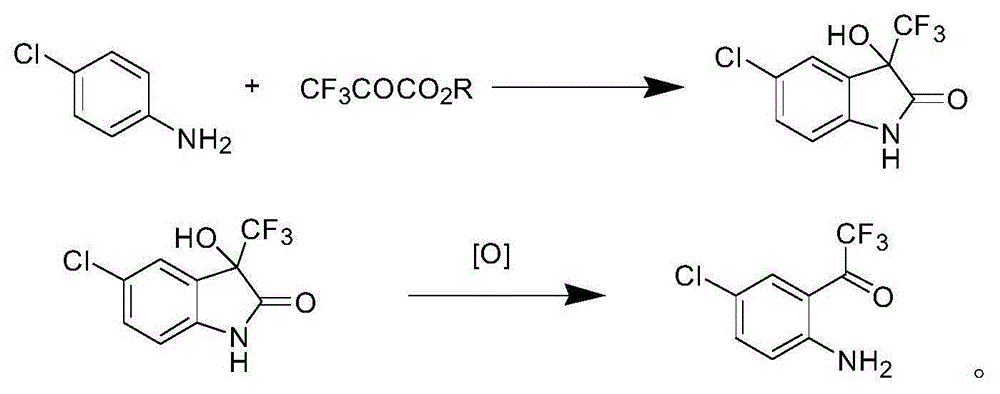
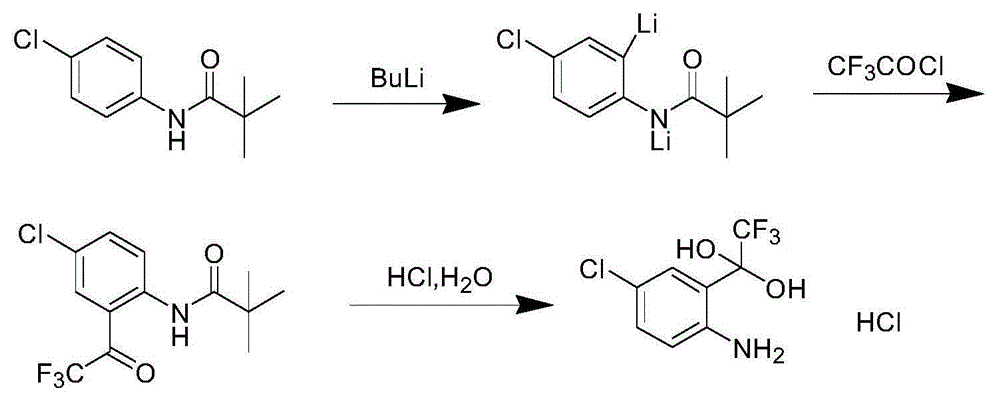
Synthetic method of 4-chlorine-2-trifluoroacetyl aniline aquo-complex hydrochloride
A technology of trifluoroacetylaniline and trifluoroacetyl, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of high price and difficulty in obtaining trifluoropyruvate, and achieve reduced production costs, reduced difficulty in sewage treatment, and easy recycling The effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 37 g of 4-chloro-N-pivaloylanilide was dissolved in 350 ml of methyl tert-butyl ether, and 2.0 M n-butyllithium was dissolved in 180 ml of methyl tert-butyl ether. When the 4-chloro-N-pivaloylanilide solution was cooled to -20°C, the n-butyllithium solution was added dropwise and reacted for 2 hours. At this temperature (-20°C), 22.9 grams of trifluoroacetyl chloride gas was introduced within 30 minutes, reacted for 1 hour after the gas was introduced, and then neutralized by adding dilute hydrochloric acid until the pH was neutral, separated to obtain an organic layer, and concentrated the organic layer. layer, the residue was added with 100 milliliters of acetic acid and 50 milliliters of concentrated hydrochloric acid and heated to 75 ° C for 4 hours, cooled to 0 ° C, crystallized to obtain 42 grams of 4-chloro-2-trifluoroacetylaniline hydrate hydrochloride, yield 87 %.
Embodiment 2
[0028] Example 2: The only difference from Example 1 is that the organic solvent methyl tert-butyl ether is replaced by n-hexane, and the other processes are the same as in Example 1, which will not be repeated here, and finally crystallized to obtain 4-chloro-2 - 39 grams of trifluoroacetylaniline hydrate hydrochloride, yield 80.7%.
Embodiment 3
[0029] Example 3: The only difference from Example 1 is that the organic solvent methyl tert-butyl ether is replaced with cyclohexane, and other processes are the same as in Example 1, which will not be repeated here, and finally crystallized to obtain 4-chloro- 40 grams of 2-trifluoroacetylaniline hydrate hydrochloride, yield 82.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com