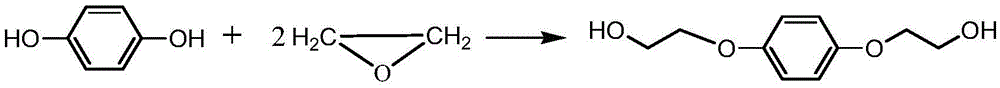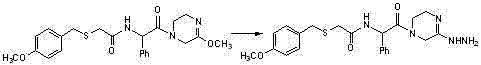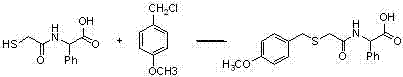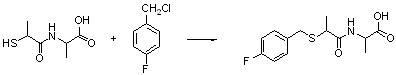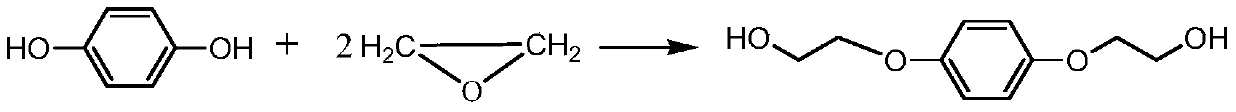Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40results about How to "The synthesis process is reasonable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
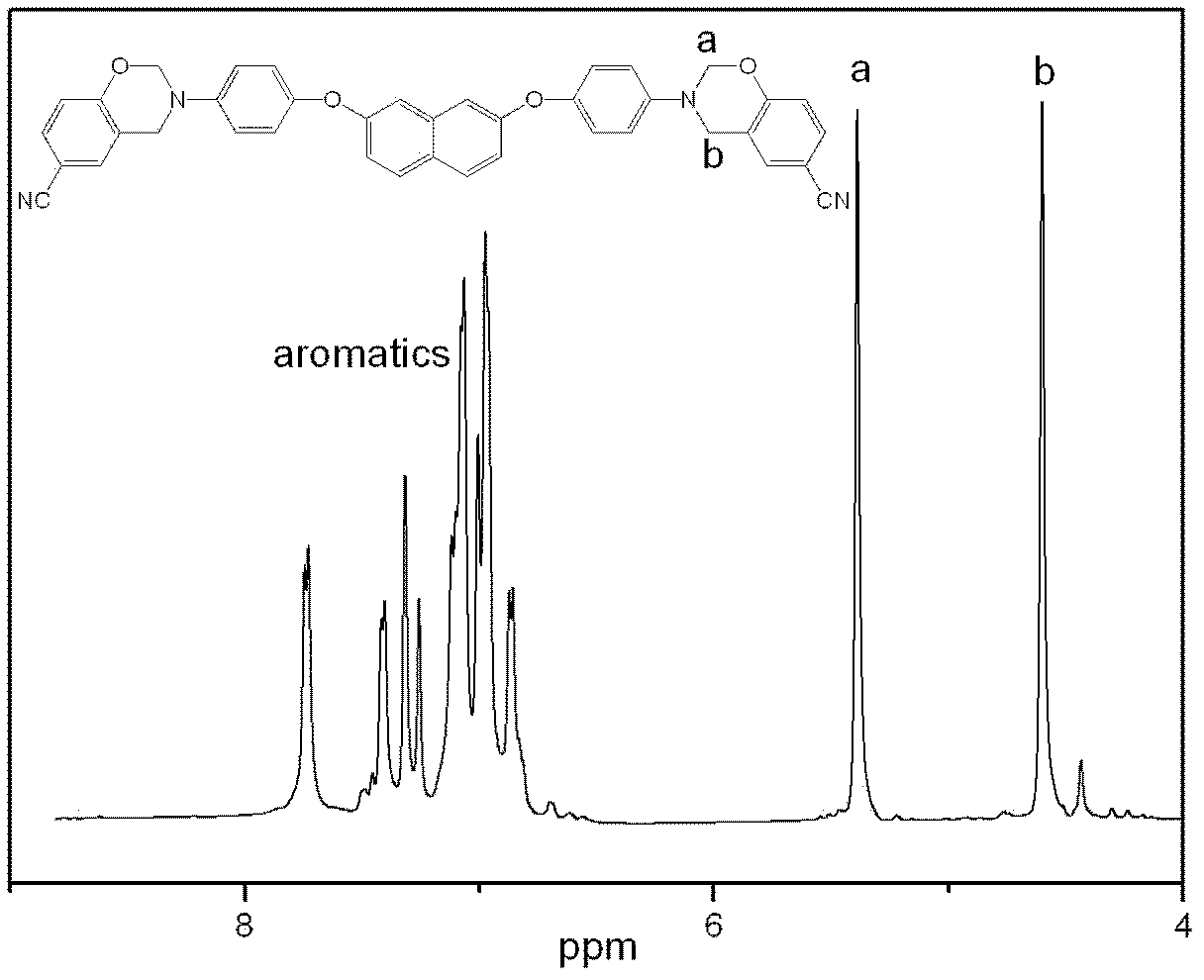
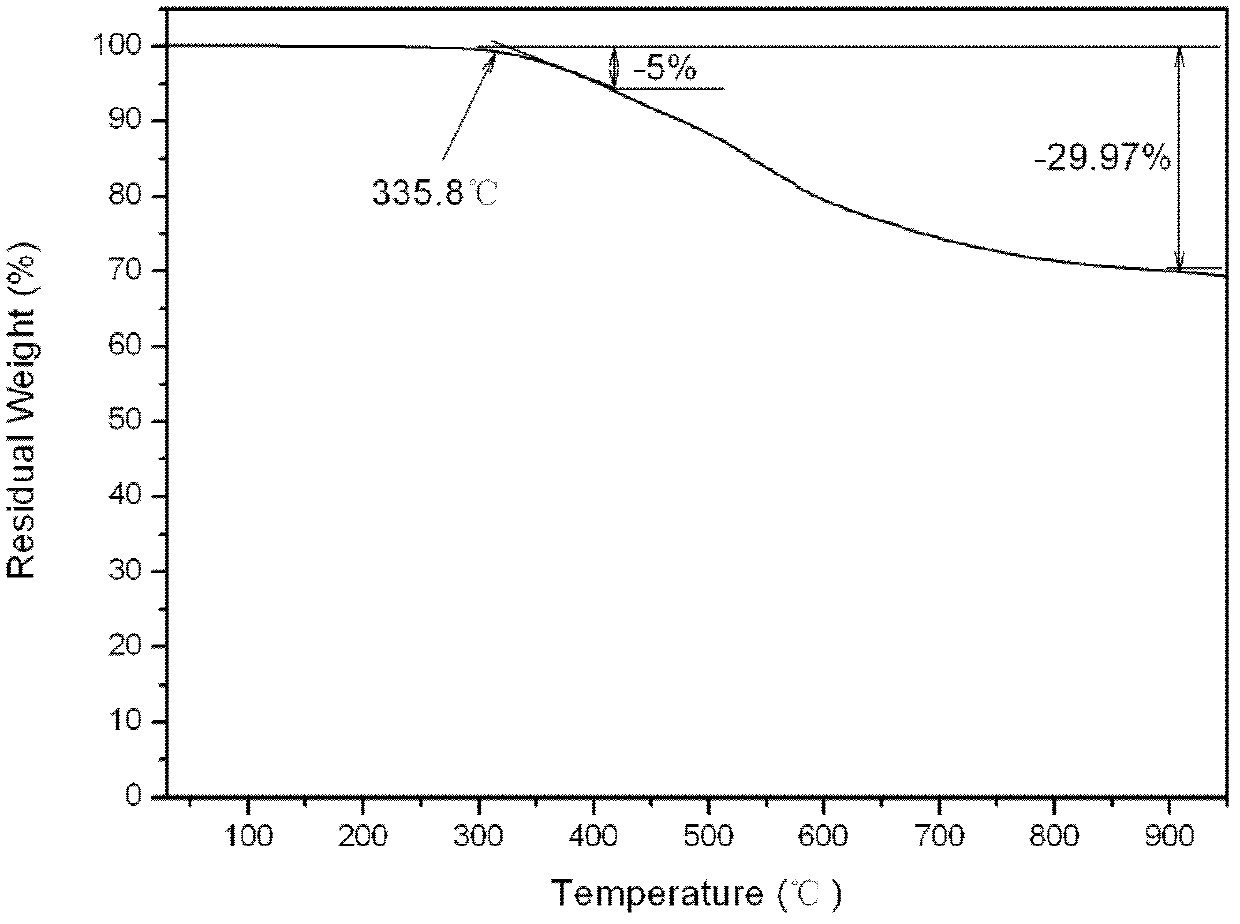
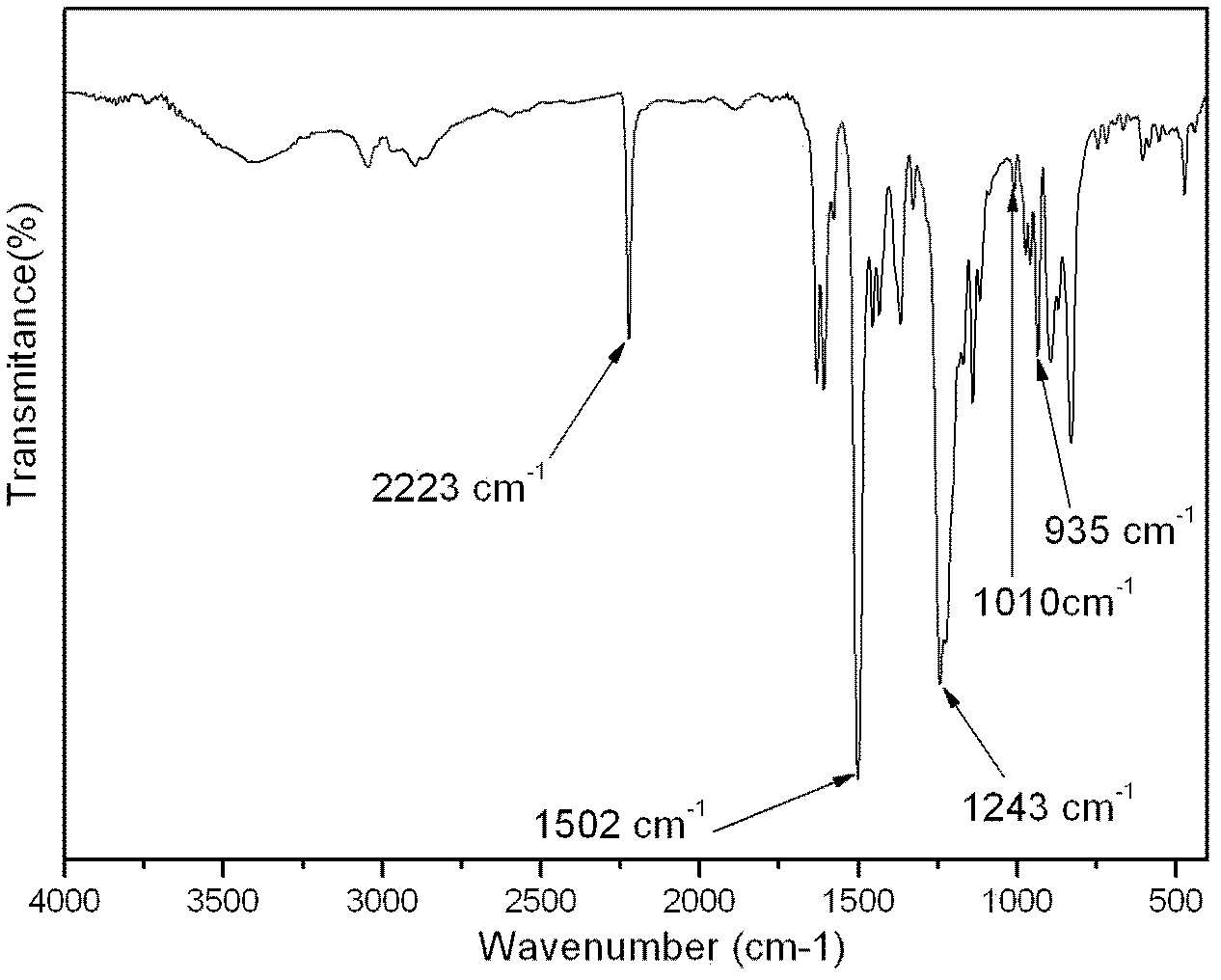
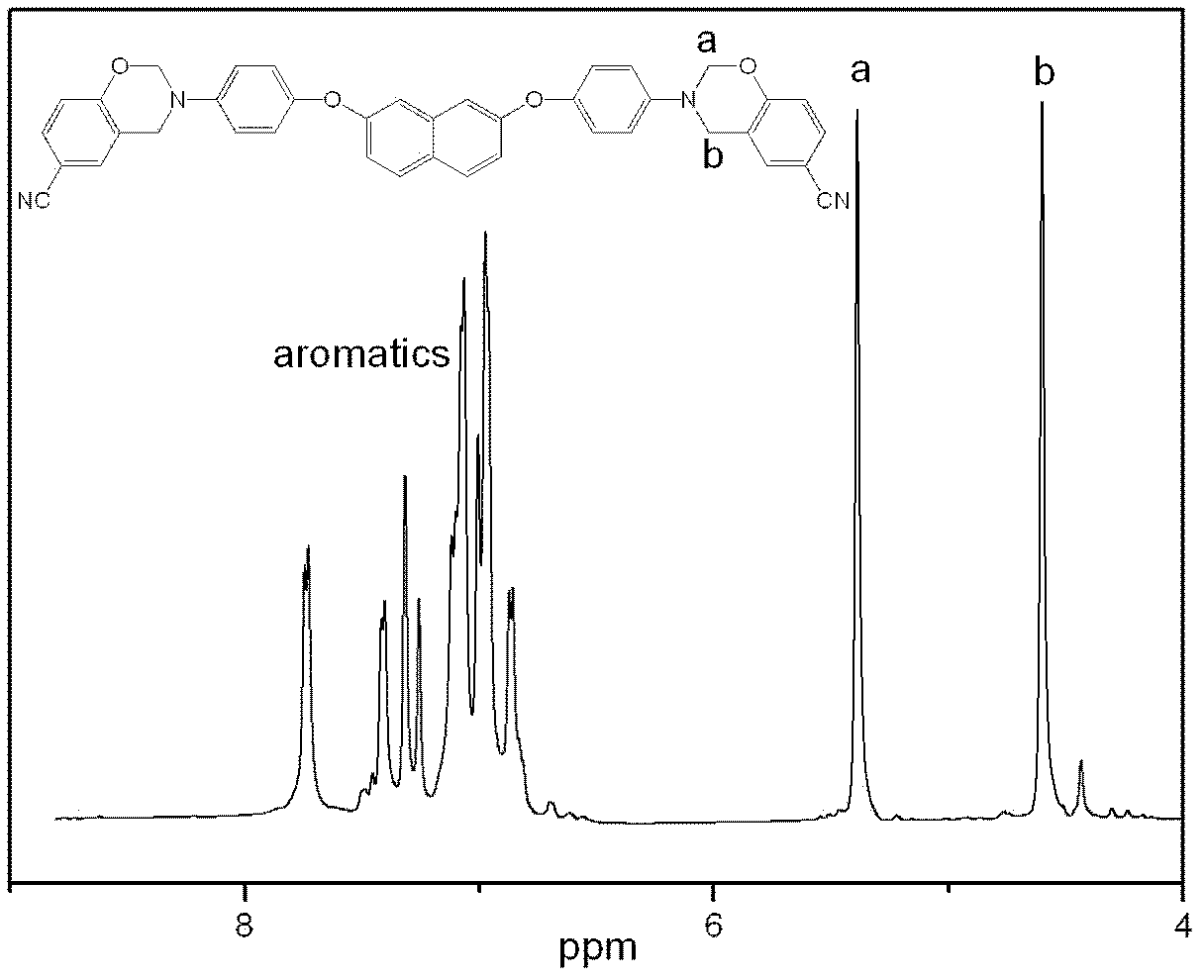
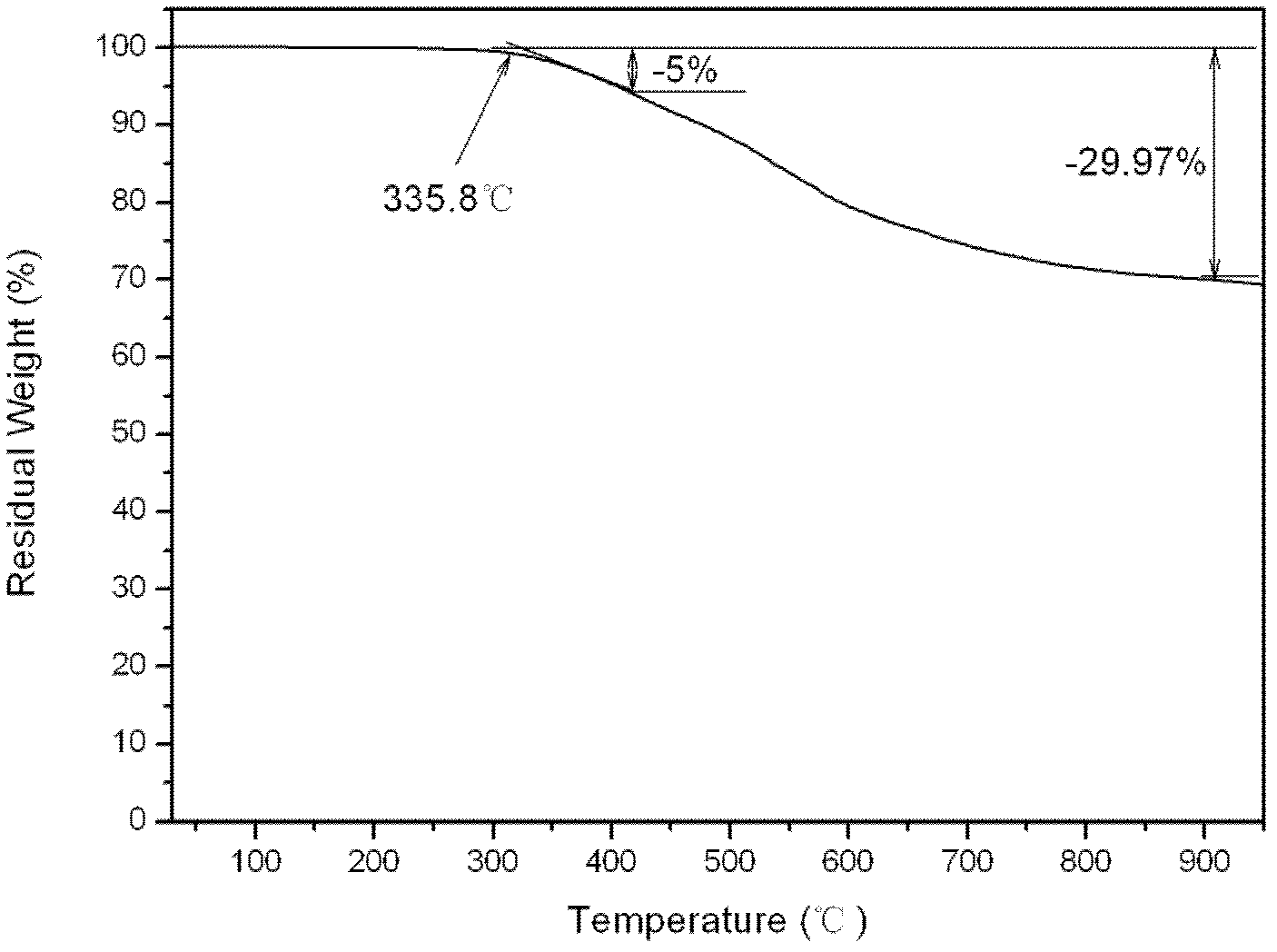
Aromatic diamine type cyano group-containing benzoxazine resin and its preparation method
The invention discloses an aromatic diamine type cyano group-containing benzoxazine resin and its preparation method. The benzoxazine resin belongs to the aromatic diamine type, its monomer main chain contains benzene rings and ether bonds, and the ends of the main chain contain cyano groups. A cured monomer has high heat resistance, high rate of combustion carbon residue, and certain toughness, thus being suitable for manufacturing high temperature resistant and flame retarding materials. The preparation method of the aromatic diamine type cyano group-containing benzoxazine resin takes an aromatic diamino compound, cyano group-containing monohydric phenol and formaldehyde as reaction raw materials to synthesize a benzoxazine monomer, which is then subjected to curing and crosslinking so as to obtain the benzoxazine resin. The monomer of the resin has reasonable synthesis process, high purity, high yield and low cost. And the monomer for synthesizing the aromatic diamine type cyano group-containing benzoxazine resin has a structural formula as the following.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Method for producing low-molecular-weight high-activity polyisobutene
InactiveCN101921352ASave the tedious processFor long-term storageHydrocarbonsHydrocarbon preparationPtru catalystOrganic solvent
The invention discloses a method for producing low-molecular-weight high-activity polyisobutene. The method comprises the following steps of: adding an organic solvent and an isobutene-containing raw material into a reaction kettle respectively; introducing gaseous-phase boron trifluoride and a liquid-phase complexing agent into the reaction kettle respectively; performing polymerization reaction at the temperature of between -30 and 15 DEG C under the pressure of between 0.05 and 0.3MPa for 0.5 to 6 hours, wherein the concentration of the isobutene monomer in the reaction process is 5 to 10 weight percent; and after the reaction is finished, processing the obtained mixture material to obtain the low-molecular-weight high-activity polyisobutene. The method overcomes the technical disadvantage that a catalyst needs to be complexed in advance, and saves the complex process for preparing the catalyst, saves the energy consumption and reduces the cost by directly adding the boron trifluoride and the complexing agent into the reaction kettle. The end-group olefin content of the prepared polyisobutene is over 70 percent and even over 80 percent; and the conversion rate of the isobutene reaches over 85 percent.
Owner:潍坊滨海石油化工有限公司
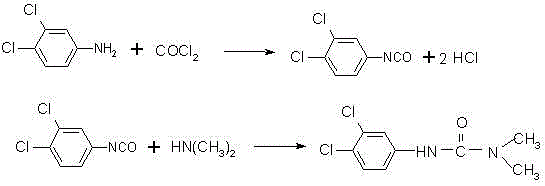
Method for preparing weedicide diuron
InactiveCN102617407AThe synthesis process is reasonableLow costUrea derivatives preparationOrganic compound preparationEvaporationPhosgene
The invention provides a method for preparing weedicide diuron. The method is characterized in that aromatic compound solvents are added in a reaction kettle, cooling and phosgene introduction are carried out, less dimethylformamide is added, 3, 4-dichloro phenylamine solution is dripped, and cocatalyst triethylene-diamine is added after the dripping completion; the temperature is raised to 80 to 100 DEG C, and the stirring is carried out under the heat insulation condition; solution consisting of 3, 4-dichloro phenyl isocyanate and the aromatic compound solvents is obtained through the reaction; and partial solvents are removed through evaporation, then, 40-percent dimethylamine is dripped, and dinitramine is prepared. Because the catalyst dimethylformamide and the cocatalyst triethylene-diamine are used in the reaction in the method provided by the invention, so the total yield of the synthesized dinitramine is higher than 91 percent, and the purity of the dinitramine raw medicine is higher than 99 percent. The synthesis process provided by the invention is more reasonable, the cost is low, the quality is high, a better environment-friendly effect is realized, the safety is higher, the international standard is reached, and the method is more suitable for industrialized production.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
Process for synthesizing 2-hydroxy-2-methyl-1-phenyl-1-propyl ketone
InactiveCN102304033AReduce pollutionThe synthesis process is reasonableOrganic compound preparationCarbonyl compound preparationKetoneAlkaline hydrolysis
The invention relates to a process for synthesizing 2-hydroxy-2-methyl-1-phenyl-1-propyl ketone, which is characterized by comprising the following steps of: obtaining a crude product of 2-hydroxy-2-methyl-1-phenyl-1-propyl ketone by using isobutyric acid as raw material via acylation, Friedel-Crafts and alkaline hydrolysis reaction; then finely steaming to obtain a finished product of the 2-hydroxy-2-methyl-1-phenyl-1-propyl ketone. The synthesis process provided by the invention is more reasonable, no sulfur dioxide gas is released in the production process, the generated sodium chloride has less environment pollution, and the product yield is high.
Owner:LIANYUNGANG SHENGNAN CHEM
Total-biology-base benzoxazine resin and preparation method thereof
InactiveCN107759615AThe synthesis process is simpleThe synthesis process is reasonableOrganic chemistryAnti-corrosive paintsRosinPetroleum
The invention provides total-biology-base benzoxazine resin and a preparation method thereof. A monomer of the resin is prepared by reacting dehydroabietylamine as a rosin derivative, biological phenol and paraformaldehyde at the temperature of 85 DEG C with a solution method for 20 hours. The preparation method comprises the following steps: cooling reactants to room temperature, washing with a sodium bicarbonate solution, washing with deionized water, and crystallizing with ethanol to obtain a purified monomer; performing ring-opening crosslinking on the monomer in a heating state or in thepresence of an added catalyst to generate the total-biology-base benzoxazine resin. The total-biology-base benzoxazine resin has the advantages of reasonable synthesis process, no need of inertial gasprotection, high product purity and environmental friendliness; compared with other biology-base benzoxazine and a part of petroleum-base benzoxazine, the obtained novel total-biology-base benzoxazine resin has great competitiveness on the aspects of ring-opening temperature and thermal stability, and shows high corrosion resistance.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing diflubenzuron serving as pesticide
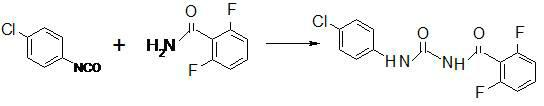
ActiveCN102180813AThe synthesis process is reasonableLow costUrea derivatives preparationOrganic compound preparationDiamineDimethyl formamide
The invention discloses a method for preparing diflubenzuron serving as a pesticide. The method is characterized by comprising the following steps of: adding an aromatic compound solvent into a reaction kettle, cooling, introducing phosgene, adding an appropriate amount of dimethyl methanamide, dripping a p-chloroaniline solution and adding triethylene diamine serving as a catalyst promoter; raising the temperature, preserving heat and stirring; reacting to obtain a solution containing rubigan isocyanate and the aromatic compound solvent; and adding 2,6-difluorobenzamide after adding the aromatic compound solvent into another reaction kettle, raising temperature and dripping a p-chloroaniline isocyanate solution to obtain the diflubenzuron. In the method, dimethyl methanamide serving as acatalyst and triethylene diamine serving as the catalyst promoter are used in a reaction, so that the total yield of synthesized diflubenzuron is over 91 percent, and the purity of a diflubenzuron active compound is over 99 percent. The method has the advantages of reasonable synthesis process, low cost, high quality and environmental friendliness, is up to the international standard, and is moresuitable for industrial production.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
Aromatic diamine type cyano group-containing benzoxazine resin and preparation method thereof
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Preparation method of 2,4-dichloro-5-isopropoxy aniline salt
InactiveCN102363597AReduce riskThe synthesis process is reasonableOrganic compound preparationAmino-hyroxy compound preparationSulfite saltNitrobenzene
The invention discloses a preparation method of 2,4-dichloro-5-isopropoxy aniline salt. The preparation method is characterized by comprising the following steps of: generating 2,4-dichloro-5-isopropoxy aniline by using 2,4-dichloro-5-isopropoxy nitrobenzene in the presence of polar solvent and water and under the reaction action of stannous chloride, sodium sulfide, sodium sulfite or sodium hyposulfate, adding aromatic hydrocarbon or chloroalkene solvent serving as an extracting agent, extracting, washing, acidifying, filtering, drying, and thus obtaining the 2,4-dichloro-5-isopropoxy aniline salt. Because the reducing agent, the polar solvent and the water are used in the reaction, risk is reduced in the production process of a production workshop, the environment is protected, massive iron cement is not produced, and the aniline salt reaches national standard. Therefore, the method is more reasonable in synthesis process, low in cost and high in quality, and is more suitable for industrialized production.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
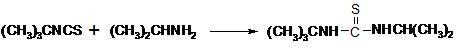
Preparation method of 1-isopropyl-3-tertiary butyl thiocarbamide
The invention relates to a preparation method of 1-isopropyl-3-tertiary butyl thiocarbamide, which is characterized by using chlorobenzene as a solvent in the process of preparing the 1-isopropyl-3-tertiary butyl thiocarbamide. The method comprises the following concrete steps: adding tert-butyl isothiocyanate to the chlorobenzene and stirring; cooling, dropwise adding isopropylamine, and insulating and reacting after dropwise adding the isopropylamine to obtain the solution of the 1-isopropyl-3-tertiary butyl thiocarbamide; and after cooling, centrifuging and drying to obtain the 1-isopropyl-3-tertiary butyl thiocarbamide. The invention has safer and simpler technological operation, no three wastes and high yield, and is more environment-friendly and more suitable for industrial production.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
Preparation method of arylamine
InactiveCN102617358AEnvironmentally friendlyThe synthesis process is reasonableOrganic compound preparationAmino compound preparationBrown iron oxidePotassium hydroxide
The invention relates to a preparation method of arylamine. The preparation method includes dissolving ferric trichloride-water (1 / 6) in distilled water, and adding dropwise an aqueous solution of sodium hydroxide or potassium hydroxide so that the pH is within the range of 7 to 8; slowly heating the solution so that the temperature is within the range of 55 DEG C to 65 DEG C, preserving heat, leaching, drying filter cakes, and grinding to obtain iron oxide hydroxide powder serving as a catalyst; and adding an aromatic nitro compound, distilled water and the catalyst into a reactor, heating the reactor to the temperature between 60 DEG C and 100 DEG C, adding hydrazine hydrate to react, allowing the reactor to stand, layering, adding an appropriate amount of water, and washing to obtain the arylamine corresponding to the aromatic nitro compound. The preparation method is safe, simple and convenient to operation, few in the three wastes, environment-friendly, high in yield and applicable to industrial production, and the product quality meets national standard.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
Method for preparing 2,5-dichlorophenol.
ActiveCN105272828ALow costPromote escapeOrganic chemistryOrganic compound preparationDichlorophenolO-chlorophenol
The invention discloses a method for preparing 2,5-dichlorophenol. In the method, o-chlorophenol is employed as a raw material, a composite catalyst is employed, acidic materials are utilized to protect hydroxys, and then chlorination and hydrolysis are carried out. When the new method for preparing 2,5-dichlorophenol is compared with present production methods in the market, the raw material cost is lower, operation is safer and simpler, the provided method is more friendly to the environment, three wastes (waste gas, waste water and industrial residue) are smaller, the yield is higher, the provided method is suitable for industrial production, and the product quality meets international standards.
Owner:兰州诚胜化工科技有限公司
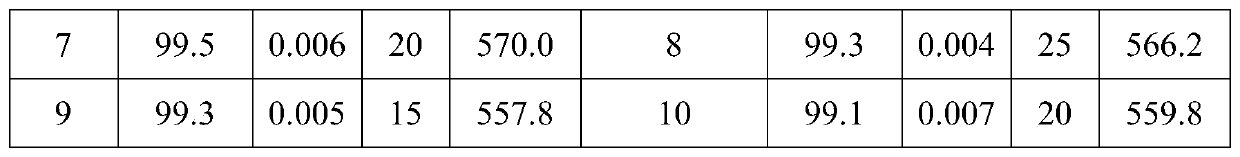
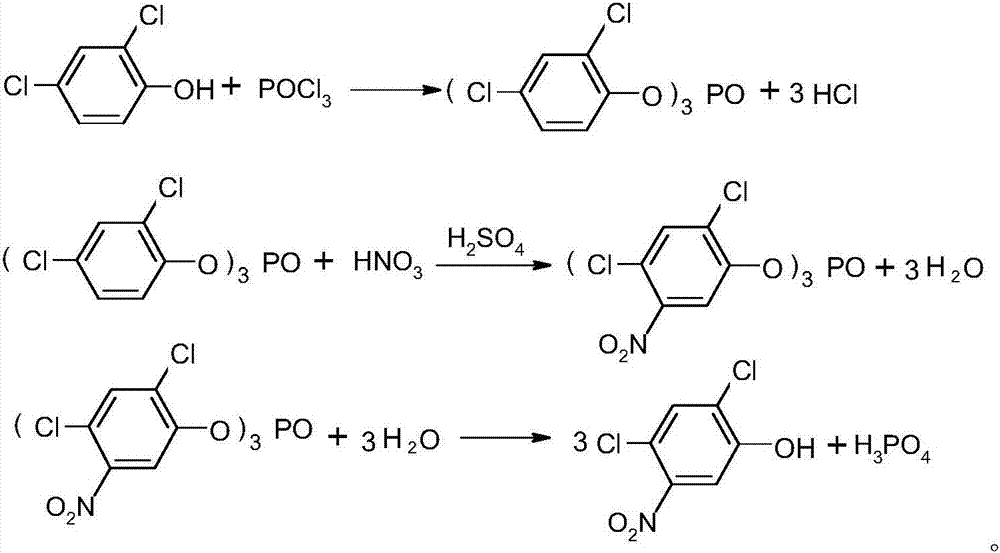
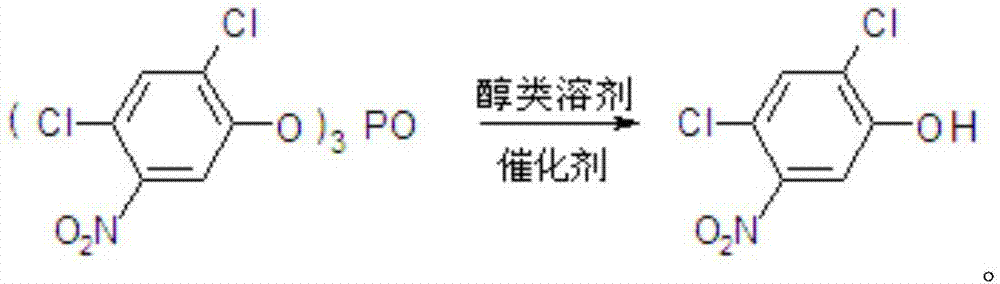
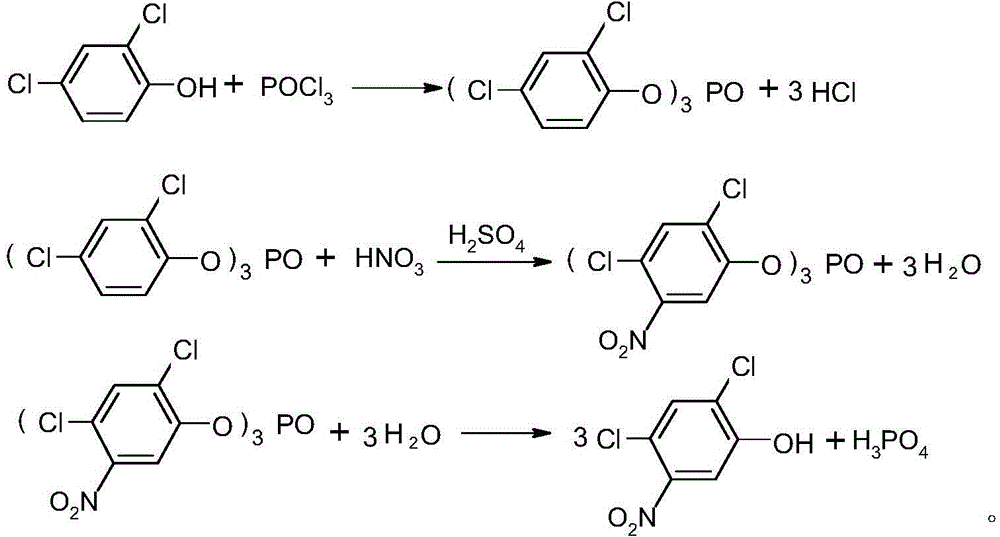
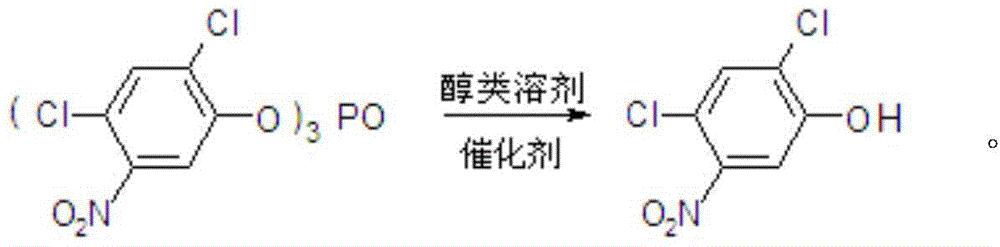
Method for preparing 2,4-dichloro-5-nitrophenol
ActiveCN105646231AAvoid pollutionThe synthesis process is reasonableOrganic chemistryOrganic compound preparationSolventPhosphate
The invention discloses a method for preparing 2,4-dichloro-5-nitrophenol. The method is as below: first, evenly mixing tris(2,4-dichloro-5-nitrophenyl) phosphate, rare earth trifluoromethyl sulfonate catalyst, a cocatalyst and an alcoholic solvent; then gradually heating to a reflux temperature, reacting for more than 3h; and cooling to room temperature after the reaction, separating the catalyst by filtration, and removing the solvent in a liquid phase by distillation to obtain the 2,4-dichloro-5-nitrophenol. In the presence of the co-catalyst, tris (2,4-dichloro-5-nitrophenyl) phosphate reacts with the alcohol solvent under the catalysis of rare earth trifluoromethyl sulfonate, so as to fundamentally avoid the environmental pollution caused by the large quantities of waste acid in the traditional hydrolysis; the reaction is more thorough; and the method increases the yield of the product 2,4-dichloro-5-nitrophenol from 88% to 98%, increases the purity from 91.5 % to 97.5%, lowers production costs, and plays a positive role for the industrial development of oxadiazon.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
Preparation method of 1-isopropyl-3-tert-butylthiourea
The invention discloses a preparation method of 1-isopropyl-3-tert-butylthiourea. Ammonium thiocyanate, tert-butyl alcohol and hydrochloric acid serve as raw materials and conduct the substitution reaction to generate a mixture of thiocyanic acid tert-butyl ester and isothiocyanic acid tert-butyl ester, the mixture stands still and is layered, water in the mixture is removed, then concentrated sulfuric acid serves as a transposition catalyst, thiocyanic acid tert-butyl ester is transposed to generate isothiocyanic acid tert-butyl ester, finally under the existence of isopropylamine, water serves as a solvent, the addition reaction is conducted, and a target product is synthesized. Water is removed in the reaction based on the principle that the specific gravity of matter is reduced to different degrees along with increase of temperature, and a large amount of heat energy is saved; the sulfuric acid serves as the transposition catalyst, and the reaction yield is increased to more than 90% from about 66% originally; the water serves as the solvent, risks are reduced in the production process of a production workshop, operation is safer, environment friendliness is achieved, cost is low, and quality is high; the total reaction yield of the method can be more than 88.9% by ammonium thiocyanate, and the method is more suitable for industrial production.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
Polyester resin impregnating agent
InactiveCN101654336AStrong solubilityThe accuracy of feeding and temperature control is improvedChemistrySolubility
The invention relates to a polyester resin impregnating agent which is widely suitable for glass fiber enterprises. The polyester resin impregnating agent is prepared by the following raw materials inparts by weight: 100-200 of dicyclopentanediol, 90-180 of neopentyl glycol, 90-200 of trimethylolpropane, 120-200 of dihydromethyl propionic acid and 70-160 of tetrafluorophthalic anhydride. The invention has high hardness, toughness, tensile strength, bending strength and dissolubility in styrene, trends to refinement in the synthesizing process, improves the feed and temperature control precision and realizes numerical control; the synthesizing process is carried out by from a one-step method to a multistep method so as to enable a molecule chain structure of a polyester film forming agentto be more reasonable and regular, and the molecular weight is as high as more than ten thousand; and the polyester resin impregnating agent is widely applied to the impregnating agent of chopped strand mat, jet, BMC and SMC yarns.
Owner:朱继业
Preparation method of diphenyl sulfide
ActiveCN104311463ALow costThe synthesis process is reasonableSulfide preparationChlorobenzeneProcess engineering
The invention discloses a preparation method of diphenyl sulfide. The method is as below: adding a certain amount of sodium sulfide in a reaction container; keeping the negative pressure at less than or equal to -0.098 MPa, and slowly heating to 190-220 DEG C; dropwise adding a solvent with insulation, stirring, transferring the sodium sulfide solution while hot to an autoclave, then dropwise adding chlorobenzene while stirring in 1.5-2.5 h, covering the lid, and displacing the air with nitrogen two times; slowly heating to 190 to 240 DEG C while stirring, preserving the temperature and stirring for reaction; slowly cooling, filtering solid, recovering the solvent, purifying and distilling to obtain diphenyl sulfide. The novel method of the invention uses chlorobenzene and sodium sulfide as raw materials to prepare diphenyl sulfide by using. Compared with the prior art in the market, the method has the advantages of low raw material cost, safe and convenient operation, environment-friendliness, less three wastes and high yield, and is suitable for industrial production; and the product quality is in line with international standards.
Owner:兰州诚胜化工科技有限公司
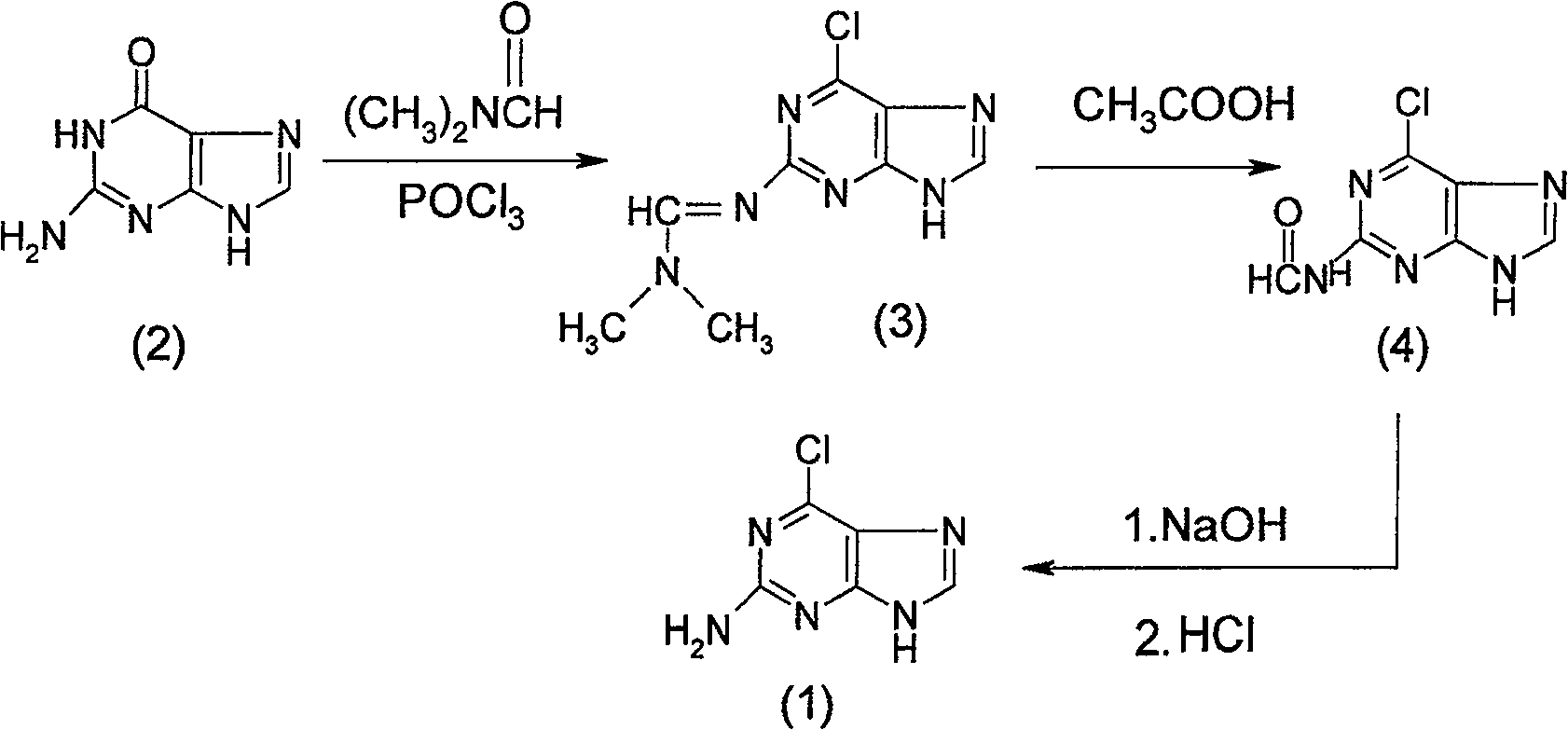
Method for synthesizing 2-amido-6-chloropurine
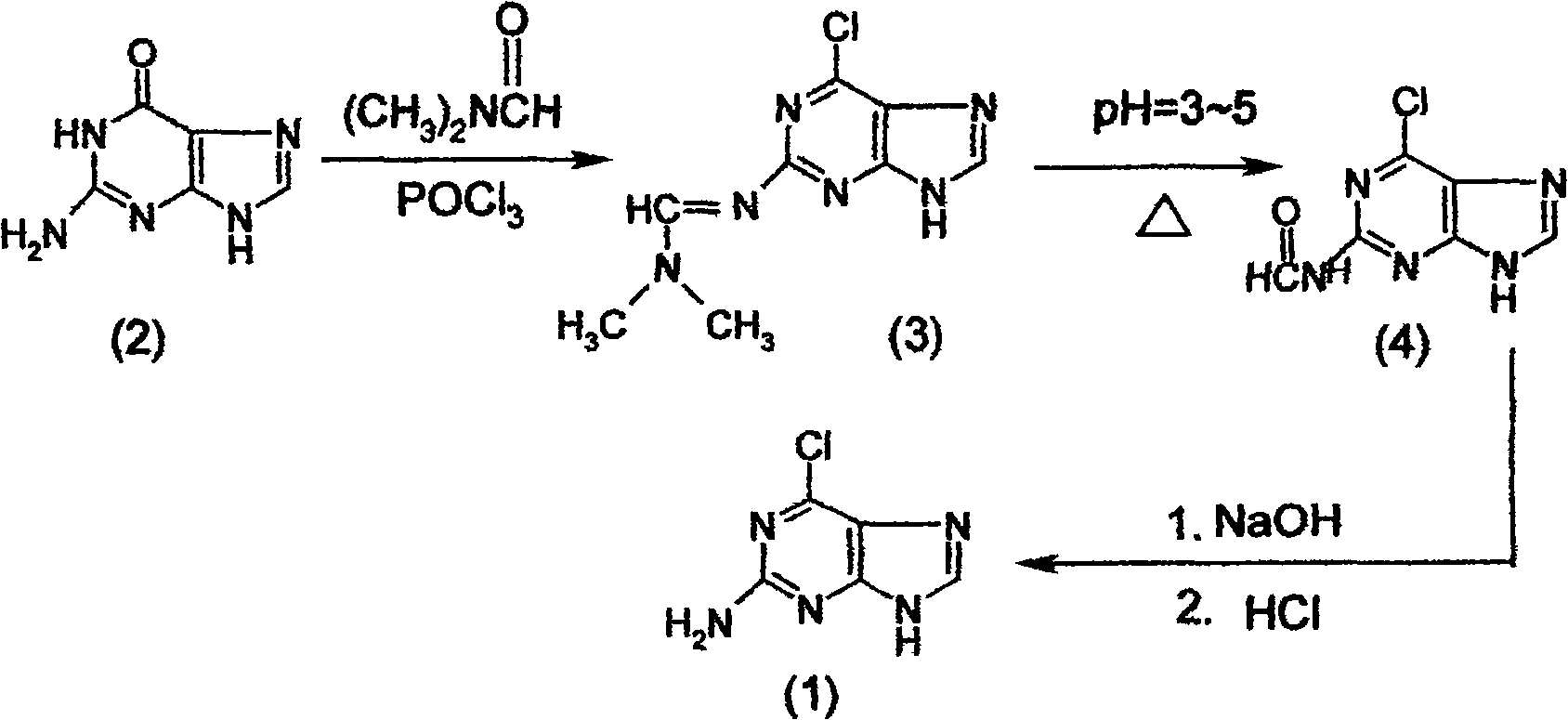
InactiveCN100549013CAvoid the disadvantage of being prone to side reactions with guanineHigh yieldOrganic chemistrySynthesis methodsEthane Dichloride
The present invention relates to a kind of synthesis method of 2-amino-6-chloropurine (1), comprising the following steps in turn: first drop phosphorus oxychloride into N,N-dimethylformamide at low temperature to form a mixed solution, Then drop into the 1,2-dichloroethane of guanine (2) and react to obtain 2-dimethylaminomethenimino-6-chloropurine (3); the reaction product (3) is dropped into water, and The organic phase is recovered and used mechanically, the pH of the aqueous phase is adjusted to 3 to 5 with an alkali metal hydroxide solution, and the reaction is heated to obtain a wet product of 2-formylamino-6-chloropurine (4); the wet product of the above reaction product (4) is then The hydrolysis reaction is carried out in an alkali metal hydroxide solution, and then the pH value is adjusted with hydrochloric acid to obtain the crude product of 2-amino-6-chloropurine (1), and finally purified to obtain the fine product of the target product (1).
Owner:LIANYUNGANG CCA CHEM CO LTD
Maleic hydrazide preparation method
InactiveCN105693623AHigh yieldThe synthesis process is reasonableOrganic chemistryHydrazine compoundRare earth
The invention discloses a maleic hydrazide preparation method. The method comprises the following steps: heating and reacting hydrazine hydrate and diluted sulfuric acid under the action of a rare earth compound catalyst, adding maleic anhydride, carrying out a ring closure reaction, and adding an inorganic alkali to neutralize the obtained product in order to obtain maleic hydrazide. The rare earth trifluoromethanesulfonate catalyst is added in the reaction, and the inorganic alkali is used to carry out neutralization treatment after the reaction in order to realize the completeness of the reaction of the raw materials hydrazine hydrate and diluted sulfuric acid, so the maleic hydrazide yield is improved by 10% or above, and the content of residual hydrazine in the maleic hydrazide is controlled to be 2ppm or below and reaches international standards. The method has the advantages of reasonable synthesis technology, low cost, high quality, and suitableness for industrial production.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
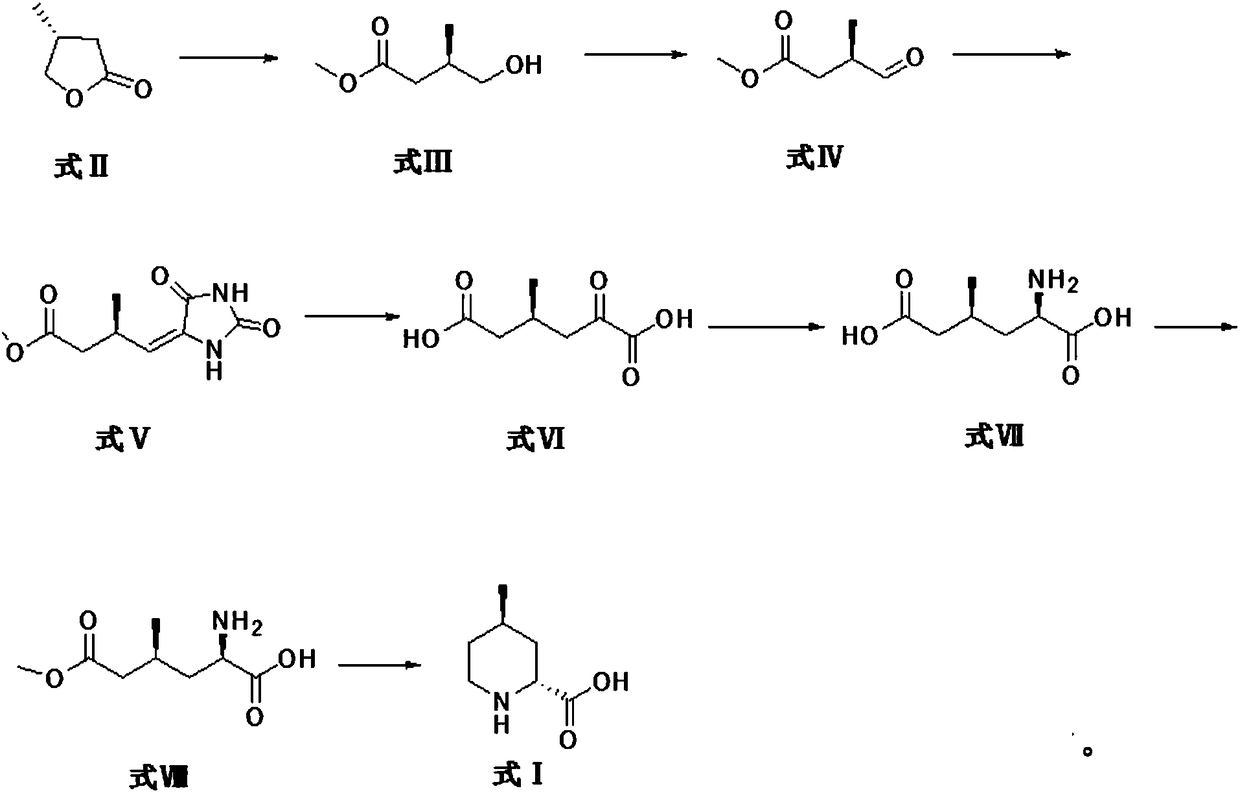
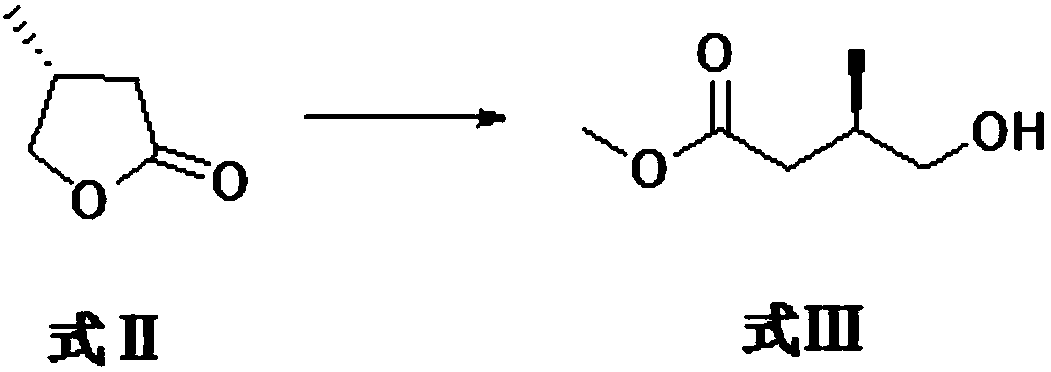
Preparation method for argatroban intermediate
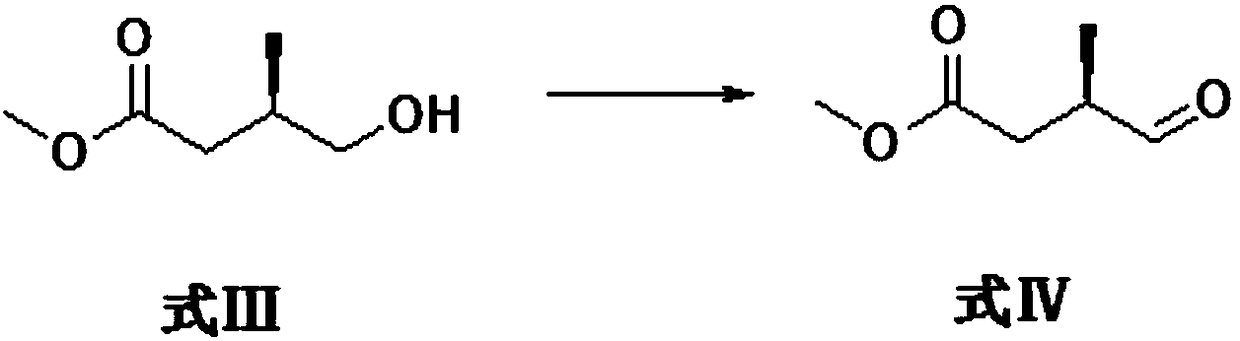
The invention discloses a preparation method for an argatroban intermediate. The preparation method comprises the following steps: subjecting (R)-4-methyldihydrofuran-2(3H)-one to ring opening and then to a reaction with sodium methoxide or potassium methoxide to form R-3-methyl-4-methyl-3-hydroxybutyrate; oxidizing R-3-methyl-4-methyl-3-hydroxybutyrate to form methyl (3R)-3-methyl-4-formylbutyrate; then reacting (3R)-3-methyl-4-formylbutyrate with hydantoin to form (3R)-3-methylene-butyric acid carbomethoxyhydantoin; successively carrying out purification, heating reflux and purification so as to obtain (4S)-4-methyl-2-oxoadipate; reacting (4S)-4-methyl-2-oxoadipate with an amino donor under the action of a catalyst, and carrying out purification so as to obtain (2R,4S)-2-amino-4-methyladipate; reacting (2R,4S)-2-amino-4-methyladipate with methanol, and carrying out purification so as to obtain (2R,4S)-2-amino-6-methoxy-4-methyl-6-oxohexanoic acid; and reacting (2R,4S)-2-amino-6-methoxy-4-methyl-6-oxohexanoic acid with a reducing agent, and carrying out purification so as to obtain the argatroban intermediate. The preparation method of the invention is mild in reaction conditions, low in cost and suitable for industrial production.
Owner:天津同华生物科技有限责任公司
Preparation method of 4, 5-dihydro-6H-cyclopenta[b]thiophene-6-ketone
InactiveCN103145692ARaw materials are cheap and easy to getReduce manufacturing costOrganic chemistryKetonePhenyl propanolamine
The invention relates to a synthetic process of 4, 5-dihydro-6H-cyclopenta[b]thiophene-6-ketone. The synthetic process comprises the following steps of: with 3-thiophene carbinal as a starting material, carrying out Knoevenagel condensation reaction, esterification and hydrogenation reduction, and finally carrying out ring formation under the action of PPA (phenyl-propanolamine) to obtain a target product. According to the preparation method, the 3-thiophene carbinal is taken as the starting material and is cheap and easily available, so that the production cost is low. The synthetic process is simple and reasonable. The conversion rate is high, and the total yield is increased to 50%. The aftertreatment is convenient and feasible. A part of intermediates do not need to be purified and can be directly used in a next reaction step, so that the aftertreatment process is reduced, simultaneously the technology difficulty and the discharge of pollutants are reduced, and the whole reaction is suitable for large-scale commercial process.
Owner:YANCHENG INST OF TECH
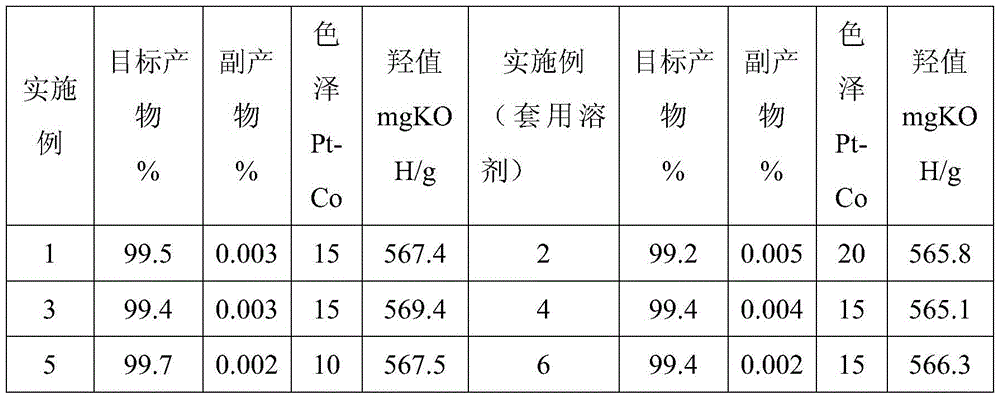
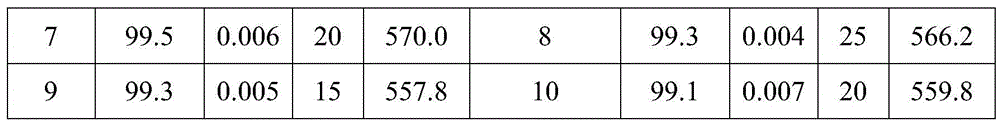
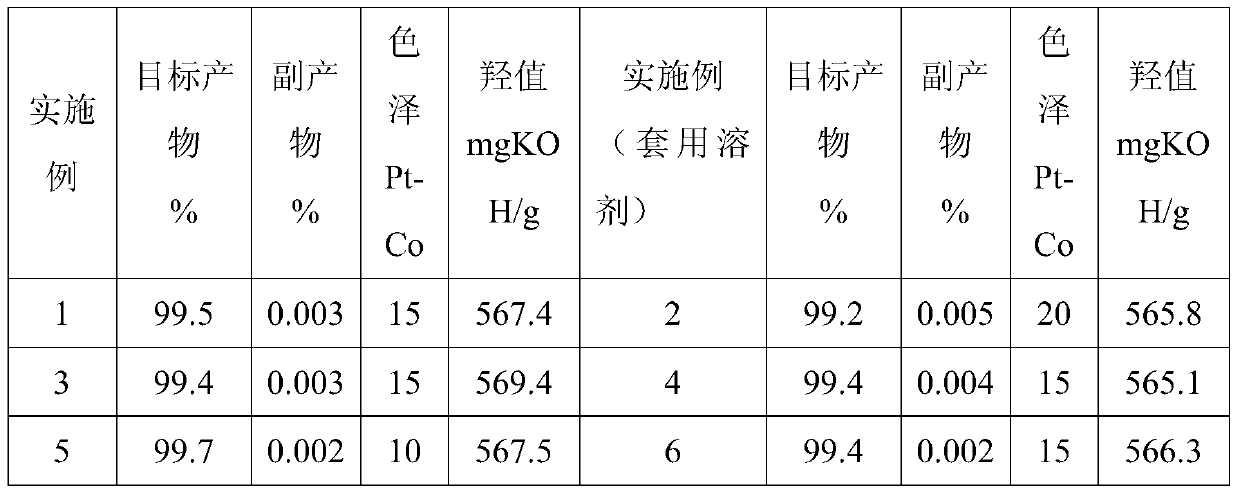
Method for synthesizing hydroquinone dihydroxyl diethyl ether
ActiveCN105523905AThe synthesis process is reasonableHigh purityEther preparation from oxiranesReaction temperatureDiethyl ether
The invention relates to a method for synthesizing hydroquinone dihydroxyl diethyl ether and belongs to the technical field of synthesis of organic compounds. The method comprises the steps: carrying out synthesis by taking hydroquinone and epoxyethane as raw materials, adding hydroquinone, a ferrocene catalyst and an ether solvent into a reactor, carrying out vacuumizing, heating the reactor until hydroquinone is completely dissolved in the ether solvent under nitrogen protection, then, adding a chain extender into the reactor, heating the reactor to a polymerization reaction temperature, and carrying out hydroquinone dihydroxyl diethyl ether synthesis under polymerization reaction pressure; and after the reaction ends, carrying out cooling, and subjecting crystallizing mother liquor to normal-pressure or reduced-pressure rectification, so as to separate out the ether solvent. The hydroquinone dihydroxyl diethyl ether is synthesized by the method, the problems in the conventional technologies that the preparation process is complicated and the quality of product is poor are solved, and the obtained product is reasonable in distribution, light in color and luster and low in byproduct content.
Owner:ZHEJIANG HUANGMA TECH
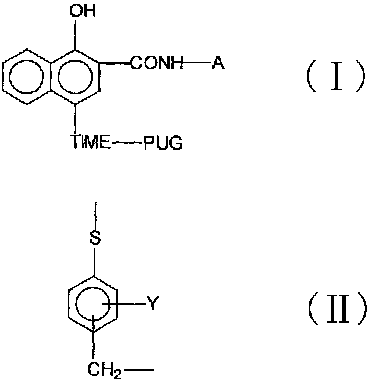
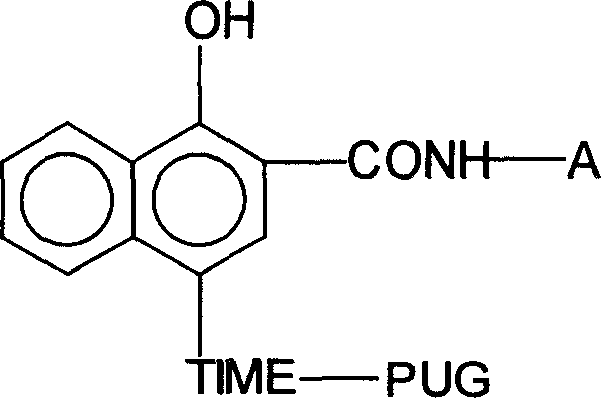
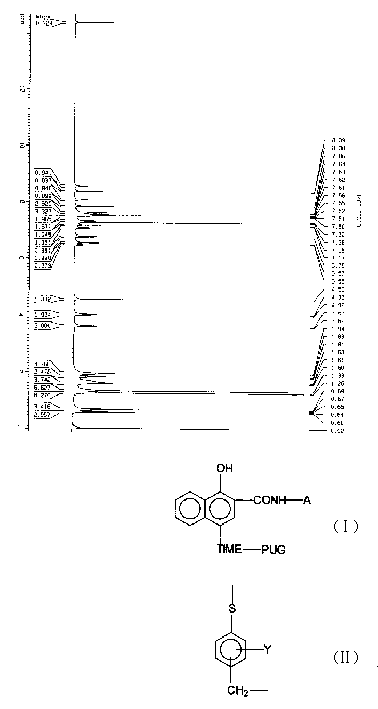
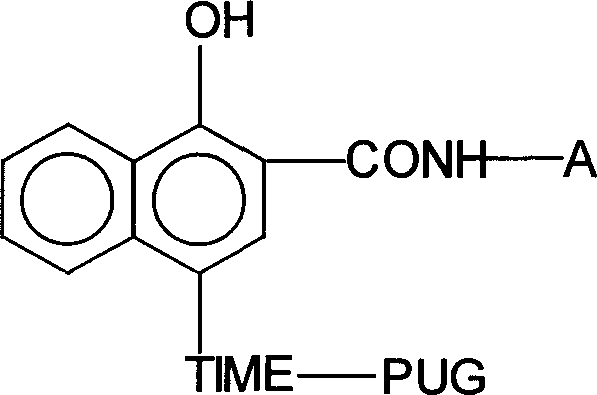
Timing release developing inhibitor cyan colour coupler and preparation thereof
InactiveCN1249519CThe synthesis process is reasonableHigh purityMulticolor photographic processingRelease timeChemistry
Owner:CHINA LUCKY FILM CORP +2
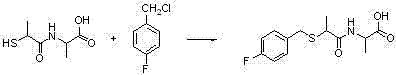
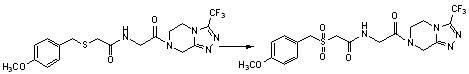
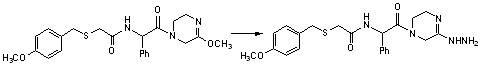
Benzylthio acetamido acetylpyrazine triazole derivative as well as preparation thereof and application thereof
ActiveCN102260268BReasonable designThe synthesis process is reasonableOrganic active ingredientsOrganic chemistryDipeptidyl peptidaseTrimethyloxonium tetrafluoroborate
Owner:HANGZHOU ADAMERCK PHARMLABS INC
Timing release developing inhibitor cyan colour coupler and preparation thereof
In the present invention, TIME group is formed by residue of the following molecular structures, compound II is used to act with developer oxide to release TIME-PUG group when compound is used in developing process, TIME-PUG grou is cracked on TIME-PUG group under certain condition to realease PUG group for improving photographic performance of the film.
Owner:CHINA LUCKY FILM CORP +2
Benzylthioacetamidoacetylpyrazinetriazole derivatives and their preparation and application
ActiveCN102260268AReasonable designThe synthesis process is reasonableOrganic active ingredientsOrganic chemistryDipeptidyl peptidaseHydrazine compound
The invention provides a benzylthio acetamido acetylpyrazine triazole derivative. A target compound (I) is obtained by condensing a sulfydryl acetamido acetic acid derivative and a benzyl chloride derivative, condensing the condensed derivatives and 2-piperazinone, reacting the obtained condensed product and trimethyloxonium tetrafluoroborate so as to obtain a methoxy imide compound, and carryingout cyclization on the methoxy imide compound and hydrazine, trifluoroaceticanhydride, acetic anhydride, propionic anhydride, trifluoroacetyl chloride, acetyl chloride or acrylyl chloride. The preparation method provided by the invention has the advantages of reasonable design, stable process, easily obtained raw materials for production, no use of high pressure hydrogenation and good production feasibility. By using the benzylthio acetamido acetylpyrazine triazole derivative provided by the invention, dipeptidyl peptidase-IV (DPP-4) can be selectively inhibited; an effect of reducing blood sugar is obtained; and the derivative can be applied to the preparation of drugs for reducing the blood sugar and has the following structural formula (I).
Owner:HANGZHOU ADAMERCK PHARMLABS INC
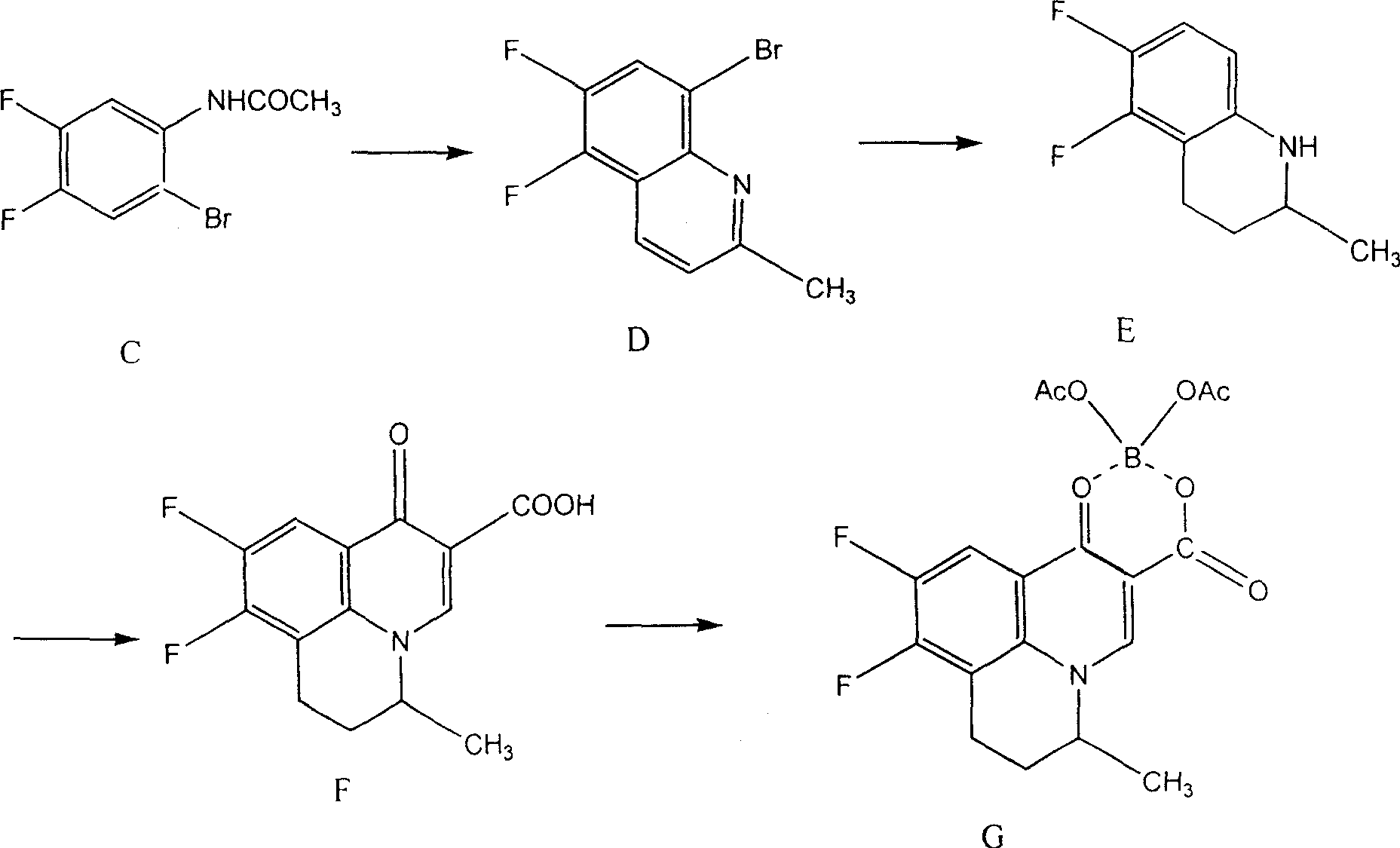
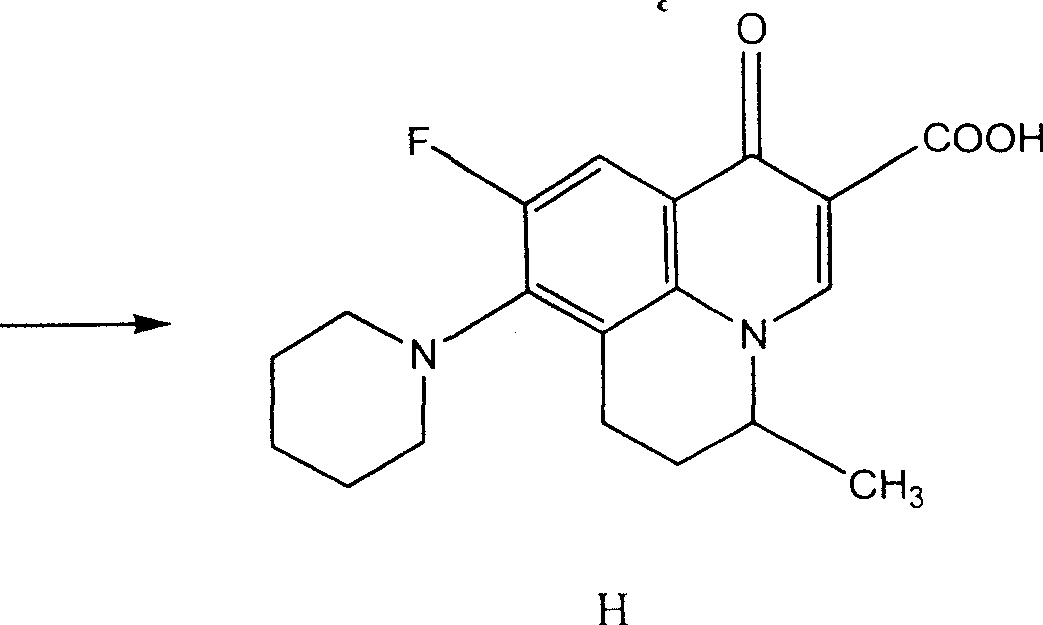
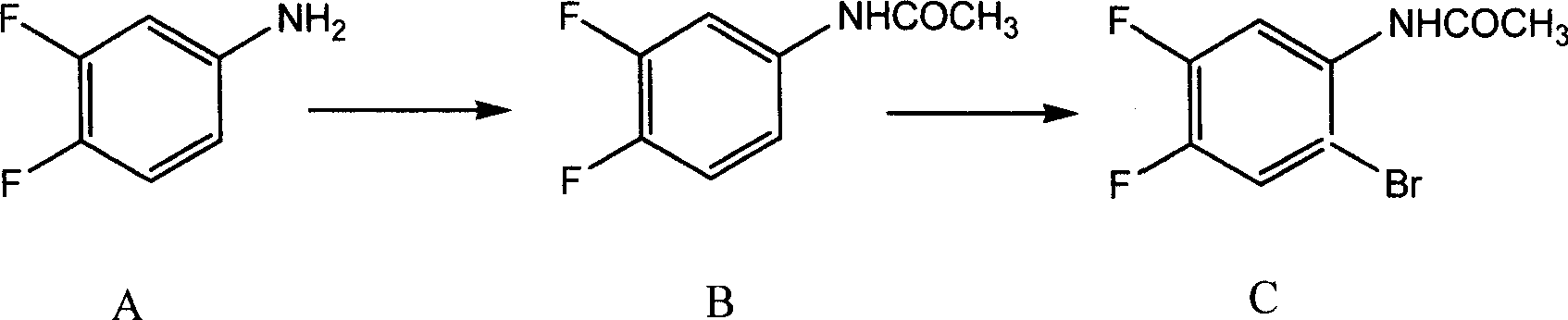
Preparing method of 8-bromo-5,6-difluoro-2-methylquinoline
The invention discloses a making method of 8-bromine-5, 6-difluo-2-methyl quinoline as key intermediate of broad spectrum antibiotic nafuxacin, which comprises the following steps: adopting 3, 4-difluo-6-difluo phenylamine as original raw material; acylating to produce 3, 4-difluo aceto phenylamine; bromidizing to make 3, 4-difluo-6-difluo aceto phenylamine as cyclic starting material; ringing 3, 4-difluo-6-difluo aceto phenylamine, oxidizer, alleviator, condensed sulfuric acid, ferric sulfate, trans-crotonaldehyde; selecting condensed sulfuric acid and condensed nitric acid as oxidizer; fitting for industrial manufacturing with low cost and high receiving rate.
Owner:CHANGZHOU YABANG PHARMA
A method for preparing 2,5-dichlorophenol
ActiveCN105272828BLow costPromote escapeOrganic chemistryOrganic compound preparationChlorideDichlorophenol
The invention discloses a method for preparing 2,5-dichlorophenol. The method is formed by using o-chlorophenol as a raw material, adopting a composite catalyst, protecting a hydroxyl group with an acidic substance, and then chlorinating and hydrolyzing it. The new method of the present invention for preparing 2,5-dichlorophenol has lower cost of raw materials than the existing production methods in the market, safe and convenient operation, and is more friendly to the environment; less waste, high yield, suitable for industrial production, Product quality meets international standards.
Owner:山东诚胜化工有限公司
A kind of synthetic method of hydroquinone dihydroxyethyl ether
ActiveCN105523905BThe synthesis process is reasonableHigh purityEther preparation from oxiranesHydroxyethyl starchReaction temperature
The invention relates to a method for synthesizing hydroquinone dihydroxyl diethyl ether and belongs to the technical field of synthesis of organic compounds. The method comprises the steps: carrying out synthesis by taking hydroquinone and epoxyethane as raw materials, adding hydroquinone, a ferrocene catalyst and an ether solvent into a reactor, carrying out vacuumizing, heating the reactor until hydroquinone is completely dissolved in the ether solvent under nitrogen protection, then, adding a chain extender into the reactor, heating the reactor to a polymerization reaction temperature, and carrying out hydroquinone dihydroxyl diethyl ether synthesis under polymerization reaction pressure; and after the reaction ends, carrying out cooling, and subjecting crystallizing mother liquor to normal-pressure or reduced-pressure rectification, so as to separate out the ether solvent. The hydroquinone dihydroxyl diethyl ether is synthesized by the method, the problems in the conventional technologies that the preparation process is complicated and the quality of product is poor are solved, and the obtained product is reasonable in distribution, light in color and luster and low in byproduct content.
Owner:ZHEJIANG HUANGMA TECH
Synthesis process of 4,6-dichloropyrimidine
InactiveCN111004184AReduce generationHigh purityOrganic chemistry methodsDimethylaniline N-oxidePtru catalyst
The invention provides a synthesis process of 4,6-dichloropyrimidine. The synthesis process takes thionyl chloride as a chlorinating agent and solvent for reactions, 4,6-dihydroxypyrimidine as a raw material, and N, N-dimethyl aniline as a catalyst. Compared with the prior art, thionyl chloride is used as a chlorinating agent and a solvent for reactions during the preparation process of 4,6-dichloropyrimidine, and no other organic solvent is introduced, so that the influence of other solvents on the product is reduced. According to the invention, less wastewater is generated; the finally generated wastewater is mainly composed of a salt solution of sodium sulfite and sodium chloride; excessive thionyl chloride is recycled in a rectification mode, the part of generated wastewater is only limited to the consumed thionyl chloride part, about 6-7 kg of wastewater is generated from 1 kg of 4,6-dichloropyrimidine product, and solid salt is obtained through concentration and crystallization in wastewater after treatment.
Owner:潍坊滨海石油化工有限公司
A kind of preparation method of 2,4-dichloro-5-nitrophenol
ActiveCN105646231BAvoid pollutionThorough responseOrganic chemistryOrganic compound preparationFiltrationDistillation
The invention discloses a method for preparing 2,4-dichloro-5-nitrophenol. The method is as below: first, evenly mixing tris(2,4-dichloro-5-nitrophenyl) phosphate, rare earth trifluoromethyl sulfonate catalyst, a cocatalyst and an alcoholic solvent; then gradually heating to a reflux temperature, reacting for more than 3h; and cooling to room temperature after the reaction, separating the catalyst by filtration, and removing the solvent in a liquid phase by distillation to obtain the 2,4-dichloro-5-nitrophenol. In the presence of the co-catalyst, tris (2,4-dichloro-5-nitrophenyl) phosphate reacts with the alcohol solvent under the catalysis of rare earth trifluoromethyl sulfonate, so as to fundamentally avoid the environmental pollution caused by the large quantities of waste acid in the traditional hydrolysis; the reaction is more thorough; and the method increases the yield of the product 2,4-dichloro-5-nitrophenol from 88% to 98%, increases the purity from 91.5 % to 97.5%, lowers production costs, and plays a positive role for the industrial development of oxadiazon.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
A kind of method for preparing diphenyl sulfide
ActiveCN104311463BLow costThe synthesis process is reasonableSulfide preparationChlorobenzeneNitrogen
The invention discloses a preparation method of diphenyl sulfide. The method is as below: adding a certain amount of sodium sulfide in a reaction container; keeping the negative pressure at less than or equal to -0.098 MPa, and slowly heating to 190-220 DEG C; dropwise adding a solvent with insulation, stirring, transferring the sodium sulfide solution while hot to an autoclave, then dropwise adding chlorobenzene while stirring in 1.5-2.5 h, covering the lid, and displacing the air with nitrogen two times; slowly heating to 190 to 240 DEG C while stirring, preserving the temperature and stirring for reaction; slowly cooling, filtering solid, recovering the solvent, purifying and distilling to obtain diphenyl sulfide. The novel method of the invention uses chlorobenzene and sodium sulfide as raw materials to prepare diphenyl sulfide by using. Compared with the prior art in the market, the method has the advantages of low raw material cost, safe and convenient operation, environment-friendliness, less three wastes and high yield, and is suitable for industrial production; and the product quality is in line with international standards.
Owner:兰州诚胜化工科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




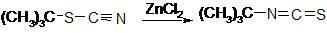
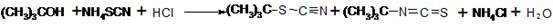
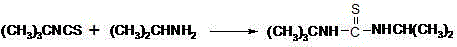
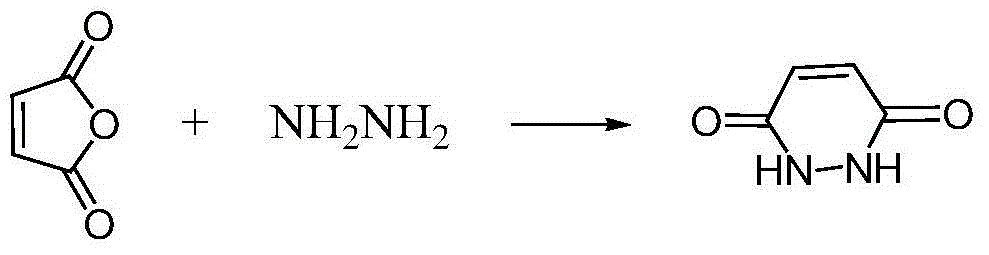
![Preparation method of 4, 5-dihydro-6H-cyclopenta[b]thiophene-6-ketone Preparation method of 4, 5-dihydro-6H-cyclopenta[b]thiophene-6-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fcbed430-d95b-4640-875f-056b8053f902/130312104625.PNG)
![Preparation method of 4, 5-dihydro-6H-cyclopenta[b]thiophene-6-ketone Preparation method of 4, 5-dihydro-6H-cyclopenta[b]thiophene-6-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fcbed430-d95b-4640-875f-056b8053f902/130312104630.PNG)
![Preparation method of 4, 5-dihydro-6H-cyclopenta[b]thiophene-6-ketone Preparation method of 4, 5-dihydro-6H-cyclopenta[b]thiophene-6-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fcbed430-d95b-4640-875f-056b8053f902/740521dest_path_image001.PNG)