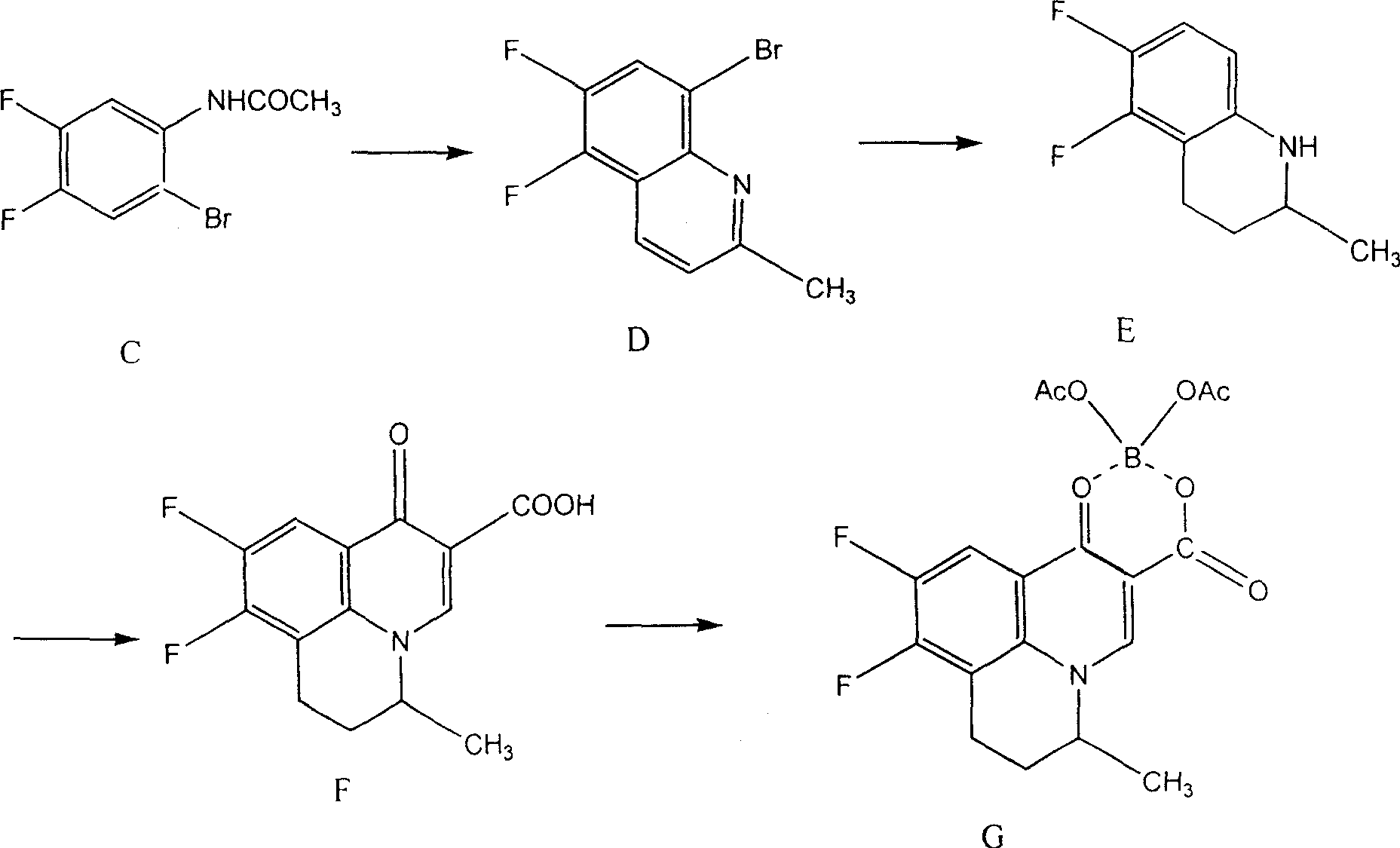
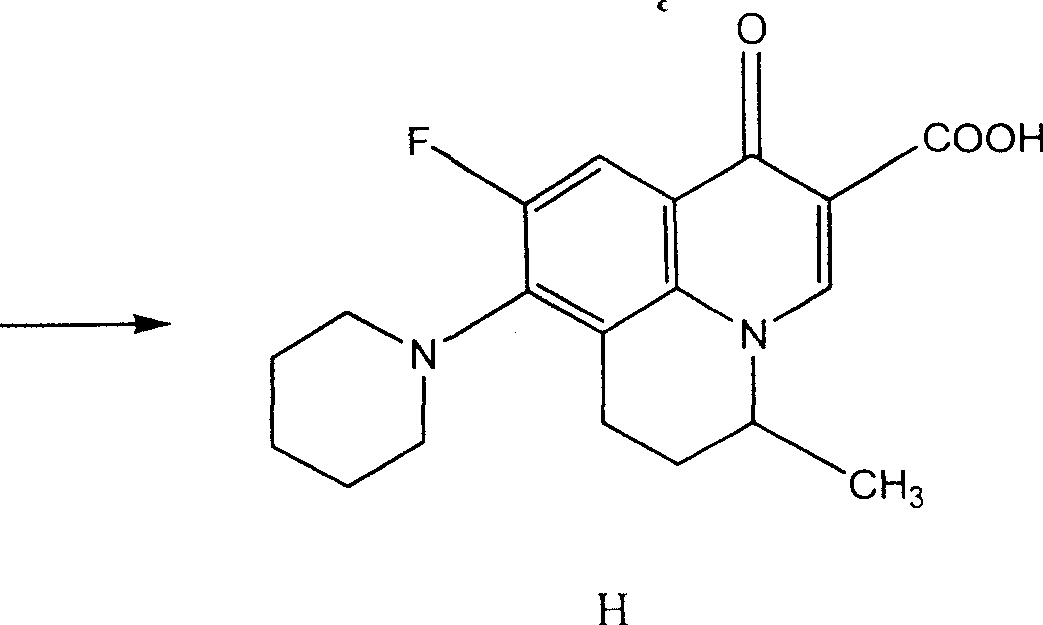
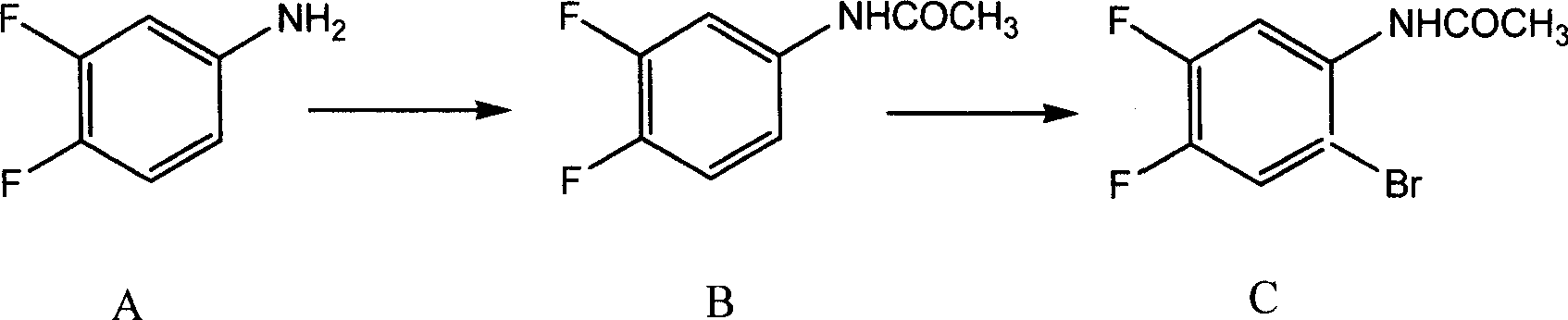
Preparing method of 8-bromo-5,6-difluoro-2-methylquinoline
A technology of methyl quinoline and difluoroacetanilide, applied to 8-bromo-5, can solve the problems of low yield in the cyclization reaction step, large consumption of alkali for neutralization, consumption of solvent, etc., and achieves low cost and low cost. And the amount of alkali, the effect of safe and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Preparation of 3,4-difluoroacetanilide (B)
[0061] In a 1000ml four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser, add 200ml of acetic acid solution containing 65g3,4-difluoroaniline (A) and 0.5g of anhydrous aluminum chloride, and cool to below 10°C. Add 90ml of acetic anhydride dropwise, gradually heat up to 60-65°C within 1 hour, react for 3 hours, cool to room temperature, add 10% dilute hydrochloric acid, stir for complete crystallization, filter with suction, wash with water, and dry to obtain 3,4- Difluoroacetanilide (B) 78.2g (mp: 124.5~106.0°C, yield 90%)
[0062] (2) Preparation of 3,4-difluoro-6-bromoacetanilide (C)
[0063] In a 1000ml four-neck flask equipped with mechanical stirring, thermometer and condenser tube, add 320ml acetic acid solution containing 77g 3,4-difluoroacetanilide (B) and 0.3g iodine, stir at room temperature, add bromine dropwise 75g, gradually heated up to 35-40°C, reacted for 12 hours, th...
Embodiment 2
[0071] (1) Preparation of 3,4-difluoroacetanilide (B)
[0072] In a 1000ml four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser tube, add 300ml acetic acid solution containing 65g 3,4-difluoroaniline and 0.8g anhydrous zinc chloride, cool to below 10°C, dropwise add Acetyl chloride 95ml, gradually heated up to 75-80°C within 1 hour, reacted for 2 hours, cooled to room temperature, added 10% dilute hydrochloric acid, stirred to crystallize completely, suction filtered, washed with water, and dried to obtain 3,4-difluoro Acetanilide (B) 78.2g (mp: 124.5~126.0°C, yield 87.1%)
[0073] (2) Preparation of 3,4-difluoro-6-bromoacetanilide (C)
[0074] In a 1000ml four-necked bottle, equipped with mechanical stirring, thermometer and condenser,
[0075] Add 77g of 3,4-difluoroacetanilide (B) and 0.6g of anhydrous magnesium chloride in 300ml of acetic acid solution, stir at room temperature, add 78g of bromine dropwise, gradually raise the tempera...
Embodiment 3
[0081] Preparation of 8-bromo-5,6-difluoro-2-methylquinoline
[0082] (The oxidizing agent is concentrated nitric acid, without catalyst)
[0083] Step (1), the preparation of 3,4-difluoroacetanilide (B) and step (2), the preparation of 3,4-difluoro-6-bromoacetanilide (C), are all the same as in Example 1 is the same, only step (3), ie the preparation of 8-bromo-5,6-difluoro-2-methylquinoline (D), is different from Example 1.
[0084] Add 95g of 3,4-difluoro-6-bromoacetanilide (C), 25ml of concentrated nitric acid, 10g of ferric sulfate, 100g of boric acid, 350ml of concentrated hydrochloric acid and 45g of trans-crotonaldehyde into a 2000ml four-necked flask with a stirring bar and a condenser , heated up to 90°C within 1 hour, reacted for 2 hours, cooled, added about 300ml of 40% sodium hydroxide, adjusted the pH to neutral, filtered, washed, and dried to obtain 8-bromo-5,6-difluoro-2-methanol Quinoline (D) 66.4g (mp; 101.1-103.2°C, yield 67.8%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com