Timing release developing inhibitor cyan colour coupler and preparation thereof
A timed release and inhibitor technology, which is applied in photography, photography technology, multi-color photography technology, etc., can solve the problems of unsuitability for industrialized large-scale production, low product yield, and difficulty in purification, and achieve a reasonable and feasible synthetic route. High yield and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
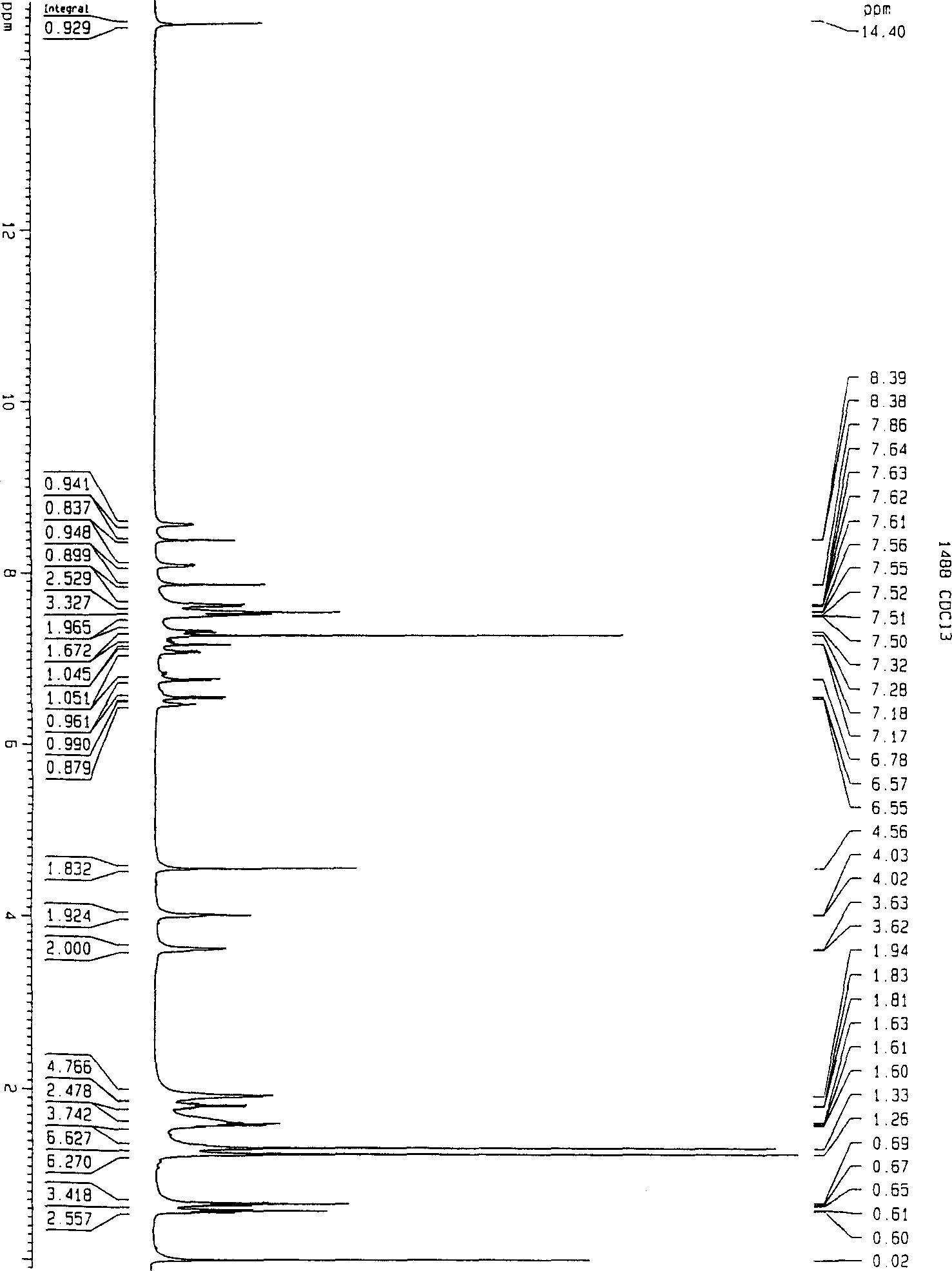
[0038] 2-(2',4'-Di-t-pentylphenyl)butanylamino-4-[2-nitro-4-methylene-(1-phenyl-5-thiotetrazolium)-benzene Synthesis of Thiolyl]-1-Naphthol
[0039] 1. Synthesis of timing precursor 2-(2', 4'-di-t-pentylphenyl) butyramide-4-(2-nitro-4-benzyl alcohol-thiol)-1-naphthol:
[0040] The reaction formula is:
[0041]
[0042] Operation method:
[0043] In a 250 ml three-necked flask equipped with a thermometer, a condenser and a stirring device, add 100 ml of ethanol and 14.7 g of 2-(2', 4'-di-t-pentylphenyl) butanylamino-4-mercapto-1- Naphthol was dissolved by stirring, 1 ml of triethylamine was added, 5.6 g of p-chloro-m-nitro-benzyl alcohol was added under stirring, and the reaction was carried out at 50° C. for three hours. Discharge in 1 liter of water, extract with 200 ml of ethyl acetate, wash once with 1N hydrochloric acid, wash twice with 100 ml of water, dry with anhydrous sodium sulfate, recover ethyl acetate by distillation under reduced pressure, and recrystallize ...
Embodiment 2
[0055] According to the synthetic method of embodiment 1, adopt corresponding intermediate, make following compound:
[0056]
Embodiment 3
[0058] According to the synthetic method of embodiment 1, adopt corresponding intermediate, make following compound:
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com