Timing release developing inhibitor cyan colour coupler and preparation thereof
A timed release and inhibitor technology, which is applied in photography, photography technology, multi-color photography technology, etc., can solve the problems of unsuitability for industrialized large-scale production, low product yield, and difficulty in purification, and achieve reasonable and feasible synthetic routes. High yield and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
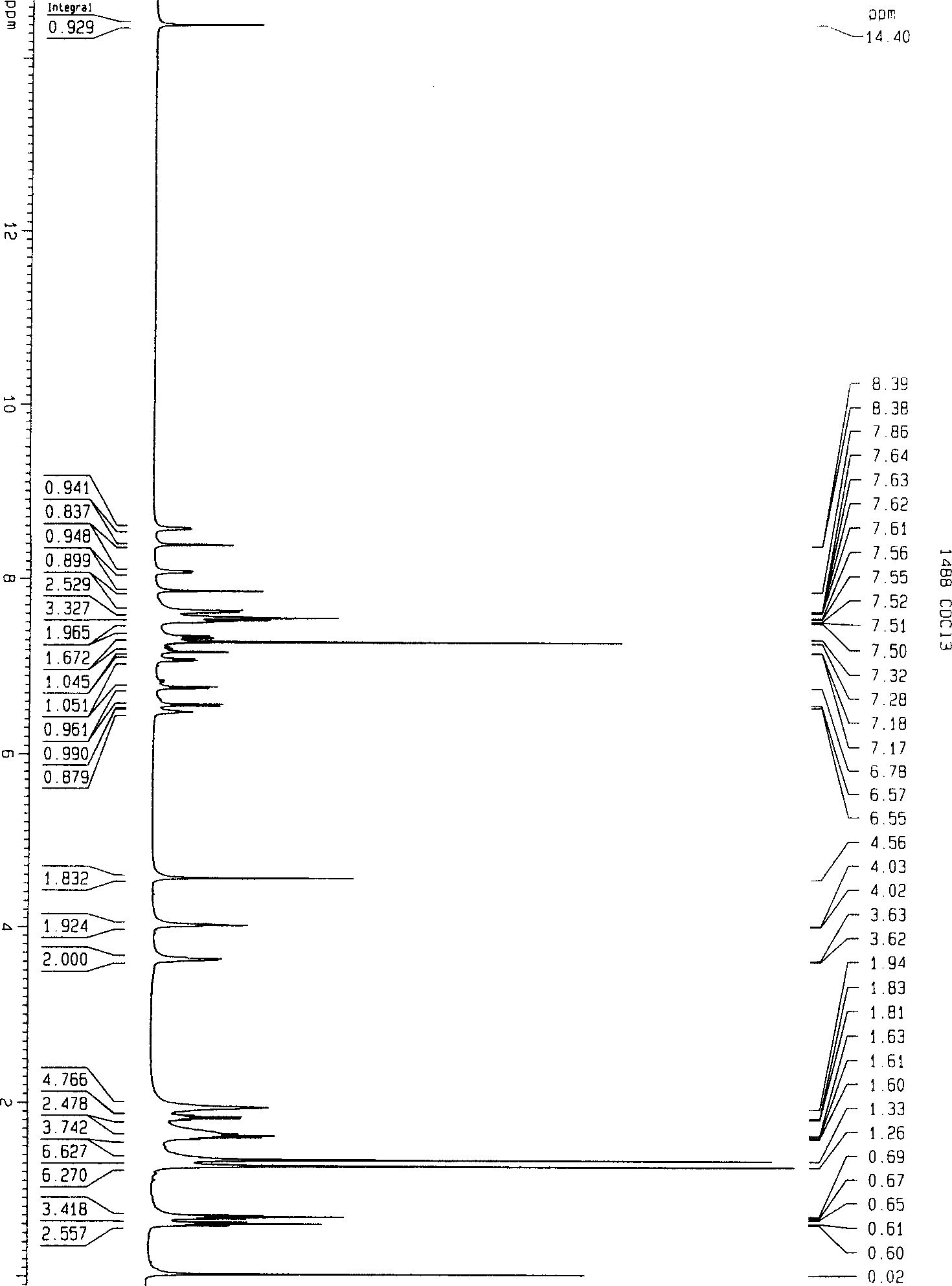
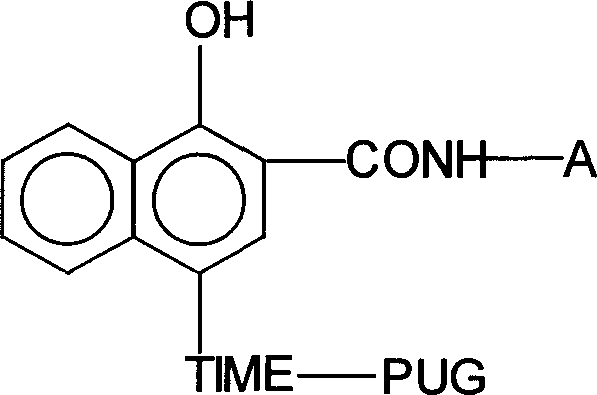
[0041] 2-(2', 4'-Di-tertpentylphenyl)butyramido-4-[2-nitro-4-methylene-(1-phenyl-5-thiotetrazolium)-benzene Synthesis of thiophenol]-1-naphthol
[0042] 1. The synthesis of timing matrix 2-(2', 4'-di-tert-amylphenyl)butyramido-4-(2-nitro-4-benzyl alcohol-thiophenol)-1-naphthol:
[0043] The reaction formula is:
[0044]
[0045] Operation method:
[0046] In a 250 ml three-necked flask equipped with a thermometer, condenser and stirring device, add 100 ml of ethanol and 14.7 g of 2-(2', 4'-di-tertpentylphenyl) butyramido-4-mercapto-1- Naphthol, stir to dissolve, add 1 ml of triethylamine, add 5.6 g of p-chloro-m-nitro-benzyl alcohol under stirring, and react at 50°C for three hours. The material is discharged in 1 liter of water, extracted with 200 ml of ethyl acetate, washed once with 1N hydrochloric acid, and then washed with 100 ml of water twice, dried with anhydrous sodium sulfate, distilled under reduced pressure to recover ethyl acetate, and recrystallized with n-hexane t...
Embodiment 2
[0058] According to the synthesis method of Example 1, using the corresponding intermediates, the following compounds were prepared:
[0059]
Embodiment 3
[0061] According to the synthesis method of Example 1, using the corresponding intermediates, the following compounds were prepared:
[0062]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com