Preparation method for argatroban intermediate
A technology for argatroban and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of unfavorable large-scale production, hydrogen flammability and explosion, unfavorable safety production, etc., and achieves low price, easy control of reaction conditions, and easy operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The biocatalyst is a genetically engineered bacterium derived from the transaminase of Vibrio fluvialis. The specific preparation method is: select the gene sequence of the transaminase derived from Vibrio fluvialis and carry out artificial design. The designed gene sequence is shown in SEQ ID NO: 1 in the sequence table shown in the nucleotide sequence; the sequence is synthesized through the whole gene, cloned into the Nde I and Xho I restriction sites of the expression vector pET28a, and transformed into the competent cell of the host bacteria E.coli BL21 (DE3); pick the positive transformation After sequencing and identification, the recombinant expression vector was obtained; the recombinant expression vector was transferred into the E. coli BL21 (DE3) strain, and the recombinant transaminase genetically engineered bacteria capable of inducing the expression of the recombinant transaminase was obtained.
[0032] Inoculate recombinant transaminase genetically enginee...
Embodiment 2
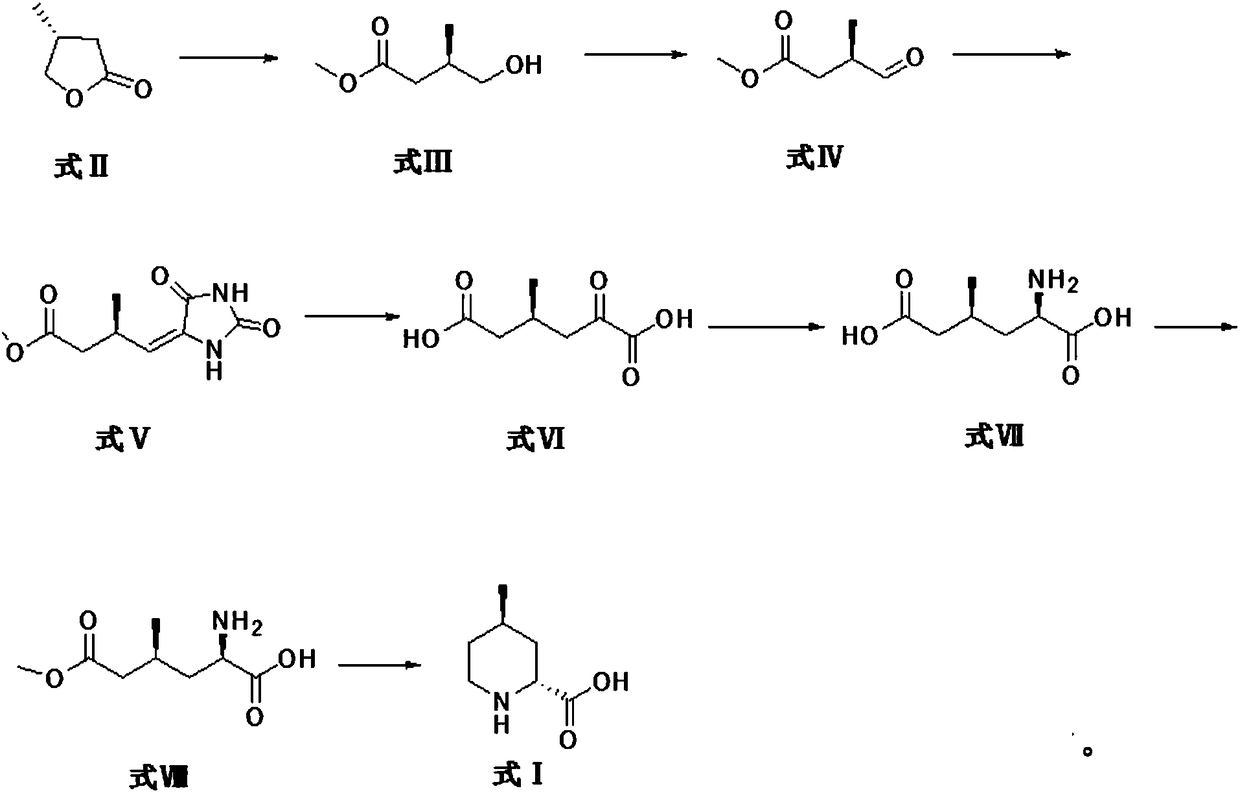
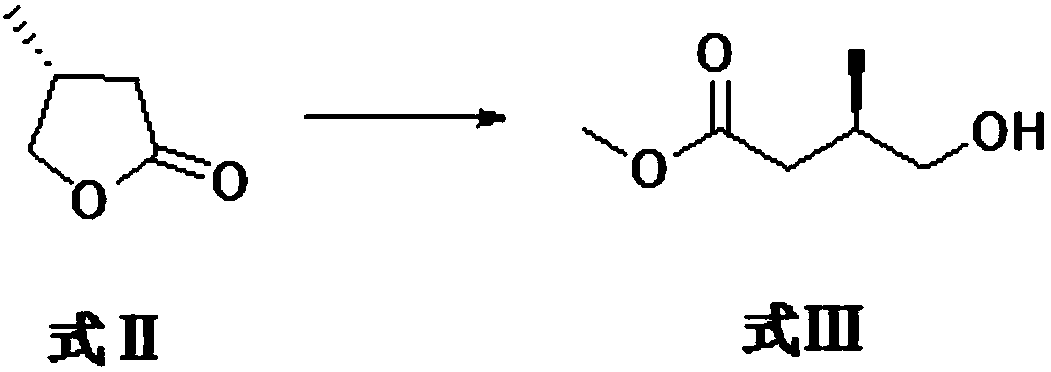
[0034] Formula II compound (R)-4methyldihydrofuran-2(3H)-one ring-opens and then reacts with sodium methoxide to generate formula III compound R-3-methyl-4-hydroxybutyric acid methyl ester.
[0035] The specific reaction process is as follows: the formula II compound (R)-4 methyl dihydrofuran-2 (3H)-one (30g, 0.3mol) was dissolved in methanol (300ml), and sodium methoxide (19.44g, 0.36 mol), and then heated to 80 ° C for 5 h. Cool down after the reaction, concentrate the methanol, adjust the pH to about 7 with ammonium chloride solution, and extract with ether. After drying and concentrating, the compound of formula III R-3-methyl-4-hydroxybutyric acid methyl ester (36.8 g, 0.279 mol) was obtained.
[0036] The reaction formula is as follows:
[0037]
Embodiment 3
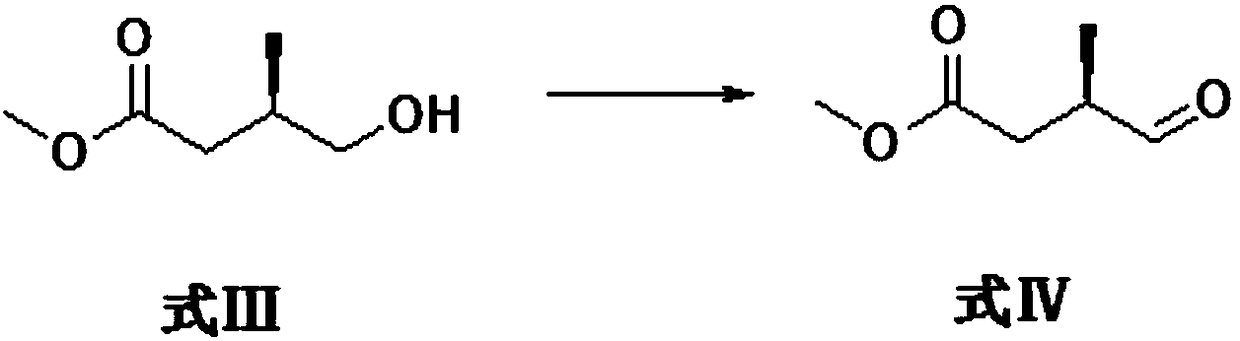
[0039] Formula III compound R-3-methyl-4-hydroxybutyric acid methyl ester is oxidized to generate formula IV compound (3R)-3-methyl-4-formyl butyric acid methyl ester.
[0040]The specific reaction process is as follows, under the protection of nitrogen, dissolve oxalyl chloride (10ml, 0.11mol) in dichloromethane (250ml), cool to -60°C, add dropwise dimethyl sulfoxide solution [dimethyl sulfoxide (17ml , 0.22mol) was dissolved in 50ml dichloromethane], dropwise reacted at -60°C for half an hour, and then added dropwise R-3-methyl-4-hydroxybutyrate methyl ester solution [the formula III compound R-3 -Methyl-4-hydroxybutyrate (13.2 g, 0.1 mol) was dissolved in 100 ml of dichloromethane], and stirred for another 1 hour after dropping. Then triethylamine (70ml, 0.5mol) was added dropwise at -60°C, reacted at -60°C for half an hour after the dropwise completion, and then warmed up to room temperature for reaction. After monitoring that the reaction is complete, add water, then ext...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com