Synthesis method of 4-ethoxy-1, 1, 1-trifluoro-3-butene-2-ketone
A synthesis method, ethoxy technology, applied in the direction of condensation preparation of carbonyl compounds, organic chemistry, etc., can solve the problems of difficult recovery of by-product trifluoroacetyl, low boiling point of trifluoroacetyl chloride, high production cost, etc., to achieve convenient recovery and simple preparation , the effect of easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
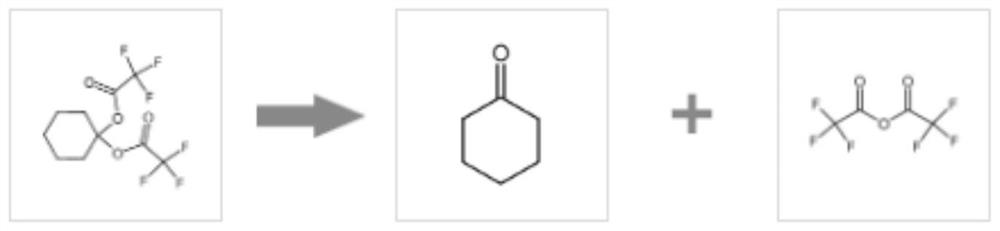
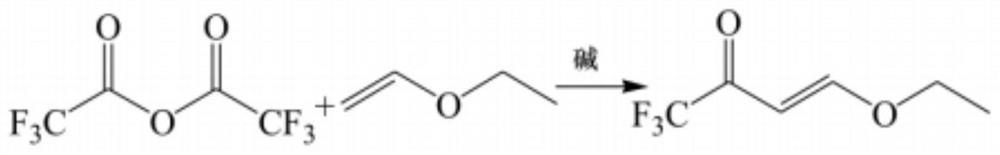
[0022] refer to Figure 1-3 , a synthetic method of 4-ethoxy-1,1,1-trifluoro-3-buten-2-one, comprising the following steps:
[0023] 1) In a reaction flask equipped with a fractionation column (connected to a condenser, and the receiving bottle is cooled with an ice-salt bath), add absolute ethanol, mercuric acetate, n-butyl vinyl ether, heat to reflux, and control the temperature at the top of the fractionation column At 36~37 ℃, distill out ethyl vinyl ether;
[0024] 2) get 0.6 moles of trifluoroacetic acid and 0.7 moles of dichloroacetic anhydride, react after adding the dehydration reactant to obtain trifluoroacetic anhydride, and the reaction equation is CF 3 COOH+(C l2 CHCO) 2O →(CF 3 CO) 2O +C l2 CHCOOH;
[0025] 3) 2-Propyn-1-ol and methyl chloroformate react under the action of dehydrating agent and catalyst at 20-40 ° C for 2 hours to obtain 4-ethoxy-1,1,1-trifluoro-3 -Buten-2-one.
[0026] The dehydration reactant in step 2) is one or more of toluene, xyle...
Embodiment 2
[0028] refer to Figure 1-3 , a synthetic method of 4-ethoxy-1,1,1-trifluoro-3-buten-2-one, comprising the following steps:
[0029] 1) In a reaction flask equipped with a fractionation column (connected to a condenser, and the receiving bottle is cooled with an ice-salt bath), add absolute ethanol, mercuric acetate, n-butyl vinyl ether, heat to reflux, and control the temperature at the top of the fractionation column At 36~37 ℃, distill out ethyl vinyl ether;
[0030] 2) get 0.4 moles of trifluoroacetic acid and 0.75 moles of dichloroacetic anhydride, add the dehydration reactant and react to obtain trifluoroacetic anhydride, and the reaction equation is CF 3 COOH+(C l2 CHCO) 2O →(CF 3 CO) 2O +C l2 CHCOOH;
[0031] 3) 2-Propyn-1-ol and methyl chloroformate react under the action of dehydrating agent and catalyst at 20-40 ° C for 2 hours to obtain 4-ethoxy-1,1,1-trifluoro-3 -Buten-2-one.
[0032] The dehydration reactant in step 2) is one or more of toluene, xylene, ...
Embodiment 3
[0034] refer to Figure 1-3 , a synthetic method of 4-ethoxy-1,1,1-trifluoro-3-buten-2-one, comprising the following steps:
[0035] 1) In a reaction flask equipped with a fractionation column (connected to a condenser, and the receiving bottle is cooled with an ice-salt bath), add absolute ethanol, mercuric acetate, n-butyl vinyl ether, heat to reflux, and control the temperature at the top of the fractionation column At 36~37 ℃, distill out ethyl vinyl ether;
[0036] 2) get 0.7 moles of trifluoroacetic acid and 0.9 moles of dichloroacetic anhydride, add the dehydration reactant and react to obtain trifluoroacetic anhydride, and the reaction equation is CF 3 COOH+(C l2 CHCO) 2O →(CF 3 CO) 2O +C l2 CHCOOH;
[0037] 3) 2-Propyn-1-ol and methyl chloroformate react under the action of dehydrating agent and catalyst at 20-40 ° C for 3 hours to obtain 4-ethoxy-1,1,1-trifluoro-3 -Buten-2-one.
[0038] The dehydration reactant in step 2) is one or more of toluene, xylene, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com