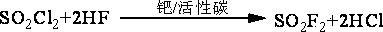Method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride
A technology of trifluoroacetyl chloride and sulfuryl fluoride, which is applied in the preparation of acyl halides, sulfur-halogen-hydrogen-oxygen compounds, organic chemistry, etc., can solve the problem of high production cost, high reaction temperature, and no by-product tetrafluoride involved. Silicon separation problems and other problems, to achieve the effect of less three wastes and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
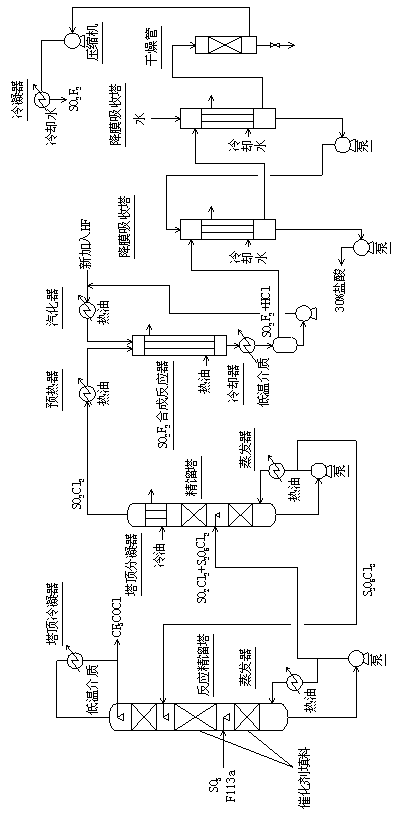
[0056] 1) Pass 600 mol / h of sulfur trioxide and 600 mol / h of trifluorotrichloroethane (F113a) continuously into the reactive distillation column (50 mm in diameter and 2000 m in total height) at a distance of 500 mm from the bottom of the tower mm, 1500 mm in the middle and bottom of the tower is filled with fluorosulfonic acid resin fillers that have been exchanged with mercury salts and mercurous salts, and the upper 500 mm is filled with glass spring packing.) To react, control the temperature of the tower kettle to 120 ° C, The reflux ratio is 2.5, and the amount of taking out trifluoroacetyl chloride from the top of the tower is 594 mol / hour;
[0057] 2) Send the sulfuryl chloride and pyrothionyl chloride in the reactor of the reactive distillation tower to the sulfuryl chloride separation tower (50 mm in diameter, 600 mm in total height, with glass spring packing inside, the upper part Packing is 400 millimeters.) Carry out rectifying separation, control tower still temp...
Embodiment 2
[0060] 1) Pass 600 mol / h of sulfur trioxide and 600 mol / h of trifluorotrichloroethane (F113a) continuously into the reactive distillation column (50 mm in diameter and 2000 m in total height) at a distance of 500 mm from the bottom of the tower mm, 1500 mm in the middle and bottom of the tower is filled with fluorosulfonic acid resin fillers that have been exchanged with mercury salts and mercurous salts, and the upper 500 mm is filled with glass spring packing.) To react, control the temperature of the tower kettle to 130 ° C, The reflux ratio is 3.0, and the amount of taking out trifluoroacetyl chloride from the top of the tower is 597 mol / hour;
[0061] 2) Send the sulfuryl chloride and pyrothionyl chloride in the reactor of the reactive distillation tower to the sulfuryl chloride separation tower (50 mm in diameter, 600 mm in total height, with glass spring packing inside, the upper part Packing is 400 millimeters.) Carry out rectifying separation, control tower still temp...
Embodiment 3
[0064] 1) Pass 600 mol / h of sulfur trioxide and 600 mol / h of trifluorotrichloroethane (F113a) continuously into the reactive distillation column (50 mm in diameter and 2000 m in total height) at a distance of 500 mm from the bottom of the tower mm, 1500 mm in the middle and bottom of the tower is filled with fluorosulfonic acid resin fillers that have been exchanged with mercury salts and mercurous salts, and the upper 500 mm is filled with glass spring packing.) To react, control the temperature of the tower kettle to 127 ° C, The reflux ratio is 2.8, and the amount of taking out trifluoroacetyl chloride from the top of the tower is 596 mol / hour;
[0065] 2) Send the sulfuryl chloride and pyrothionyl chloride in the reactor of the reactive distillation tower to the sulfuryl chloride separation tower (50 mm in diameter, 600 mm in total height, with glass spring packing inside, the upper part Packing is 400 millimeters.) Carry out rectifying separation, control tower still temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com