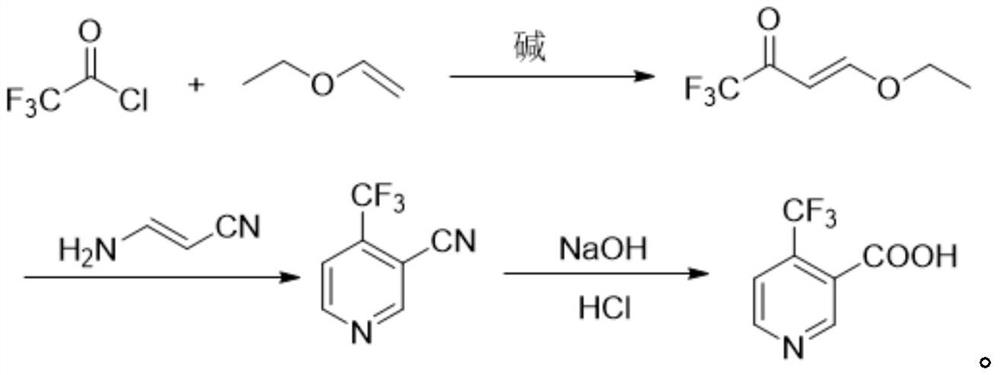
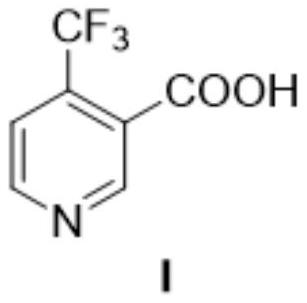
The preparation method of 4-trifluoromethyl nicotinic acid
A technology of trifluoromethyl nicotinic acid and trifluoroacetyl chloride, which is applied in the field of chemical drug intermediate preparation, can solve the problems of difficulty in industrialized production, long process routes, increased steps, etc., and achieves high product yield and easy operation of the method. , the effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 4-ethoxy-1,1,1-trifluoro-3-en-2-one.
[0021] In a 1000mL airtight four-necked reaction flask, add 72.1g of vinyl ether, 87.0g of pyridine and 500mL of toluene, stir evenly, control the temperature at 10-15°C, slowly add 145.8g of trifluoroacetyl chloride dropwise, and the dropping time lasts for 1 hour , after the dropwise addition was completed, the stirring was continued for 3 hours, the reaction was completed, and 300 mL of ice water was added to the reaction system to quench, the organic phase was washed twice with saline, and the solvent toluene was distilled off under reduced pressure to obtain a light yellow liquid 4-ethoxy-1 , 1,1-trifluoro-3-en-2-one 115.2 g, yield 68.5%.
Embodiment 2
[0022] Example 2: Preparation of 4-ethoxy-1,1,1-trifluoro-3-en-2-one.
[0023] In a 1000mL airtight four-necked reaction flask, add 72.1g of vinyl ether, 87.0g of pyridine and 500mL of toluene, stir evenly, control the temperature at 0-5°C, slowly add 145.8g of trifluoroacetyl chloride dropwise, and the dropping time lasts for 1 hour , after the dropwise addition was completed, the stirring was continued for 3 hours, the reaction was completed, and 300 mL of ice water was added to the reaction system to quench, the organic phase was washed twice with saline, and the solvent toluene was distilled off under reduced pressure to obtain a light yellow liquid 4-ethoxy-1 , 1,1-trifluoro-3-en-2-one 145.7 g, yield 86.7%.
Embodiment 3
[0024] Example 3: Preparation of 4-ethoxy-1,1,1-trifluoro-3-en-2-one.
[0025] In a 1000mL airtight four-necked reaction flask, add 72.1g of vinyl ether, 87.0g of pyridine and 500mL of toluene, stir evenly, control the temperature at 0-5°C, and slowly add 159.0g of trifluoroacetyl chloride dropwise for 1 hour , after the dropwise addition was completed, the stirring was continued for 3 hours, the reaction was completed, and 300 mL of ice water was added to the reaction system to quench, the organic phase was washed twice with saline, and the solvent toluene was distilled off under reduced pressure to obtain a light yellow liquid 4-ethoxy-1 , 1,1-trifluoro-3-en-2-one 155.2 g, yield 92.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com