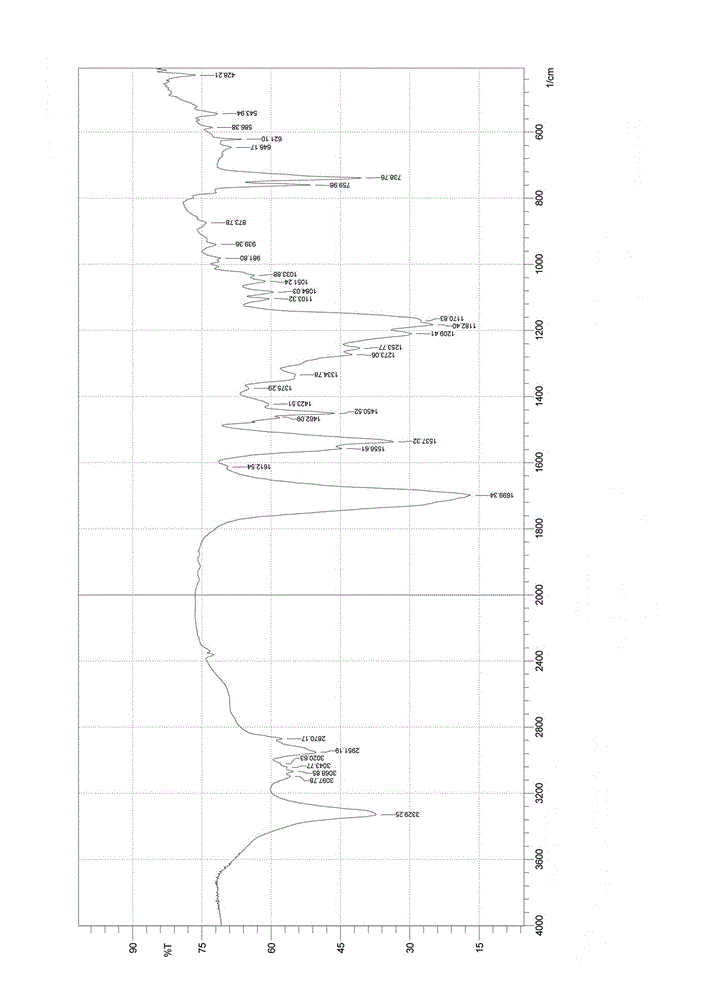
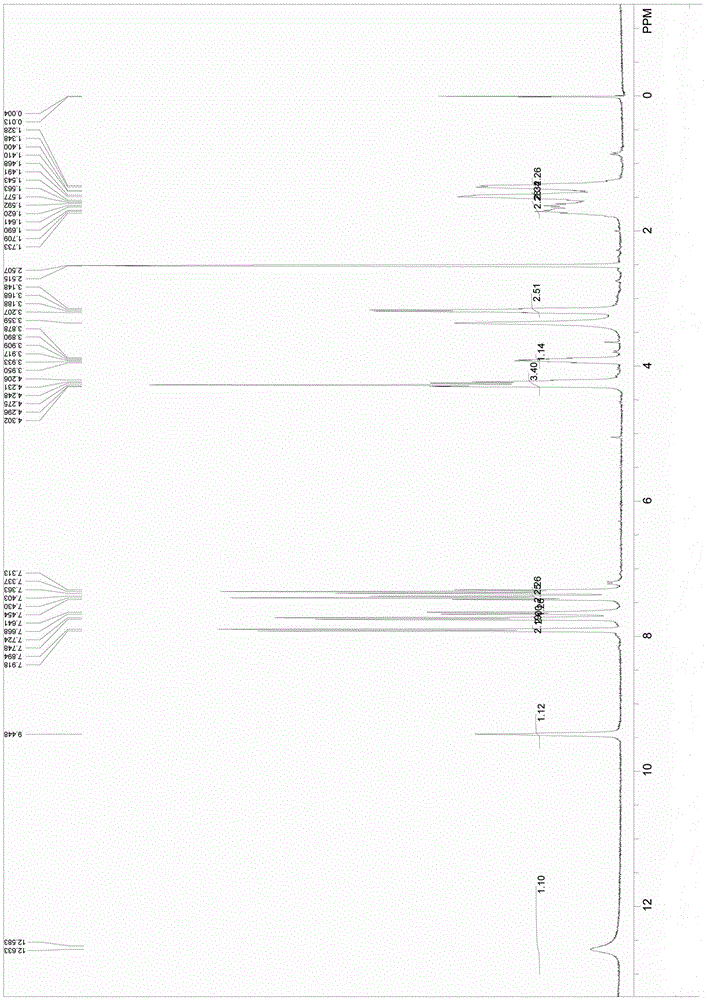
Nalpha-fluorenylmethyloxycarbonyl-Nepsilon-trifluoroacetyl-lysine preparation method
The technology of fluorene methoxycarbonyl and trifluoroacetyl is applied in the field of preparation of lysine, can solve problems such as difficulty in production and operation, and achieve the effects of stable and reliable product quality, avoiding difficult washing and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Dissolve 1 equivalent of Nα-fluorenylmethoxycarbonyl-Nε-tert-butoxycarbonyl-L-lysine in dichloromethane, add 1 part of a dichloromethane solution with a mass percentage of 15% trifluoroacetic acid, Stir vigorously, cool with tap water externally, detect the reaction is complete by thin-layer chromatography, distill off the solvent and trifluoroacetic acid under reduced pressure, wash with petroleum ether, pour off the petroleum ether and dissolve in tetrahydrofuran, add N-methylmorpholine until the pH is weakly alkaline ( 7﹤pH≤8, the same as below) and stir; take another bottle, add trifluoroacetic acid and dimethylformamide to it, stir in the peripheral ice bath, add thionyl chloride dropwise, generate gas and pass into Nα-fluorenylmethoxycarbonyl - In L-lysine tetrahydrofuran solution, after the dropwise addition, nitrogen gas is blown into the gas generating container, so that the generated gas further enters the following reaction, the progress of the re...
Embodiment 2
[0020] Example 2: Dissolve 1 equivalent of Nα-fluorenylmethoxycarbonyl-Nε-tert-butoxycarbonyl-L-lysine in ethanol or ether, pass in hydrogen chloride gas, stir vigorously, cool the outside with tap water, and check that the reaction is complete by thin-layer chromatography. Filter off the solvent, wash with ether, dissolve in tetrahydrofuran, add N,N-diisopropylethylamine until the pH is weakly alkaline and stir; take another bottle, add trifluoroacetic acid and dimethylformamide to it, stir, and ice Bath, add thionyl chloride dropwise, the generated gas is passed into the Nα-fluorenylmethoxycarbonyl-L-lysine tetrahydrofuran solution, after the dropwise addition, nitrogen is blown into the gas generating container, so that the generated gas further enters the following reaction , thin-layer chromatography to detect the reaction progress, pH test paper to detect the acidity of the reaction system, and maintain the pH value at 10. After the reaction, concentrate the reaction so...
Embodiment 3
[0021] Example 3: 1 equivalent of Nα-fluorenylmethoxycarbonyl-Nε-tert-butoxycarbonyl-D-lysine was dissolved in dichloromethane, and 3 parts of trifluoroacetic acid in dichloromethane with a mass percent content of 35% were added, Vigorously stir, cool with tap water externally, complete reaction by TLC, distill off solvent and trifluoroacetic acid under reduced pressure, wash with petroleum ether, pour off petroleum ether and dissolve in tetrahydrofuran, add N-methylmorpholine until the pH is weakly alkaline and stir ; Take another bottle, add trifluoroacetic acid and dimethylformamide to it, stir in the peripheral ice bath, add thionyl chloride dropwise, generate gas and pass it into the Nα-fluorenylmethoxycarbonyl-D-lysine tetrahydrofuran solution, After the dropwise addition is completed, nitrogen is blown into the gas generating container so that the generated gas further enters the following reaction, the reaction progress is detected by thin layer chromatography, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com