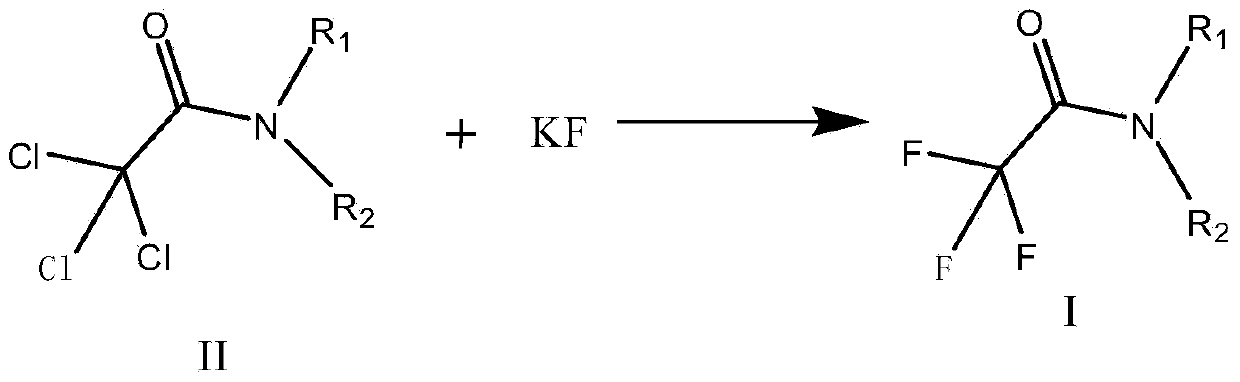Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
94 results about "Trichloroacetyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trichloroacetyl chloride is the acyl chloride of trichloroacetic acid. It can be formed by reacting chlorine with acetyl chloride or acetaldehyde in the presence of activated charcoal. It is used in the manufacture of pharmaceuticals and plant protection compounds.
Method for preparing trichlorideacetyl chloride from mother liquor of chloroactic acid
InactiveCN1562941ARaw materials are easy to getReduce manufacturing costOrganic compound preparationCarboxylic compound preparationBoiling pointChloride
Mother liquor is refined by distilation at temp. of 140 deg.C to remove low boiling point matters, then under the function of sulfur monochloride, pumping in chlorine gas to proceed acyl-chlorization to abtain acetic chloride mixture. After rectifications, the mixture of monoacetic chloride and diacetic chloride is obtained. The mixture is then chloridized by pumping-in chlorine gas using catalyst of pyridine and assistant catalyst active carbon. After rectification the final product trichloro-acetic chloride is obtd. The advantages are: raw material is available easily, simple process, safety, high pureness and high yield.
Owner:大庆市富杰化工有限公司
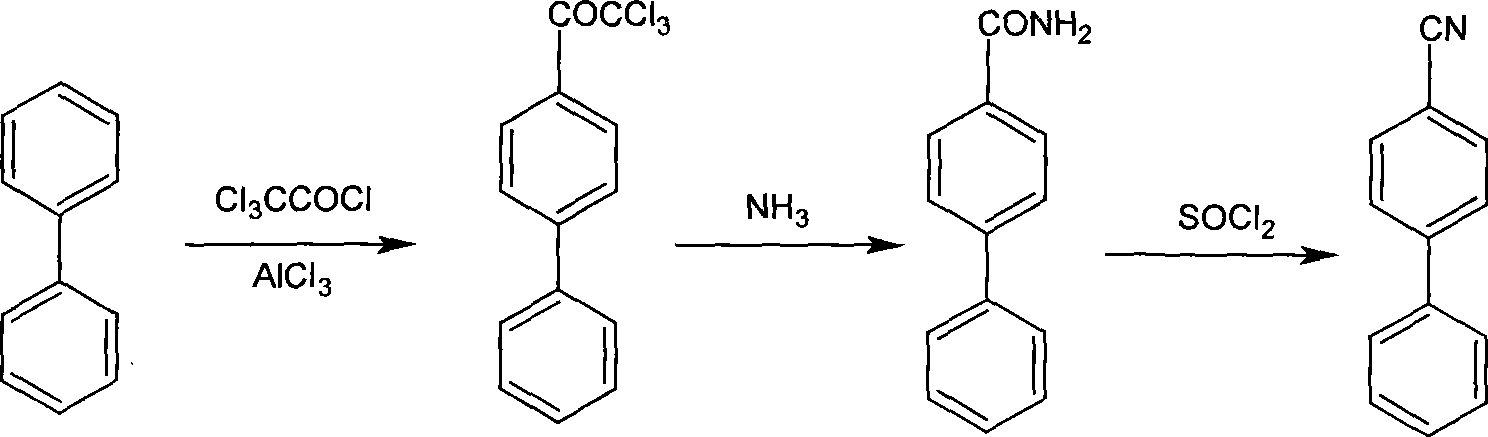
Process for producing p-phenyl cyanophenyl
InactiveCN101429137AHigh purityHigh yieldPreparation by carboxylic acid amide dehydrationAminolysisChloride
The invention provides a method for preparing terephthalic benzonitrile. The method comprises the steps of taking biphenyl and trichloro-acetic chloride as raw materials and preparing theterephthalic benzonitrile through acylation, aminolysis and dehydration reaction. The method overcomes the disadvantages that the prior art is expensive in raw materials, high in intermediate toxicity, harsh in reaction conditions, low in reaction yield, poor in purity and the like, provides a terephthalic benzonitrile synthesizing process which is easy to industrialize, convenient to operate, high in yield and high in product purity, and provides a key raw material intermediate for producing the pigment red 264 with bright color and high quality.
Owner:CINIC CHEM SHANGHAI
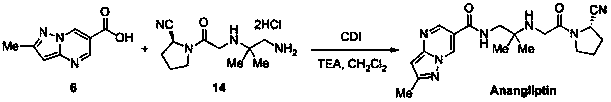
Synthesis method of anagliptin
InactiveCN105503878AHigh reaction yieldEasy post-processingOrganic chemistryBulk chemical productionDiabrezideNucleophilic substitution
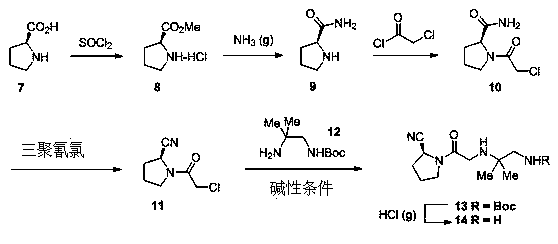
The invention relates to a synthesis method of bulk drug of anagliptin for treating II type diabetes, and aims to solve the problem that at present there is no industrial synthesis method of anagliptin. The synthesis method comprises the following steps: (1) taking vinyl ethyl ether and trichloroacetic chloride as the raw materials, carrying out three-step reactions to obtain an intermediate (4) with a protected aldehyde group; carrying out dehydration condensation between the intermediate (4) and 3-amino-5-methylpyrazole to obtain pyrazolopyrimidine parent nucleus; and hydrolyzing carboxyl ethyl ester to obtain 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carboxylic acid6; (2) taking L-proline as the raw material, subjecting L-proline to methyl esterification, ammoniation, acetylation, and cyaniding reactions to obtain a chiral cyanopyrrole intermediate (11); making the chiral cyanopyrrole intermediate (11) and a diamine segment (12) carry out nucleophilic substitution reactions under an alkaline condition to obtain an intermediate (13), and finally removing the Boc protective group from the intermediate (13) in the presence of hydrochloric acid to obtain a cyanopyrrole amine intermediate (14); (3) coupling 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carboxylic acid 6 with the cyanopyrrole amine intermediate (14) under condensation conditions so as to obtain the bulk drug anagliptin.
Owner:南通佰康生物医药有限公司
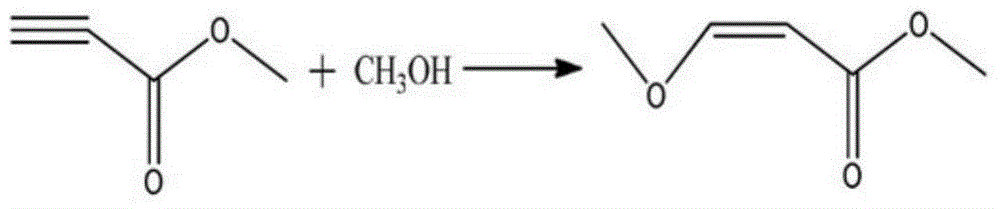
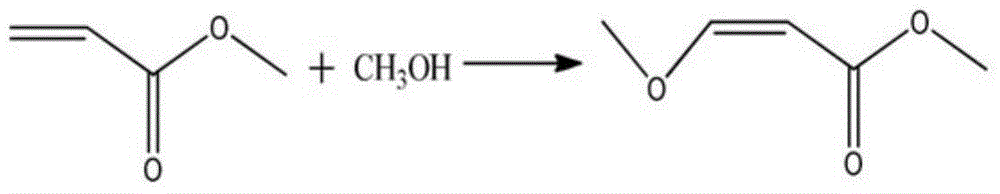
Synthesizing method for 3-methoxyacrylate
InactiveCN105418421AFew reaction stepsEasy to operateOrganic compound preparationCarboxylic acid esters preparationOrganic solventSolvent
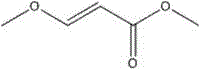
The present invention relates to a synthesizing method for 3-methoxy-3-ethoxy methyl propionate, and belongs to the field of chemistry. The synthesizing step comprises the following steps: introducing methyl vinyl ether into trichloroacetyl chloride, and insulating until a reaction is completed; combining BJ01 with anhydrous methanol, adding an acid-binding agent I, and insulating until a reaction is completed; and mixing BJ02 with an organic solvent 2, adding a catalyst, and insulating until a reaction is completed to obtain the 3-methoxyacrylate. The synthesizing method for the 3-methoxyacrylate has the advantages of few reaction steps, easiness in operation, less waste gas, waste water and waste residues, high yield, high purity of products, and inexpensive and easily available raw materials. All the steps have no harsh conditions and are simple in operation and environmentally-friendly, and the solvent are easily recycled and reused, so that the synthesizing method is suitable for industrial production.
Owner:吴清来
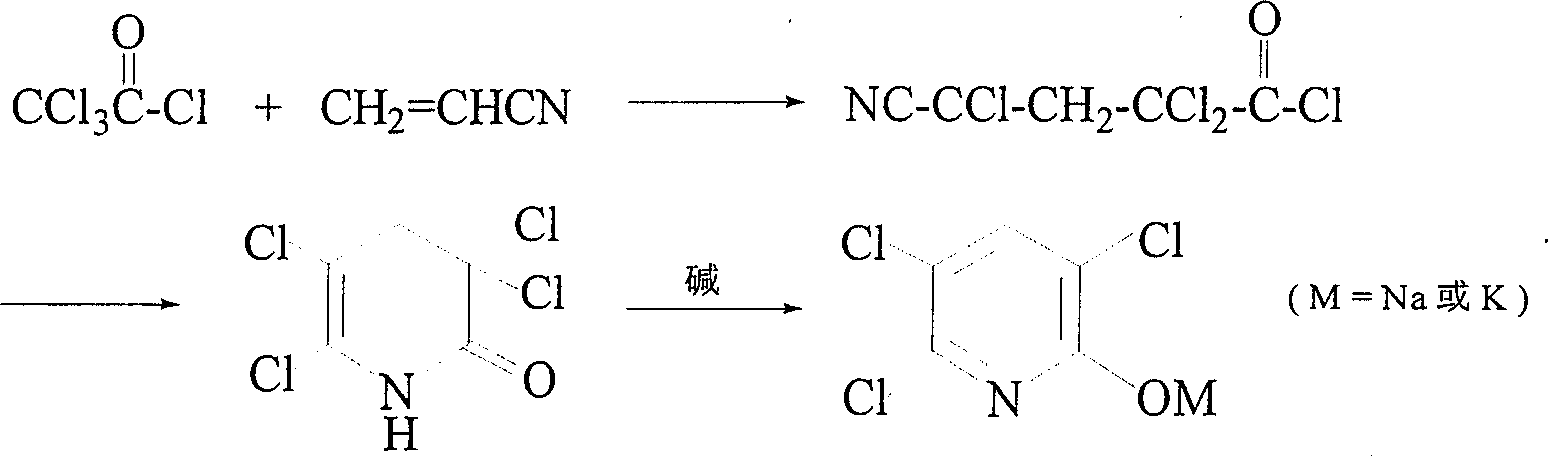
Production method of 3,5,6-trichloro pyridine-2-phenolate
InactiveCN1513840ARaw materials are easy to getHigh yieldOrganic chemistryOrganic solventAcrylonitrile
A process for preparing 3,5,6-trichloropyridine-2-phenoxide from trichloroacetyl chloride and acrylonitrile includes addition and cyclizing reactions under existance of solvent and catalyst, and aromatizing and neutralizing in alkaline condition. Its advantages are simple process and high output rate.
Owner:ZHEJIANG UNIV OF TECH
Novel process for producing trichloro-acetic chloride
InactiveCN103086870AReduce pollutionSimple processOrganic compound preparationCarboxylic compound preparationChloroacetic acidsEthyl Chloride
The invention relates to a method for preparing trichloro-acetic chloride by using chloroacetic acid waste liquid as a raw material, and belongs to the field of organic chemicals and preparation technology. The method comprises the following steps of (1) pumping chloroacetic acid waste liquid refined by a rectifying tower into an acylation kettle, adding a proper amount of sulphur, and passing a chlorine gas at a certain temperature for reaction, and thus mixed acid chloride is obtained after the reaction, wherein to make the chlorine gas reacted adequately, cross chloride is adopted by using a main kettle and an assistant kettle; and (2) adding the mixed acid chloride into a chlorination reaction vessel, reacting by using pyridine as a catalyst and 4-dimethylamino pyridine as a cocatalyst and passing the chlorine gas for reaction at a certain temperature, and thus trichloro-acetic chloride is formed. The novel process has wide raw material source and low production cost and is suitable for industrialized production.
Owner:SHANXI SANWEI FENGHAI CHEM
Method for treating 4 chloro pyridine in solvent
ActiveCN1978429AEliminate separation and purificationThe process is simple and reliableOrganic chemistryPolyethylene glycolPotassium hydroxide
This invention relates to a treatment method of tetrachloro pyridine in solvent. The stated solvent is chlorpyrifos intermediate for producing trichloro pyridine sodium alcoholate salt or kalium salt. Its characteristic is including that under effect of the phase-transfer catalyst, solvent containing by-product tetrachloro pyridine is transformed into trichloro pyridine sodium alcoholate salt or kalium salt by using water solution of natrium hydroxydatum or caustic potash. The mentioned phase-transfer catalyst utilizes carbowax of low molecular. In this invention, there is no need to separate and purify the by-product tetrachloro pyridine existing in solvent in the technology of using trichloroacetyl chloride route to synthesize trichloro pyridine sodium alcoholate salt or kalium salt as intermediate, but directly using lye to process to transform it into trichloro pyridine sodium alcoholate salt or kalium salt. The method reduces the procedure of separation and purification in present technique. Technology is simple and reliable, yield is near theoretical value.
Owner:SHANDONG TIANCHENG BIOTECH
Aqueous-phase synthesis method of chlorpyrifos by using trichloro-acetic chloride as initial material
InactiveCN102993237ASimple methodEasy to operateGroup 5/15 element organic compoundsFiltrationAcrylonitrile
The invention discloses an aqueous-phase synthesis method of chlorpyrifos by using trichloro-acetic chloride as an initial material. The method comprises steps of: reacting trichloro-acetic chloride, acrylonitrile, orthodichlorobenzene and a compound catalyst to obtain a 3,5,6-Trichloropyridin-2-ol sodium crude product; adding the 3,5,6-Trichloropyridin-2-ol sodium crude product into a reaction kettle with water, a catalyst and alkali, heating for reflux for 3 h, standing to separate out a solvent, and cooling, crystallizing and filtering an aqueous-phase to obtain the 3,5,6-Trichloropyridin-2-ol sodium; and adding water into the reaction kettle, adding a dispersing agent, 3,5,6-Trichloropyridin-2-ol sodium, ethyl chloride and a catalyst with stirring, reacting, cooling, crystallizing, washing and conducting suction filtration to obtain a chlorpyrifos active compound product. The invention employs trichloro-acetic chloride as the initial material to produce high-purity 3,5,6-Trichloropyridin-2-ol sodium, which is used directly as a raw material for the synthesis of chlorpyrifos. The sodium alkoxide produced by the method does not require refining, purifying or drying; water is used as the medium; and reaction materials are added at once for synthesis of chlorpyrifos. The method has advantages of simpleness, easy operation, environmental protection, energy saving, increased product yield, and improved product purity.
Owner:JIANGSU QIHENG AGRI & CHEM TECHCO
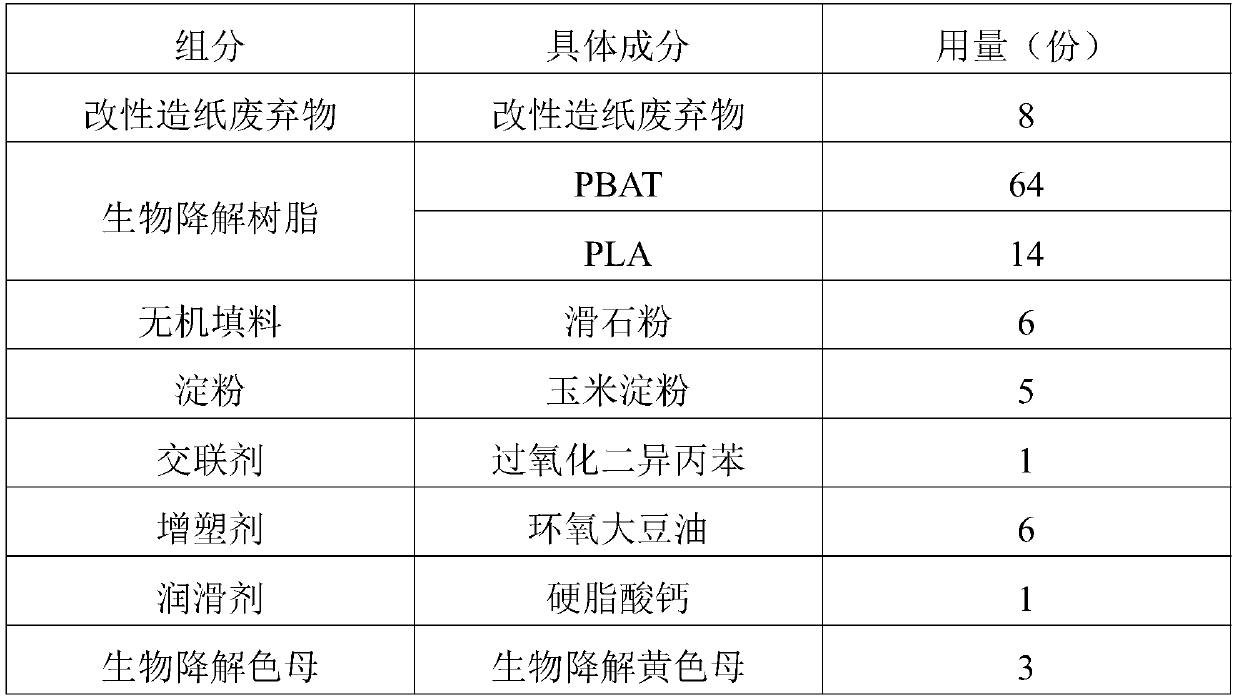
Application of raw material composition in preparation of biodegradable insect trapping plate
InactiveCN111234477AHigh tensile strengthHigh elongation at breakInsect catchers and killersBiotechnologyChloroacetyl chloride
The invention discloses an application of a raw material composition in preparation of a biodegradable insect trapping plate. The raw material composition comprises the following components in parts by weight: 5-20 parts of modified papermaking waste, 50-90 parts of a biodegradable resin and 1-10 parts of auxiliaries, wherein the modified papermaking waste comprises 100 parts of papermaking waste,1-50 parts of a modifier and 0.1-5 parts of a phase transfer agent, and the modifier is one or more of stearoyl chloride, acetyl chloride, benzoyl chloride, oxalyl chloride, chloroacetyl chloride andtrichloroacetyl chloride. The master batch of the biodegradable insect trapping plate and the biodegradable insect trapping plate can be completely degraded by microorganisms in the nature, so that the environment is not polluted, waste utilization can be achieved, and the cost is reduced. The modified papermaking waste is adopted, so that the compatibility of the modified papermaking waste and biodegradable resin can be effectively improved, and the tensile strength and elongation at break of the biodegradable insect trapping plate are improved. The insect trapping amount of the biodegradable insect trapping plate is obviously superior to that of a traditional PP insect trapping plate and that of a traditional PVC insect trapping plate.
Owner:SHANGHAI CHANGFA NEW MATERIAL CO LTD
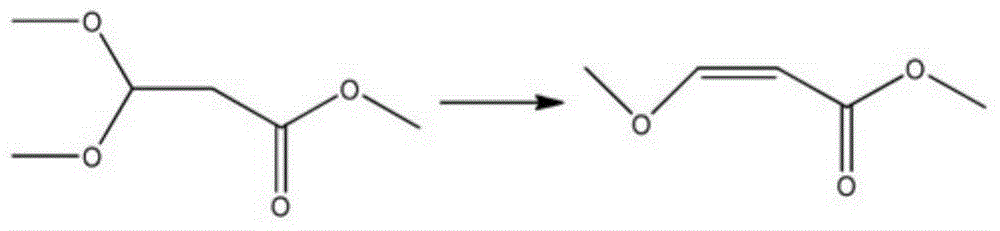
Preparation method of methyl 3-methoxyacrylate
InactiveCN104058960AConveniently preparedHigh boiling pointOrganic compound preparationCarboxylic acid esters preparationHydrogen SulfateBoiling point
The invention discloses a preparation method of methyl 3-methoxyacrylate. Methyl 3-methoxy-3-R-oxypropionate and methanol used as raw materials react to prepare the methyl 3-methoxyacrylate by a one-pot process under the action of a catalyst potassium hydrogen sulfate, sodium bisulfate or p-toluenesulfonic acid. The chemical equation is disclosed in the specification, wherein R represents propyl or butyl group. The raw material methyl 3-methoxy-3-R-oxypropionate can be conveniently prepared from vinyl R ether and trichloro-acetic chloride; and the vinyl R ether has higher boiling point, and thus, is convenient for transportation and storage. The methyl 3-methoxy-3-R-oxypropionate and methanol are synthesized into the target product by the one-pot process under the action of the catalyst; and thus, the method is simple and easy to operate, and is suitable for industrial production.
Owner:HUNAN HAILI CHEM IND
Synthesis process of trichloro pyridyl sodium alcoholate
InactiveCN101020656AAvoid addition reactionsSave raw materialsOrganic chemistryAcrylonitrileNitrobenzene
The present invention is synthesis process of trichloro pyridyl sodium alcoholate. The synthesis process includes the following steps: reaction of trichloroacetyl chloride, acrylonitrile, nitrobenzene and catalyst in a reactor at 40 -60 deg.c for 20-40 min; adding HCl gas release controlling agent and refluxing at 70-160 deg.c for 6-10 hr; adding 15-25 % concentration NaOH solution, heating to 75-85 deg.c and refluxing for 3-4 hr; cooling to 25-35 deg.c and filtering to obtain coarse product; decompressing the filtrate to recover solvent, re-crystallizing the coarse product for purifying at 40-70 deg.c in a internally circular crystallizer; centrifugally separating the mother liquid and drying to obtain the product; and returning the mother liquid to the crystallizer for reuse. The present invention has high product quality, high yield, less wastes and low production cost.
Owner:BLUESTAR LEHIGH ENG INST CO LTD
Method for preparing 3, 5, 6-trichloropyridin-2-ol sodium
The invention discloses a method for preparing 3, 5, 6-trichloropyridin-2-ol sodium. The method comprises the steps of esterification reaction, addition reaction, cyclization reaction, hydrolysis reaction and the like. In the method, trichloroacetic chloride is used as the chlorinating agent of the cyclization reaction; and by dropwise adding alcohol, the residual trichloroacetic chloride is converted to trichloroacetate. Therefore, the problem of cyclization material conversion can be solved and the damage of the chlorinating agent with high corrosivity to human body can be reduced. The method is characterized in that trichloroacetate can further be used as the addition raw material, thus the utilization rate of trichloroacetic chloride can be greatly increased, the emission of the phosphorus-containing wastewater can be reduced and the molar yield of sodium alkoxide can reach 68%.
Owner:JIANGSU JIUJIUJIU TECH
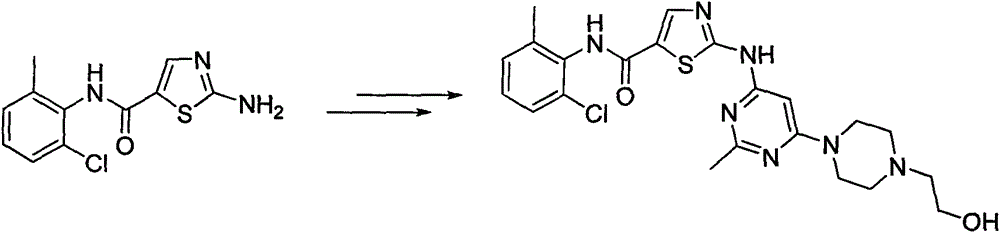
A kind of synthetic method of 2-amino-n-(2-chloro-6-methylphenyl) thiazole-5-carboxamide
The invention relates to a 2-amino-N-(2-chloro-6-methyl phenyl)thiazole-5-carboxamide synthesis method, wherein the compound 2-amino-N-(2-chloro-6-methyl phenyl)thiazole-5-carboxamide is a key intermediate for synthesizing dasatinib. The synthesis method comprises that: 1) trichloroacetyl chloride and ethyl vinyl ether are subjected to a nucleophilic substitution and hydrolysis reaction one-pot method to synthesize 3-ethoxyacrylic acid; 2) 3-ethoxyacrylic acid is subjected to an acyl chlorination and amidation reaction one-pot method to synthesize N-(2-chloro-6-methyl phenyl)-3-ethoxy acrylamide; and 3) N-(2-chloro-6-methyl phenyl)-3-ethoxy acrylamide, NBS and thiourea are subjected to a reaction to obtain 2-amino-N-(2-chloro-6-methyl phenyl)thiazole-5-carboxamide. The synthesis method has characteristics of yield increase, cost reduction, operation simplifying, and high implementation value.
Owner:ZHEJIANG LUOXING IND CO LTD
Synthetic method of chlorpyrifos
ActiveCN102775443AIncrease valueReduce pollutionGroup 5/15 element organic compoundsAlkali metal chloridesChlorpyrifosAcrylonitrile
The invention discloses a synthetic method of chlorpyrifos, which comprises the following steps of: enabling trichloro-acetic chloride, acrylonitrile, o-dichlorobenzene and a CuCl catalyst to react to obtain a crude product; adding the crude product into a reaction kettle containing water, a catalyst and alkali, raising the temperature and backwardly flowing for 3-4 hours, standing and separating out a solvent, cooling and crystallizing a water phase, centrifuging to obtain a trichloropyridine alcohol potassium tide product and drying to obtain a trichloropyridine alcohol potassium finished product; and adding the trichloropyridine alcohol potassium finished product into the reaction kettle, adding water, boric acid and a catalyst, agitating, raising the temperature to 75-80 DEG C, maintaining the pH to be equal to 9-10, dripping ethyl chloride, maintaining for 3-5 hours, standing and phase separation, separating out an oil phase, adding the oil phase into absolute ethyl alcohol for recrystallization, filtering and air-drying to obtain a chlorpyrifos finished product. The synthetic method has the advantages of easiness in operation, high yield and reduced environment pollution and provides guarantee for sustainable development.
Owner:JIANGSU JIUJIUJIU TECH
Method for preparing 3,5,6-trichloro pyridine-2-sodium alcoholate without solvent
The invention discloses a making method of 3, 5, 6-trichloropyridine-2-sodium alcoholate, which comprises the following steps: reacting trichloroacetyl chloride and acrylonitrile under copper halide at 80-100 deg.c; aromatizing in the alkali solution to obtain the product. The invention avoids operator from danger, which recycles high-toxic solvent and catalyst.
Owner:EAST CHINA UNIV OF SCI & TECH +1
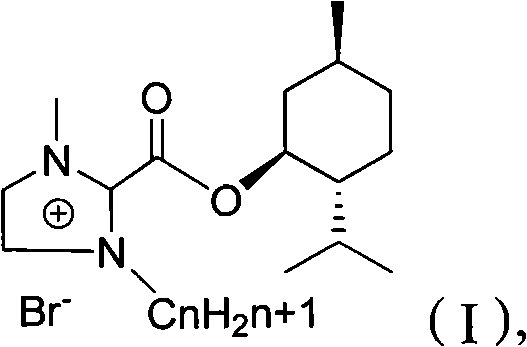
Preparation method of imidazole chiral ionic liquid replaced by C-2
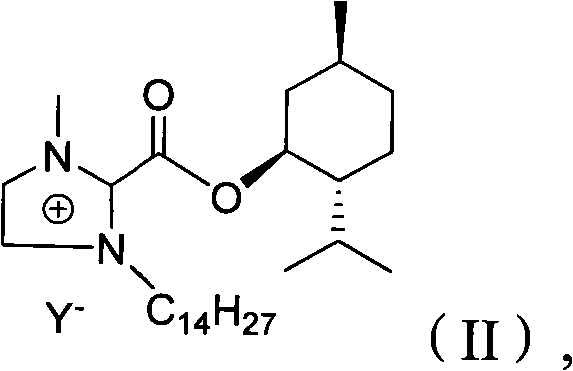
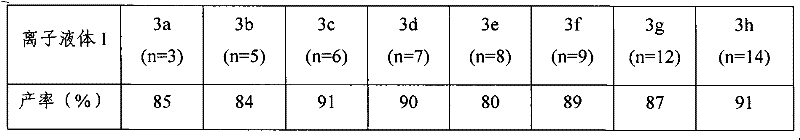
InactiveCN102516180AEasy to prepareRaw materials are easy to getOrganic chemistryMenthol1-Methylimidazole
The invention relates to a preparation method of imidazole chiral ionic liquid replaced by C-2, which comprises the following steps of: acylating 1-methylimidazole and trichloroacetic chloride, esterfying with chiral menthol and carrying out chiral fragment reaction at 1-methylimidazole C-2. According to the preparation method of the imidazole chiral ionic liquid replaced by the C-2, natural menthol is introduced into a chiral center by adopting a green chemical thinking method and utilizing a chiral pool method to prepare the imidazole chiral ionic liquid replaced by the C-2 under the microwave-assisted catalysis. The preparation method has the advantages of simplicity, easiness for raw material obtaining, low cost and short reaction time, thereby having a wide development prospect.
Owner:中国人民解放军63975部队
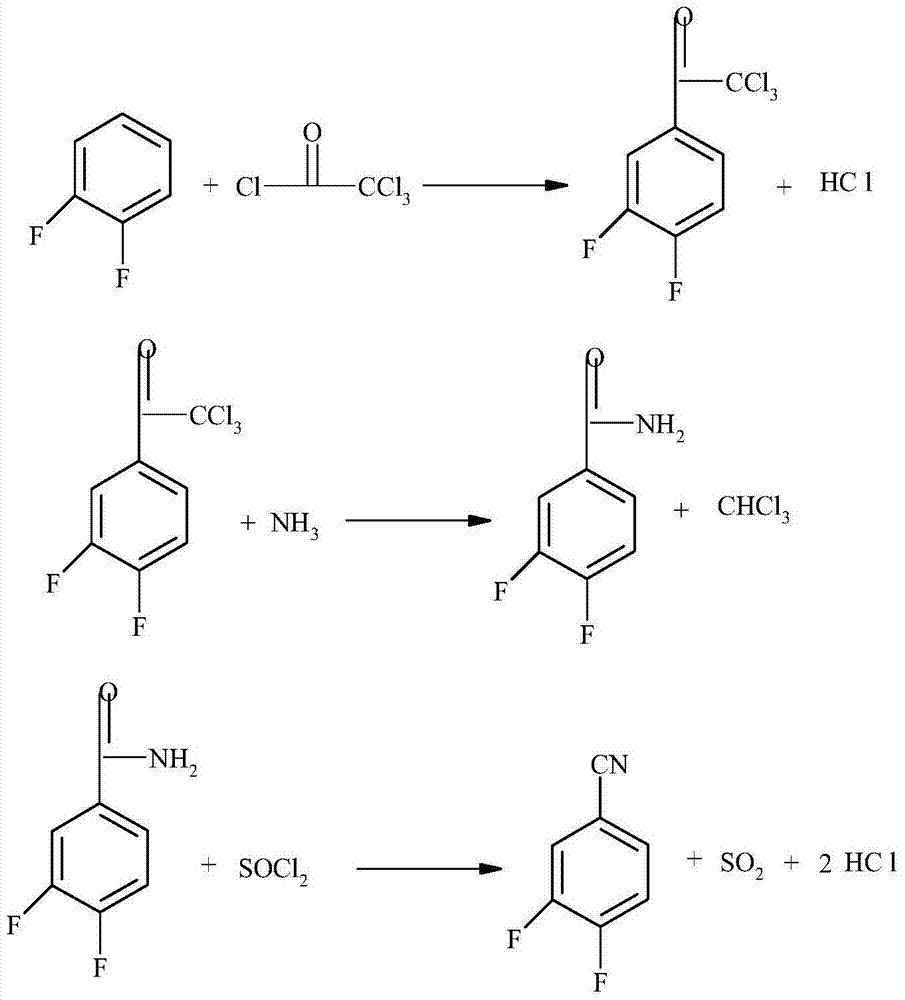
3, 4-difluorobenzonitrile preparation method
ActiveCN103709071AHigh purityHigh yieldPreparation by carboxylic acid amide dehydrationLewis acid catalysisTrichloroacetyl chloride
The invention discloses a 3, 4-difluorobenzonitrile preparation method comprising the following steps: (1) reacting 1, 2-difluorobenzene with trichloroacetic chloride in the presence of a lewis acid catalyst at 0-40 DEG C for synthesis of 3, 4-difluoro-(alpha, alpha, alpha-trichloroacetic) benzene; (2) reacting the 3, 4-difluoro-(alpha, alpha, alpha-trichloroacetic) benzene with ammonia at -10-60 DEG C to obtain 3, 4-difluorobenzamide; (3) reacting the 3, 4-difluorobenzamide with a halogen-containing dehydration reagent and a catalyst at 30-80 DEG C to obtain 3, 4-difluorobenzonitrile. The 3, 4-difluorobenzonitrile preparation method overcomes the defects that in the prior art the raw material price is high, intermediates are highly toxic, reaction conditions are harsh, the reaction yield is poor, the purity is low, and the like, and is a 3, 4-difluorobenzonitrile synthesis process having the advantages of being easy to industrialization, simple in operation, high in yield and high in product purity.
Owner:JIANGSU FLAG CHEM IND
Novel process for producing chlopyrifos from pyridone by using aqueous phase method
InactiveCN103086960AHigh yieldSave raw materialsBiocideGroup 5/15 element organic compoundsChemical synthesisTrichloroacetyl chloride
The invention relates to a novel process for producing chlopyrifos from pyridone by using an aqueous phase method, and belongs to the field of chemical synthesis. The novel process is finished by the following steps of mixing trichloro-acetic chloride, acrylonitrile and a solvent according to a certain molar ratio to form the pyridine under the effect of a catalyst; putting desalinized water, pyridone, sodium chloride, caustic soda flake (adjusting pH value of the system within 8 to 10), boric acid, an emulsifier and a phase-transfer catalyst into a condensation kettle successively; stirring uniformly; adding a certain amount of 0,0-diethyl thiophosphoryl chloride slowly; reacting at a temperature of 45-50 DEG C to form a chlopyrifos solution; layering; dehydrating; press filtering; cooling; crystallizing; centrifuging for separation and drying to obtain a raw powder of the chlopyrifos with a purity higher than 99%. The novel process reduces production cost, has little process wastewater and can synthesize the raw powder of the chlopyrifos with relatively high purity.
Owner:SHANXI KANGDELI FINE CHEM
Chlorpyrifos aqueous-phase synthesizing method with trichloro-acetic chloride as primary raw material
PendingCN105348323ASimple methodEasy to operateGroup 5/15 element organic compoundsChlorpyrifosFiltration
The invention discloses a chlorpyrifos aqueous-phase synthesizing method with trichloro-acetic chloride as a primary raw material. The chlorpyrifos aqueous-phase synthesizing method includes the steps that the trichloro-acetic chloride, acrylonitrile, ortho-dichlorobenzene and a composite catalyst are reacted to obtain crude sodium trichloro pyridinol; the crude sodium trichloro pyridinol is put into a reaction still filled with water, a catalyst and alkali, heating and refluxing are carried out for 3 hours, a solvent is separated through standing, and sodium trichloro pyridinol is obtained in an aqueous-phase mode through cooling crystallizing and filtering; water is put into the reaction still, a dispersing agent, the sodium trichloro pyridinol, ethyl chloride and a catalyst are added while stirring and reacted, cooling crystallizing is carried out, and a chlorpyrifos raw-medicine finished product is obtained in a washing and suction-filtration mode. According to the chlorpyrifos aqueous-phase synthesizing method, the high-purity sodium trichloro pyridinol is prepared with the trichloro-acetic chloride as the primary raw material, and directly used as a raw material for synthesizing chlorpyrifos, and the sodium trichloro pyridinol produced with the method does not need to be refined, purified and dried. The water serves as a medium, the reaction materials are once added to synthesize the chlorpyrifos, the method is simple, operation is easy, environmental protection and energy saving are achieved, the product yield is increased, and product purity is improved.
Owner:山东谦诚工贸科技有限公司
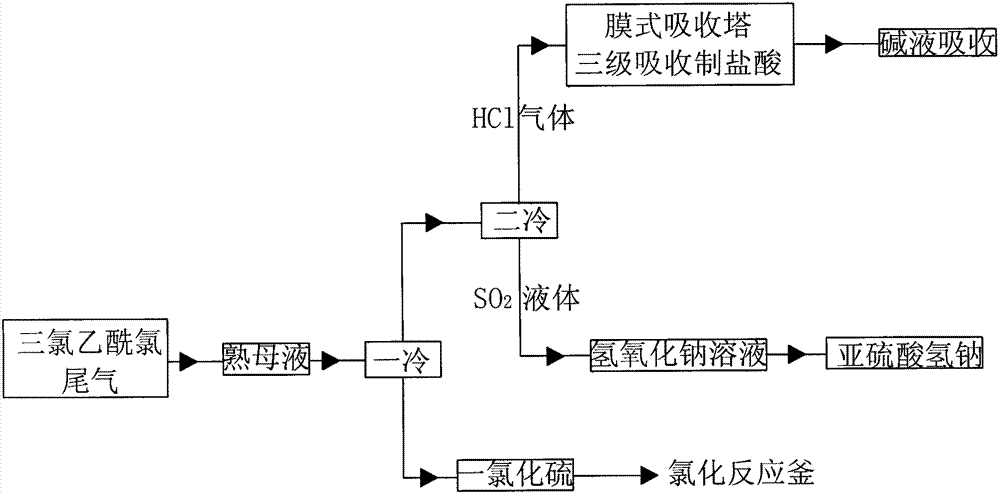
Novel method for absorbing tail gas from trichloro-acetic chloride production through chlorination process
InactiveCN102764572AEfficient separationEfficient recyclingOrganic compound preparationDispersed particle separationDisulfur dichlorideSodium bisulfite
The present invention relates to a novel method for absorbing tail gas from trichloro-acetic chloride production through a chlorination process. The method can realize separation and recovery of hydrogen chloride, sulfur dioxide, sulfur, disulfur dichloride, and chlorine generated in the production process of trichloro-acetic chloride; the method is safe and easily controlled, has no harm to the environment, and generates by-products containing hydrochloric acid with concentration higher than 30% and a sodium bisulfite solution. The invention belongs to the technical field of environmental protection.
Owner:SHANXI SANWEI FENGHAI CHEM
Synthesis method of 3,5,6-trichloropyridyl-2-sodium alkoxide
The invention provides a synthesis method of 3,5,6-trichloropyridyl-2-sodium alkoxide, which comprises the following steps: by using trichloro-acetic chloride and acrylonitrile as raw materials and cuprous chloride and tetrabutyl ammonium chloride as catalysts, adding a cocatalyst, carrying out addition, cyclization and aromatization reaction to prepare the 3,5,6-trichloropyridyl-2-sodium alkoxide. The method maximally controls the generation of the side reaction, and the yield of the 3,5,6-trichloropyridyl-2-sodium alkoxide can reach higher than 80%. Meanwhile, the method has the advantages of accessible raw materials, low price and mild technological conditions, and is beneficial to industrial production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
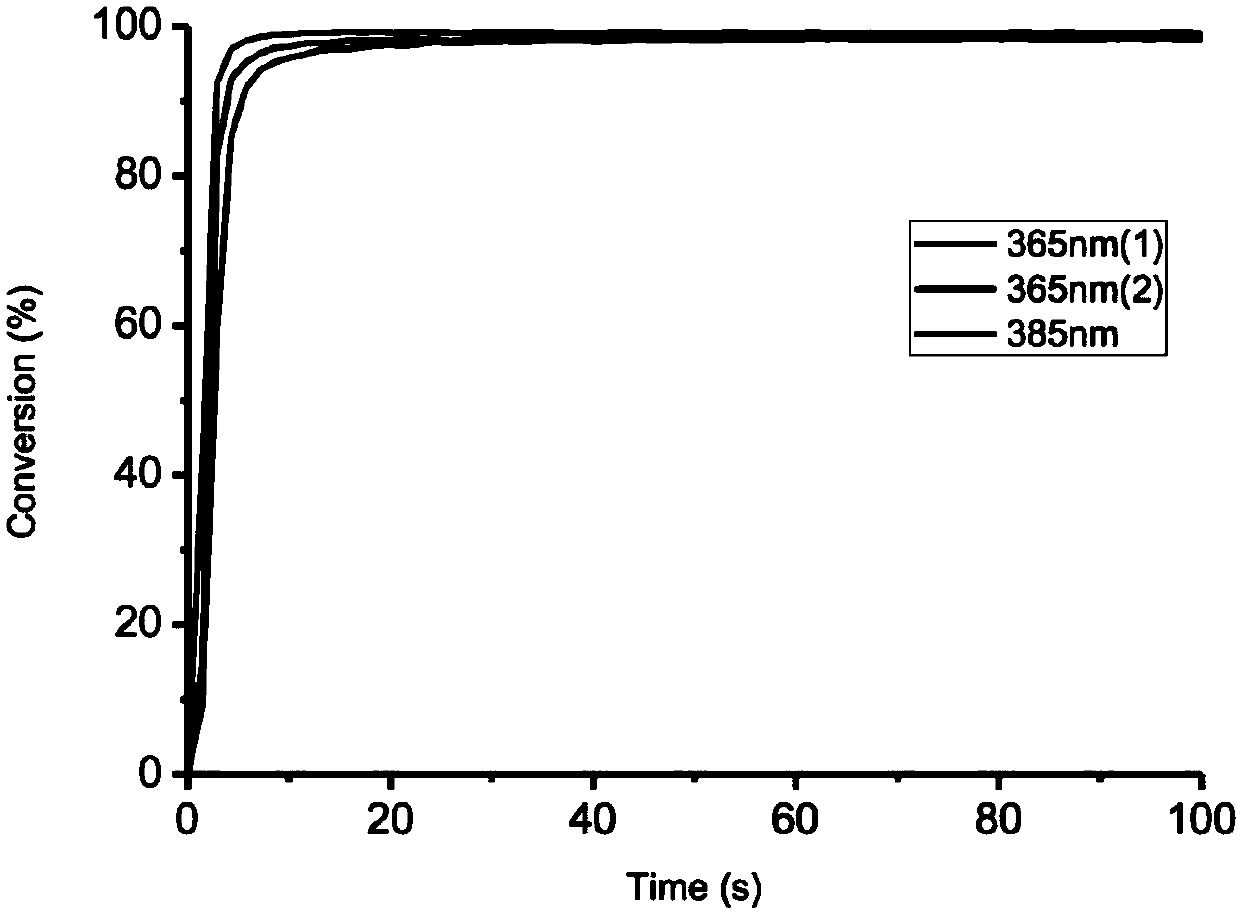
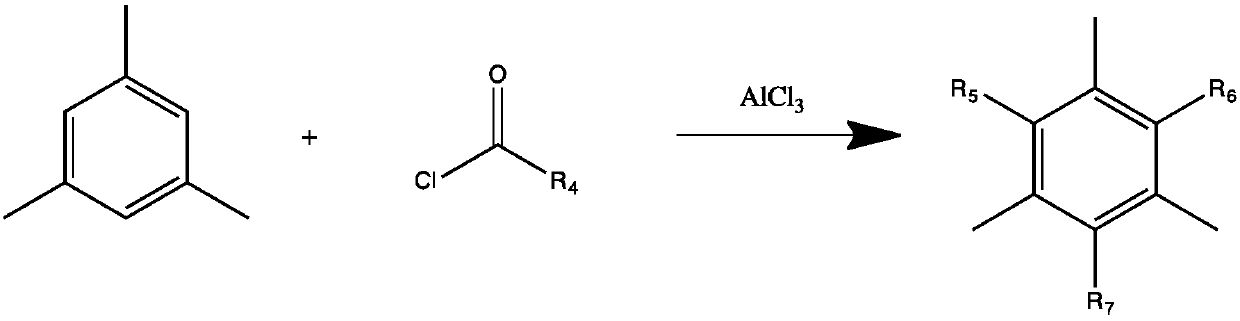
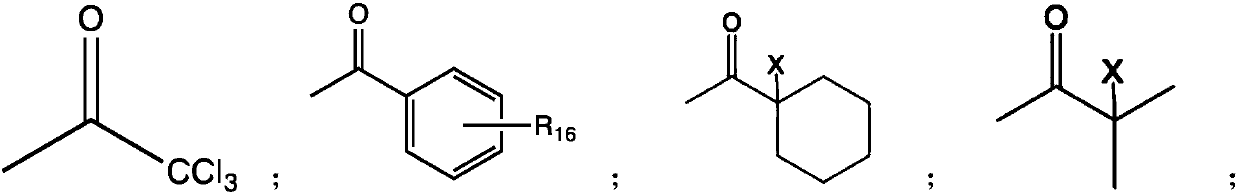
Preparation method of macromolecular low-migration phosphine oxide UV-LED photoinitiators
ActiveCN109897063ASolving High Migration ProblemsSolve the problem of surface curing and non-dryingGroup 5/15 element organic compoundsPhotosensitive materials for photomechanical apparatusPhosphine oxidePhosphorus trichloride
The invention provides a preparation method of macromolecular low-migration phosphine oxide UV-LED photoinitiators. The preparation method comprises the steps of using 1,3,5-trimethylbenzene as a rawmaterial, enabling the raw material to be subjected to a Friedel-Craft reaction with trichloro-acetic chloride or benzoyl chloride under the catalytic action of Lewis acid or solid superacid, hydrolyzing the obtained product under acidic or basic conditions to obtain a mono-, di-, or tricarboxy-substituted 2,4,6-trimethylbenzene, enabling the mono-, di-, or tricarboxy-substituted 2,4,6-trimethylbenzene to interact with phosphorus trichloride, thionyl chloride or phosgene to form acyl chloride, enabling the acyl chloride and ethoxyphenylphosphine or diethoxyphenylphosphine to be subjected to phosphine oxide reaction to obtain macromolecular low-migration phosphine oxide UV-LED photoinitiators; the invention claims a new structure of new type of macromolecular phosphine oxide initiators, andobtains a polyacyl chloride substituent by an innovative method, thereby obtaining a polyfunctional macromolecule phosphine oxide initiator.
Owner:TIANJIN MOSEN TECH CO LTD
New synthesis technique of 3,5,6-trichloropyridyl-2-sodium alkoxide
InactiveCN104177290AHigh selectivitySolve the problem of low yield and many by-productsOrganic chemistryPtru catalystAcetyl chloride
The invention provides a new synthesis technique of 3,5,6-trichloropyridyl-2-sodium alkoxide, which comprises the following steps: by using trichloro-acetic chloride and acrylonitrile as raw materials, carrying out one-step addition cyclization under the actions of a specific solvent and a specific catalyst to obtain 3,3,5,6-tetrachloro-4,4-dihydropyridyl-2-one, desolventizing, crystallizing, and carrying out alkali resolution to obtain the 3,5,6-trichloropyridyl-2-sodium alkoxide. The technique has the characteristics of lower cost of production equipment, simple production equipment, short process, higher reaction yield and the like, and is simple to operate and suitable for industrial production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Synthetic methods of 7-azaspiro[3,5]-nonane-2-ol and hydrochloride compound thereof
The invention discloses 7-azaspiro-[3,5]-nonane-2-ol and a hydrochloride compound thereof. Concretely, N-Boc-4-piperidone is used as a raw material, a wittig reaction is carried out in order to prepare N-Boc-4-methylenepiperidine, zinc / copper is used for catalysis of trichloroacetyl chloride in order to carry out [2+2] cyclisation for preparing N-Boc-7-azaspiro ketone; the azaspiro ketone intermediate is reduced into N-Boc-7-azaspiro-ol by sodium borohydride at a room temperature, and finally 2mol / L hydrochloric acid-ethyl acetate is used for removing Boc in order to prepare a target product hydrochloride whose purity reaches 98%. The product has the advantages of economical and easily available reagents and raw materials which are needed, simple operation, and high product purity; and the product is suitable for batch production.
Owner:苏州汉德创宏生化科技有限公司
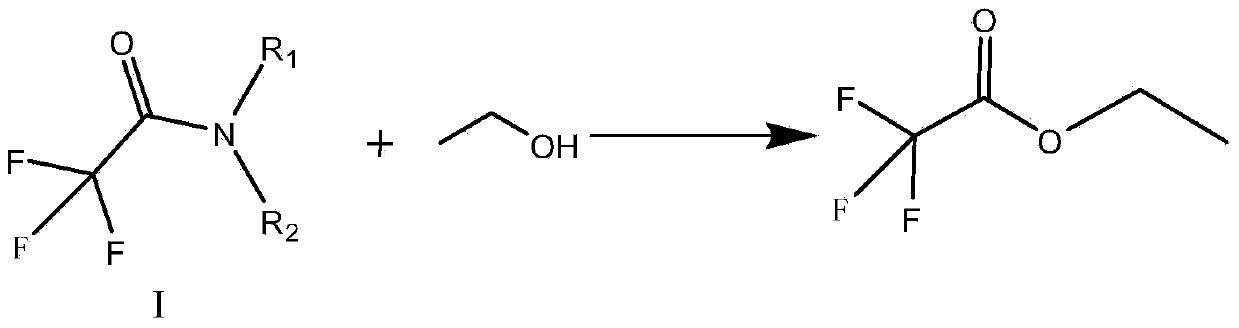
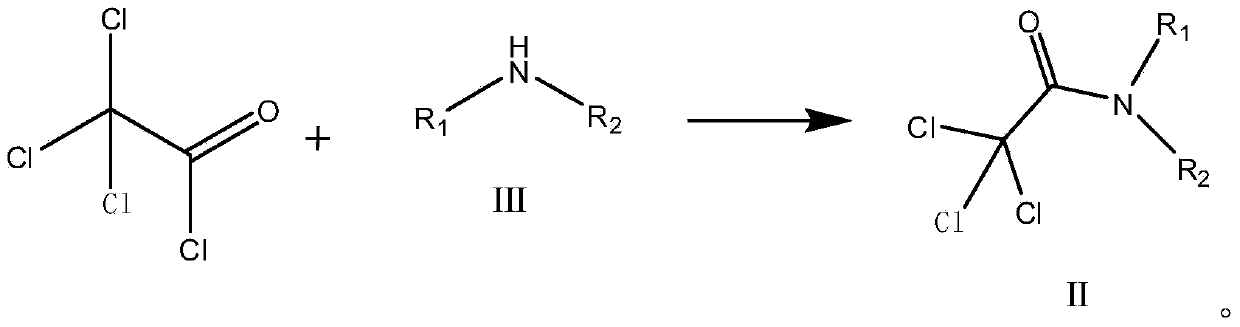
Preparation method of trifluoroacetic acid ethyl ester and preparation method of intermediate of trifluoroacetic acid ethyl ester
InactiveCN103694118AInhibitionShort reaction timeOrganic compound preparationCarboxylic acid esters preparationSolventIonic liquid
The invention discloses a preparation method of a trifluoroacetic acid ethyl ester and a preparation method of an intermediate of the trifluoroacetic acid ethyl ester. The preparation method of the trifluoroacetic acid ethyl ester comprises the following steps: adding dropwise a mixed solution of a compound I and ethyl alcohol into concentrated sulfuric acid at the temperature of 95-100 DEG C, and keeping the temperature at 95-100 DEG C to react until distilling generated trifluoroacetic acid ethyl ester in the dropping process, wherein R1 and R2 independent represent phenyl or C1-3 linear chain or branched chain alkyl, and when R1 and R2 are respectively independent C1-3 linear chain or branched chain alkyl, R1 and R2 are different. The preparation method takes trichloro-acetic chloride and secondary amine as starting materials, the production process is simple and convenient, a green solvent ionic liquid is used in the fluoridation, the three wastes are few, the reaction condition is mild, the energy consumption is reduced, the yield is high, and the preparation method is applicable to industrial production.
Owner:联化科技(上海)有限公司 +3
3,5,6-trichloropyridin-2-ol sodium synthesis method
InactiveCN104163789AReduce generationIncrease contentOrganic chemistryWater dischargeSynthesis methods
The invention discloses a novel method for fractional step method-based preparation of 3,5,6-trichloropyridin-2-ol sodium from trichloro-acetic chloride and acrylonitrile as main raw materials. Trichloro-acetic chloride and acrylonitrile undergo a series of reactions under the action of a solvent and a catalyst to produce 3,5,6-trichloropyridin-2-ol sodium, and tetrachloropyridine as a side product does not need separation and purification and can be directly treated and transformed into 3,5,6-trichloropyridin-2-ol sodium as a target product by an alkali liquid so that a yield is greatly improved. The 3,5,6-trichloropyridin-2-ol sodium synthesis method is simple, has a less waste water discharge capacity, realizes high-efficiency 3,5,6-trichloropyridin-2-ol sodium synthesis by simple and easy processes, produces the high-purity high-yield product and is suitable for large-scale industrial production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
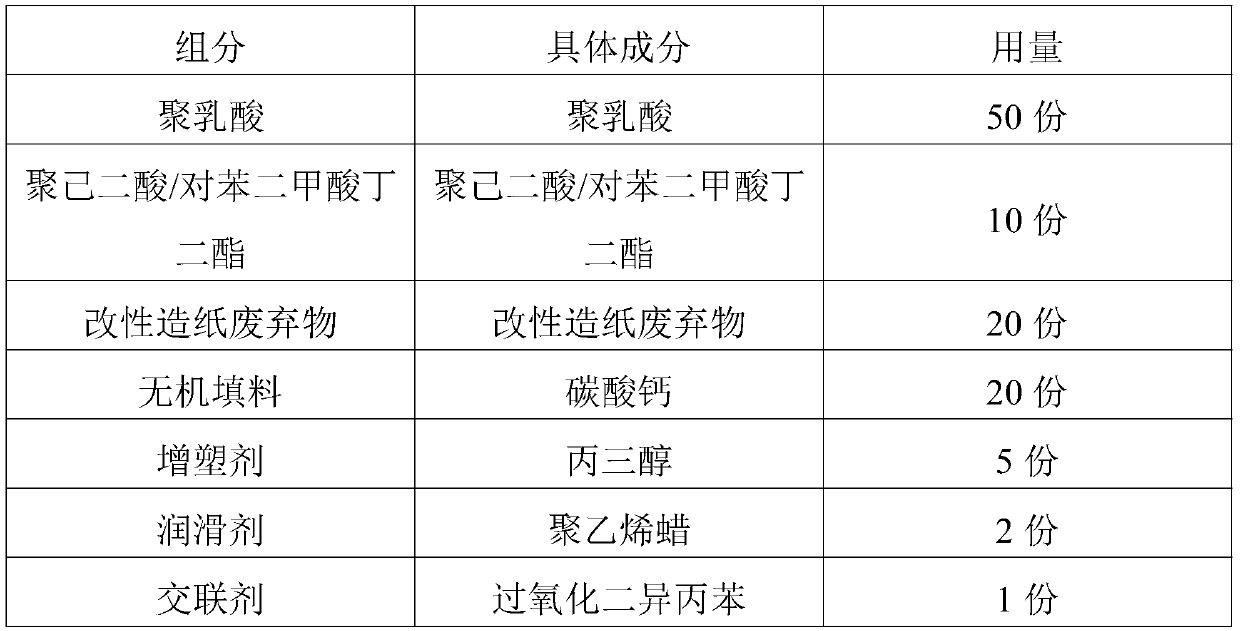
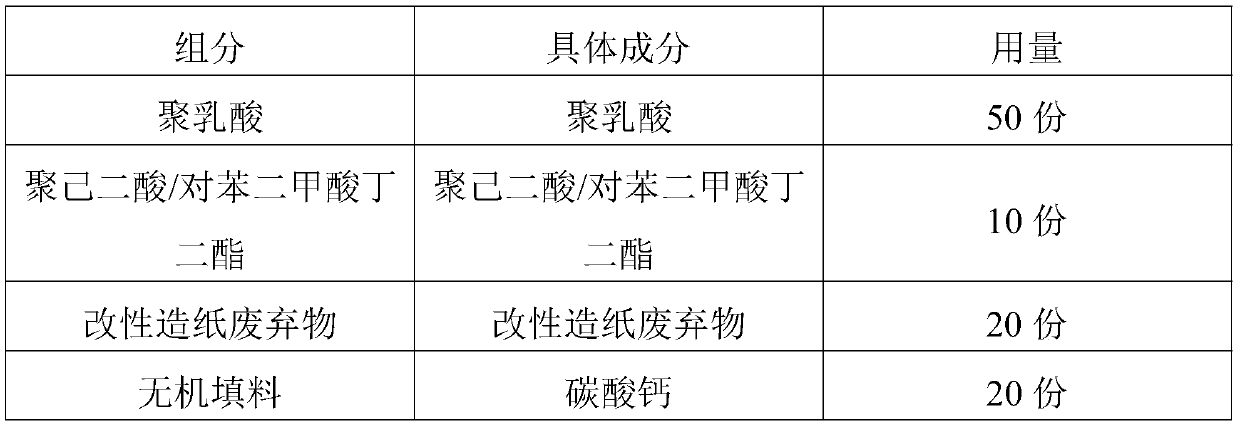
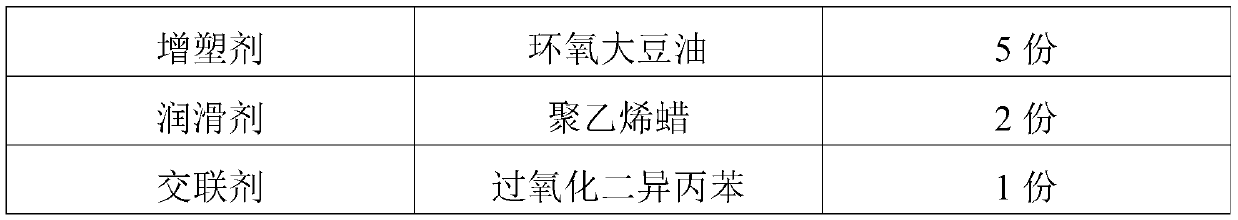
Raw material composition and master batch of biodegradable material and preparation method and application thereof
The invention discloses a raw material composition and master batch of a biodegradable material and a preparation method and application thereof. The raw material composition of the biodegradable material comprises the following components in parts by weight: 20-80 parts of polylactic acid, 1-40 parts of poly(butylene adipate-co-terephthalate), 1-40 parts of modified papermaking waste and 1-20 parts of an auxiliary agent, and the raw material composition of the modified papermaking waste comprises the following components in parts by weight: 100 parts of papermaking waste, 1-50 parts of a modifier and 0.1-5 parts of a phase transfer agent, wherein the modifier is one or more of stearoyl chloride, acetyl chloride, benzoyl chloride, oxalyl chloride, chloroacetyl chloride and trichloroacetylchloride. The biodegradable master batch and the material are easy to degrade in nature, the raw materials are renewable, and the cost is low. The prepared biodegradable material has good bending strength, flexural modulus and notch-free impact strength.
Owner:SHANGHAI CHANGFA NEW MATERIAL CO LTD
Production technology of trichloroacetyl chloride
PendingCN105152914AReduce pollutionSimple processOrganic compound preparationCarboxylic compound preparationChloroacetic acidsEthyl Chloride
The invention relates to a production technology of trichloroacetyl chloride. Chloroacetic acid waste liquor is used as a raw material and belongs to the technical field of organic chemicals and preparation. The production method comprises steps as follows: (1) the chloroacetic acid waste liquor refined by a rectifying column is pumped into an acylation kettle with vacuum, a certain amount of sulfur is added, chlorine is fed at a certain temperature for a reaction, cross chlorination with main and auxiliary tanks is adopted to enable the chlorine to fully react, and mixed acyl chloride is produced through the reaction; (2) the mixed acyl chloride is added to a chlorination kettle, pyridine is used as a catalyst, 4-dimethylamino pyridine is used as a promoter, the chlorine is fed for a reaction at a certain temperature, and the trichloroacetyl chloride is produced. The raw material is wide in source, the production cost is low, and the production technology is suitable for industrial production.
Owner:山东谦诚工贸科技有限公司
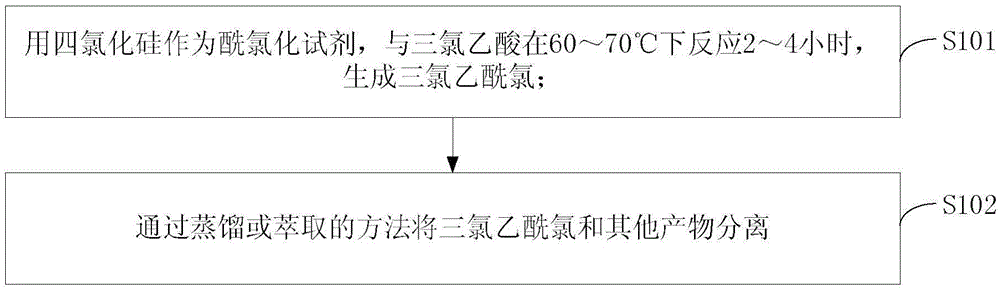
Method of preparing trichloro-acetic chloride from silicon tetrachloride
InactiveCN105601501ALow costOrganic compound preparationCarboxylic compound preparationDistillationChloride
The invention discloses a method of preparing trichloro-acetic chloride from silicon tetrachloride. Silicon tetrachloride serves as an acylating chlorination reagent and reacts with trichloroacetic acid for 2-4 hours at 60 DEG C-70 DEG C to generate trichloro-acetic chloride, and then trichloro-acetic chloride and other products are separated through distillation or extraction. A lot of byproduct silicon tetrachloride generated in polycrystalline silicon production and organic silicon production is effectively converted through the method, and high-additional-value trichloro-acetic chloride is produced; byproduct silicon tetrachloride which is brought by production development of polycrystalline silicon and is difficult to treat is converted, and meanwhile the cost for preparing trichloro-acetic chloride is obviously lowered.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
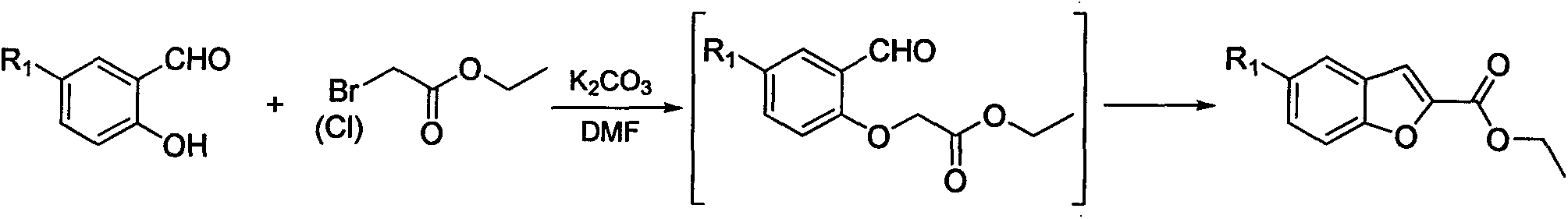
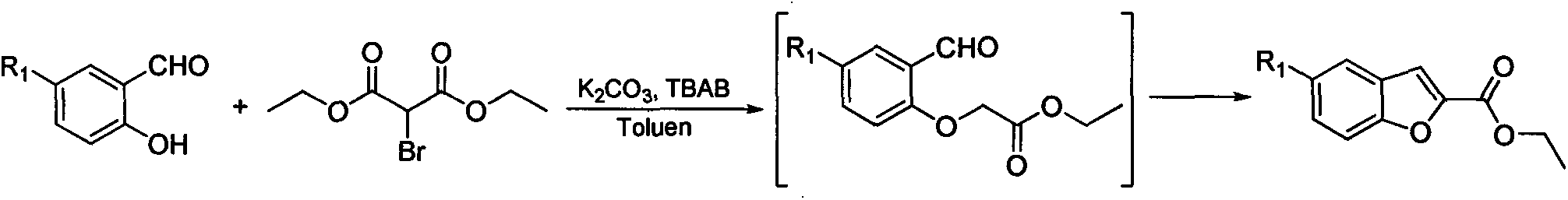
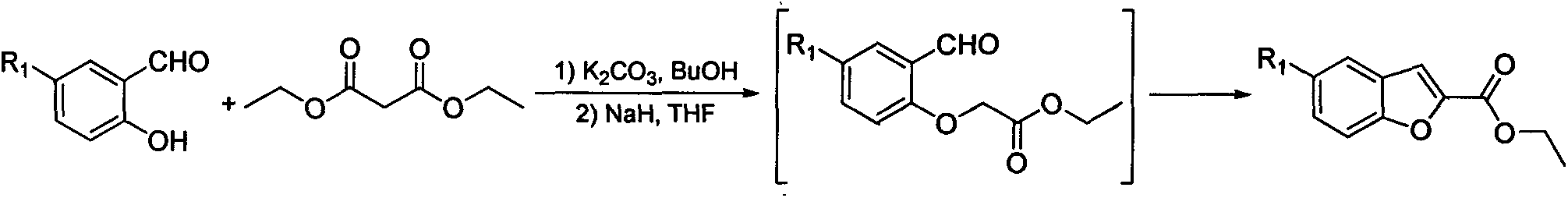
Synthesis method for 5-substituted benzofuran-2-carboxylic acid and derivatives thereof
InactiveCN103450125AMild reaction conditionsSimple processOrganic chemistryPhosphoniumTriphenylphosphine
The invention discloses a synthesis method for 5-substituted benzofuran-2-carboxylic acid and the derivatives thereof. The synthesis method comprises the following steps of: 1) performing a chloromethylation reaction or a bromomethylation reaction on 4-substituted phenol to generate 2-halogenated methyl-4-substituted phenol; 2) reacting 2-halogenated methyl-4-substituted phenol with triphenylphosphine to generate 2-hydroxyl-5-substituted halogenated benzyltriphenyl phosphonium; 3) performing a ring-closing reaction on 2-hydroxyl-5-substituted halogenated benzyltriphenyl phosphonium and trichloroacetyl chloride in an alkaline condition to obtain 2-trichloromethyl-5-substituted benzofuran; and 4) performing alcoholysis on 2-trichloromethyl-5-substituted benzofuran in an acidic condition to obtain 5-substituted benzofuran-2-carboxylic ester, and then performing functional group conversion to obtain a series of derivatives. According to the synthesis method provided by the invention, cheap and easily-available raw materials are used, thus reducing production cost; and moreover, the synthesis method is simple and convenient in process, easy to enlarge, and high in implement value.
Owner:CHIM LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
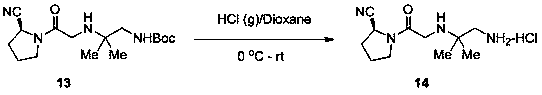
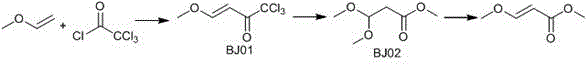


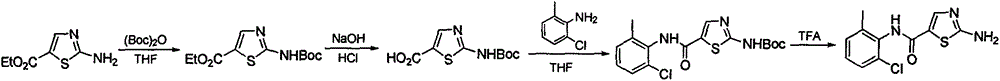

![Synthetic methods of 7-azaspiro[3,5]-nonane-2-ol and hydrochloride compound thereof Synthetic methods of 7-azaspiro[3,5]-nonane-2-ol and hydrochloride compound thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5f0ae501-3aba-491b-8b16-330936c5a129/161215110137.png)
![Synthetic methods of 7-azaspiro[3,5]-nonane-2-ol and hydrochloride compound thereof Synthetic methods of 7-azaspiro[3,5]-nonane-2-ol and hydrochloride compound thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5f0ae501-3aba-491b-8b16-330936c5a129/BDA0001181222100000021.png)
![Synthetic methods of 7-azaspiro[3,5]-nonane-2-ol and hydrochloride compound thereof Synthetic methods of 7-azaspiro[3,5]-nonane-2-ol and hydrochloride compound thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5f0ae501-3aba-491b-8b16-330936c5a129/BDA0001181222100000022.png)